13.3
Impact Factor
Theranostics 2012; 2(9):850-870. doi:10.7150/thno.4547 This issue Cite
Research Paper
Syntheses and Photodynamic Activity of Pegylated Cationic Zn(II)-Phthalocyanines in HEp2 Cells
Louisiana State University, Department of Chemistry, Baton Rouge LA, 70803, USA
Received 2012-5-2; Accepted 2012-6-11; Published 2012-9-20
Abstract
Di-cationic Zn(II)-phthalocyanines (ZnPcs) are promising photosensitizers for the photodynamic therapy (PDT) of cancers and for photoinactivation of viruses and bacteria. Pegylation of photosensitizers in general enhances their water-solubility and tumor cell accumulation. A series of pegylated di-cationic ZnPcs were synthesized from conjugation of a low molecular weight PEG group to a pre-formed Pc macrocycle, or by mixed condensation involving a pegylated phthalonitrile. All pegylated ZnPcs were highly soluble in polar organic solvents but were insoluble in water; they have intense Q absorptions centered at 680 nm and fluorescence quantum yields of ca. 0.2 in DMF. The non-pegylated di-cationic ZnPc 6a formed large aggregates, which were visualized by atomic force microscopy. The cytotoxicity, cellular uptake and subcellular distribution of all cationic ZnPcs were investigated in human carcinoma HEp2 cells. The most phototoxic compounds were found to be the α-substituted Pcs. Among these, Pcs 4a and 16a were the most effective (IC50 ca. 10 μM at 1.5 J/cm2), in part due to the presence of a PEG group and the two positive charges in close proximity (separated by an ethylene group) in these macrocycles. The β-substituted ZcPcs 6b and 4b accumulated the most within HEp2 cells but had low photocytoxicity (IC50 > 100 μM at 1.5 J/cm2), possibly as a result of their lower electron density of the ring and more extended conformations compared with the α-substituted Pcs. The results show that the charge distribution about the Pc macrocycle and the intracellular localization of the cationic ZnPcs mainly determine their photodynamic activity.
Keywords: phthalocyanine, PDT, pegylation, cationic photosensitizer
Introduction
Phthalocyanines (Pcs), also known as tetraaza-benzoporphyrins, are a class of synthetic tetrapyrrolic compounds related to the naturally occurring porphyrins; they contain an extended 18 π-electron system. Due to their strong absorptions in the near-IR, Pcs have found multiple applications in biology, medicine, and materials science, for example as colorant dyes, molecular wires, optical sensors, bioimaging agents, and as photosensitizers for the photodynamic therapy (PDT) of cancers and for inactivation of bacteria and viruses [1-4]. PDT involves light activation of a photosensitizer with subsequent in situ production of singlet oxygen, and other reactive oxygen species (ROS), which destroy photosensitizer-accumulated cells via necrosis and/or apoptosis [5,6]. Photofrin is a FDA-approved porphyrin, a derivative of hematoporphyrin IX, that has been used for nearly two decades in the PDT treatment of various cancers, including lung, skin, cervical and bladder [7,8]. PDT has several advantages over surgery and radiation therapy, in that it is relatively non-invasive and is a localized form of therapy, in part due to the natural tendency of porphyrin compounds for preferential accumulation in cancer tissues combined with precise light delivery, normally from a diode laser. However, Photofrin has some drawbacks because porphyrins typically absorb only weakly in the red region of the spectrum (λmax = 630 nm) where light penetrates deeper into tissue; in addition Photofrin is a complex mixture of porphyrin monomers and oligomers which tend to persist for long time periods in healthy tissues following systemic administration, causing unwanted side-effects such as patient photosensitivity for several weeks post-PDT. Pcs have emerged as promising second-generation photosensitizers due to their intense absorptions at long wavelengths (λmax > 670 nm), and their unique abilities for crossing cellular membranes and for producing ROS upon light activation. Sulfonated Al(III)Pcs, designated Photosense, and a Si(IV)Pc, designated Pc4, have been evaluated in clinical trials for PDT [9-11]. These Pcs and other potential Pc-based photosensitizers contain peripheral water-solubilizing substituents and/or axial ligands for minimizing aggregation and increasing their solubility in aqueous media, thereby improving their photodynamic activity. Of particular interest are polyethylene glycol (PEG) groups, which can be used as delivery vehicles [12-14] or may be covalently attached to Pcs [15-17] for improved delivery to target tissues. The pegylation of photosensitizers has been shown to increase their water-solubility, serum life and tumor accumulation, while reducing their uptake by the reticuloendothelial system [18-23]. On the other hand, positively charged photosensitizers are of particular interest for PDT [24-31] and for photoinactivation of virus and bacteria [32-34], because of their potential stronger interactions with negatively charged cell membranes and targeted biomolecules (e.g. DNA and RNA), which can result in effective photodamage and overall enhanced photodynamic efficacy. We have recently reported that di-cationic α-substituted ZnPcs are the most phototoxic among a series of Pcs bearing one to eight positively-charged trimethylaminophenoxy groups, and the most promising photosensitizers for PDT [35]. In our continuing investigation of photosensitizers with enhanced biological effectiveness, we report herein the synthesis of a new series of di-cationic phthalocyanines, containing a PEG group or a diglycolic spacer between the ZnPc and the positively charged quaternary ammonium groups. Our studies show that the pegylated di-cationic ZnPcs are more soluble than the non-pegylated analogues, which were observed to form large aggregates by atomic force microscopy.
Methods and Materials
Syntheses
All reagents and solvents were purchased from commercial sources and used without further purification, unless otherwise noted. Silica gel 60 (230×400 mesh, Sorbent Technologies) was used for column chromatography and Sephadex G-100 and LH-20 (Amersham Biosciences) were used for purification of the water-soluble ZnPcs. Analytical thin-layer chromatography (TLC) was carried out using polyester backed TLC plates 254 (precoated, 200 μm) from Sorbent Technologies. NMR spectra were recorded on an AV-400 LIQUID Bruker spectrometer (400 MHz for 1H, 100 MHz for 13C). The chemical shifts are reported in δ ppm using the following deuterated solvents as internal references: Acetone-d6 2.05 ppm (1H), 29.92 ppm (13C); DMF-d7 8.03 ppm (1H), 163.15 ppm (13C); THF-d8 3.58 ppm (1H), 67.57 ppm (13C); CDCl3 7.27 ppm (1H), 77.23 ppm (13C); DMSO-d6 2.50 ppm (1H), 39.51 ppm (13C). MALDI-TOF mass spectra were recorded on a Bruker UltrafleXtreme (MALDI-TOF/TOF) using 4-chloro-α-cyanocinnamic acid as the matrix; high resolution ESI mass spectra were obtained on an Agilent Technologies 6210 TOF LC/MS. Absorption spectra were measured on a UV-vis NIR scanning spectrophotometer using 10 mm path length quartz cuvettes. Stock solutions (1.0 mM, 1.0 mL each) of all Pcs in HPLC grade DMF solvent were prepared and dilutions obtained by spiking 20 - 80 µL of each stock solution into solvent (10 mL). Emission spectra were obtained on a Fluorolog® - HORIBA JOBINVYON, Model LFI-3751 spectrofluorimeter. The optical densities of the solutions used for emission studies ranged between 0.04 - 0.05 at excitation wavelengths to minimize re-absorption by the photosensitizers. All measurements were performed within 4 h of solution preparation and carried out at room temperature (23-25 °C). ZnPcs 1a,b and 2a,b were synthesized as we have previously described [36].
ZnPc 3a. To a solution of ZnPc 2a (20.0 mg, 0.017 mmol) in DMF (0.4 mL) were added in the following order: Et3N (3.3 mg, 0.032 mmol), HOBt (4.7 mg, 0.035 mmol), 1,4-bis-Boc-triazaheptane (7 mg, 0.023 mmol), and EDCI (4.0 mg, 0.026 mmol). The reaction mixture was stirred at room temperature for 4 days, diluted with ethyl acetate (40 mL) and washed with water (2 × 20 mL). The organic phase was dried over anhydrous Na2SO4 and the solvent removed under reduced pressure. The crude product was purified by column chromatography using CH2Cl2/methanol for elution, to afford a blue solid (20.2 mg, 81.8%). 1H NMR (DMSO-d6): δ 9.33-9.03 (m, 6H, Ar-H), 8.30-8.07 (m, 5H, Ar-H), 7.84-7.44 (m, 5H, Ar-H), 7.20 (br, 1H, N-H), 6.79 (br, 1H, N-H), 4.14-3.98 (m, 4H, CH2O), 3.53-3.50 (m, 4H, CH2O), 3.44-3.38 (m, 12H, CH2NH), 3.36-3.22 (m, 2H, CH2NH), 3.00 (br, 2H, CH2NH), 2.31-2.20 (m, 2H, CH2CO), 1.80-1.76 (m, 27H, C(CH3)3), 1.37-1.34 (m, 18H, -OC(CH3)3). 13CNMR (DMSO-d6): δ 171.0, 170.1, 168.9, 167.4, 167.3 (C=O), 155.6, 155.3, 154.7, 152.9, 152.6, 151.5, 150.8, 150.4, 150.2, 140.2, 138.1, 137.9, 137.6, 135.8, 135.2, 133.1, 132.4, 130.6, 128.3, 127.4, 127.0, 122.5, 122.0, 121.8, 121.5, 118.9, 118.6, 117.7, 117.4, 115.8 (Ar-C), 78.5, 77.5 (-OC(CH3)3), 70.6, 70.5, 70.4, 69.6, 69.5, 68.8, 66.7 (OCH2), 46.7, 46.2, 38.2, 36.1, 35.7, 35.6 (N-CH2), 31.8 (Ar-C(CH3)3), 28.2, 28.0 (O-C(CH3)3). MS (MALDI-TOF) m/z 1455.650 [M]+, calcd for C77H93N13O12Zn 1455.636. The blue solid (20.2 mg, 0.014 mmol) was dissolved in a 1:1 mixture of CH2Cl2/trifluoroacetic acid (TFA) (6 mL) and stirred at 0 ºC for 3 h. Solvent was removed and the residue treated with 2N NaOH (10 mL) to afford the title ZnPc as a blue solid (15.5 mg, 89.0 %). 1H NMR (DMF-d7): δ 9.59-9.07 (m, 6H, Ar-H), 8.41-8.32 (m, 4H, Ar-H), 8.12-8.06 (m, 1H, Ar-H), 7.92-7.85 (m, 2H, Ar-H), 7.60-7.53 (m, 2H, Ar-H), 6.96 (br, 1H, N-H), 4.23-4.10 (m, 4H, CH2O), 3.57-3.28 (m, 20H, CH2O), 2.13-2.03 (m, 2H, CH2CO), 1.79-1.75 (m, 27H, C(CH3)3). 13CNMR (DMF-d7): δ 171.2, 171.1, 170.4, 168.8, 168.7, 157.3, 156.8, 155.9, 155.7, 155.5, 155.4, 155.0, 154.8, 154.6, 154.3, 154.2, 153.9, 153.7, 152.9, 152.7, 152.3, 152.1, 142.6, 140.1, 139.8, 137.7, 137.4, 134.7, 134.3, 131.7, 131.5, 130.6, 128.50, 128.3, 124.0, 123.6, 123.4, 123.2, 122.6, 122.4, 120.7, 120.1, 120.0, 119.8, 119.6, 119.0118.3, 117.6, 117.1 (Ar-C), 72.4, 72.13, 72.08, 71.1, 71.0, 70.9, 70.24, 70.20, 68.3, 68.0 (O-C(CH3)3), 48.9, 48.7, 39.6, 39.3, 38.7, 37.5, 37.3, 36.8 (N-CH2), 32.7, 32.6 (m, 27H, C(CH3)3). MS (MALDI-TOF) m/z 1255.633 [M]+, calcd for C67H77N13O8Zn 1255.531. UV-vis (DMF): λmax (log ε) 350 (5.00), 612 (4.77), 680 (5.52) nm.
ZnPc 3b. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 2b (20.0 mg, 0.017 mmol), DMF (0.4 mL), Et3N (3.2 mg, 0.032 mmol), HOBt (4.7 mg, 0.035 mmol), 1,4-bis-Boc-triazaheptane (7 mg, 0.023 mmol) and EDCI (4.0 mg, 0.026 mmol). The crude product was purified as described above to afford a blue solid (19.7 mg, 79.6%). 1H NMR (DMF-d7): δ 9.32-8.67 (m, 8H, Ar-H), 8.35-8.30 (m, 4H, Ar-H), 8.15-8.04 (m, 2H, Ar-H), 7.94-7.82 (m, 2H, Ar-H), 7.64-7.59 (m, 2H, Ar-H), 6.90 (br, 1H, N-H), 4.39 (d, J = 13.6, 2H, CH2O), 4.30 (d, J = 9.76, 2H, CH2O), 3.73-3.68 (m, 4H, CH2O), 3.62-3.56 (m, 14H, CH2O), 3.51-3.47 (m, 2H, CH2NH), 3.35-3.19 (m, 8H, CH2NH), 2.47-2.42 (m, 2H, CH2CO), 1.86-1.82 (m, 27H, C(CH3)3), 1.45-1.36 (m, 18H, -OC(CH3)3). 13C NMR (DMF-d7): δ 172.4, 171.5, 170.6, 169.2, 169.1 (C=O), 161.0, 160.4, 157.1, 156.3, 154.4, 153.9, 139.1, 138.9, 136.7, 136.3, 133.7, 128.7, 125.3, 123.5, 122.8, 122.0, 121.5, 121.1, 120.8, 120.3, 115.0, 112.8, 111.6 (Ar-C), 79.9, 78.7 (O-C(CH3)3), 72.4, 72.20, 72.16, 71.3, 71.2, 71.12, 71.06, 70.4, 68.2, 67.9 (OCH2), 48.6, 48.3, 46.1 39.7, 38.9, 37.6, 37.5, 36.9, 36.6 (N-CH2), 32.6 (Ar-C(CH3)3), 29.0, 28.9 (O-C(CH3)3). MS (MALDI-TOF) m/z 1355.693 [M-tBu]+, 1255.650 [M-2 tBu]+; calcd for C72H85N13O10Zn 1355.583, C67H77N13O8Zn 1255.531. The blue solid (19.0 mg, 0.013 mmol) was dissolved in 1:1 CH2Cl2/TFA (6 mL) and stirred at 0 ºC for 3 h. The crude was treated as described for ZnPc 3a above to afford a blue solid (14.9 mg, 90.7 %). 1H NMR (DMF-d7): δ 9.56-8.89 (m, 8H, Ar-H), 8.39-8.31 (m, 4H, Ar-H), 8.08-7.92 (m, 2H, Ar-H), 7.55-7.51 (m, 2H, Ar-H) 4.36 (d, J = 6.88, 2H, CH2O), 4.26 (d, J = 6.64, 2H, CH2O), 3.56-3.25 (m, 24H, CH2O), 2.38-2.22 (m, 2H, CH2CO), 1.80-1.76 (m, 27H, C(CH3)3). 13CNMR (DMF-d7): δ 171.3, 170.6, 169.2, 169.1, 160.6, 160.3, 155.2, 155.1, 154.8, 154.0, 153.4, 141.5, 140.0, 139.8, 137.7, 136.5, 136.2, 135.1, 134.9, 134.7, 128.3, 125.1, 123.3, 122.8, 121.3, 120.7, 119.8, 115.0, 112.6, 111.5, 111.0 (Ar-C), 72.4, 72.2, 71.2, 71.1, 70.9, 70.3, 68.1 (O-C(CH3)3), 48.9, 48.1, 39.7, 39.3, 37.5, 37.3 (N-CH2), 32.6 (m, 27H, C(CH3)3). MS (MALDI-TOF) m/z 1255.555 [M]+, calcd for C67H77N13O8Zn 1255.531. UV-VIS (DMF): λmax (log ε) 351 (4.81), 612 (4.53), 677 (5.28) nm.
ZnPc 4a. ZnPc 3a (20.0 mg, 0.015 mmol), DIPA (0.015 mL, 0.107 mmol) and CH3I (0.2 mL) were dissolved in dry DMF (0.5 mL), and the final solution stirred at room temperature for 2 days. The solvent was removed under reduced pressure and the resulting residue purified by Sephadex G-100 using CH2Cl2/methanol for elution to afford a blue solid (17.2 mg, 68.3%). 1H NMR (DMF-d7): δ 9.57-9.06 (m, 7H, Ar-H), 8.38-8.18 (m, 5H, Ar-H), 7.94-7.84 (m, 3H, Ar-H), 7.61-7.54 (m, 2H, Ar-H), 4.29-4.13 (m, 8H, CH2O), 3.62-3.44 (m, 18H, CH2O/N-CH2/N-CH3), 3.28 (s, 9H, N-CH3), 2.47-2.30 (m, 2H, COCH2), 1.78 (s, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 172.6, 170.5, 168.8, 168.7 (C=O), 157.1, 156.4, 155.8, 155.5, 155.3, 154.5, 154.2, 154.1, 153.9, 153.8, 153.5, 152.8, 152.7, 152.3, 142.7, 140.3, 140.2, 139.9, 137.8, 137.5, 134.9, 134.4, 131.4, 130.5, 129.7, 128.3, 128.1, 123.5, 123.3, 123.2, 123.1, 122.7, 122.5, 121.9, 120.4, 120.0, 119.8, 119.7, 118.9, 117.5 (Ar-C), 72.2, 72.0, 71.1, 71.0, 67.9 (CH2O), 64.6, 64.4, 58.6, 57.1, 54.3, 52.4 (N-CH3), 39.6, 37.3, 36.8 (N-CH2) 32.7, 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1493.711 [M-I+K]+, 1441.654 [M-I-CH3+H]+; calcd for C72H89IKN13O8Zn+ 1493.493, C71H88IN13O8Zn+ 1441.522. UV-Vis (DMF): λmax (log ε) 348 (4.81), 611 (4.55), 679 (5.32) nm.
ZnPc 4b. A procedure similar to the one described for ZnPc 4a was used: ZnPc 3b (20.0 mg, 0.015 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry DMF (0.5 mL). The title ZnPc was obtained as a blue solid (16.2 mg, 64.3%). 1H NMR (DMF-d7): δ 9.57-9.26 (m, 7H, Ar-H), 8.38-8.18 (m, 5H, Ar-H), 7.94-7.84 (m, 2H, Ar-H), 7.61-7.48 (m, 2H, Ar-H), 4.35-4.23 (m, 8H, CH2O), 3.81-3.48 (m, 18H, CH2O/N-CH2/N-CH3), 3.46 (s, 9H, N-CH3), 2.47-2.30 (m, 2H, COCH2), 1.85-1.76 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 173.0, 172.6, 172.4, 170.6, 169.2, 169.1 (C=O), 160.6, 160.2, 155.1, 154.9, 154.6, 154.0, 153.9, 153.2, 141.4, 140.0, 139.9, 137.6, 137.4, 136.5, 136.2, 134.9, 134.6, 129.5, 128.7, 128.4, 125.0, 123.3, 122.8, 121.3, 121.1, 120.7, 119.8, 115.0, 112.6, 111.5 (Ar-C), 72.4, 72.2, 71.2, 71.1, 70.3, 68.0 (CH2O), 64.6, 64.4, 58.6, 57.2, 55.9, 54.4, 52.5, 49.9, 48.0 (N-CH3), 43.2, 39.7, 37.5, 36.8 (N-CH2) 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1493.710 [M-I+K]+, calcd for C72H89IKN13O8Zn+ 1493.493. UV-Vis (DMF): λmax (log ε) 351 (4.84), 610 (4.54), 677 (5.30) nm.
ZnPc 5a. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 1a (40.0 mg, 0.04 mmol), DMF (0.5 mL), Et3N (5.2 mg, 0.051 mmol), HOBt (7.5 mg, 0.055 mmol), 1,4-bis-Boc-triazaheptane (15.5 mg, 0.051 mmol) and EDCI (9.8 mg, 0.051 mmol). The protected ZnPc was obtained as a blue solid (38.6 mg, 77.0%). 1H N MR (DMF-d7): δ 9.60-9.03 (m, 6H, Ar-H), 8.40-8.20 (m, 5H, Ar-H), 7.96-7.79 (m, 3H, Ar-H), 7.61-7.50 (m, 2H, Ar-H), 6.73 (br, 1H, N-H), 4.26-4.12 (m, 4H, CH2O), 3.38-3.26 (m, 6H, CH2NH), 3.20-3.16 (m, 2H, CH2NH), 1.82-1.78 (m, 27H, C(CH3)3), 1.42-1.32 (m, 18H, -OC(CH3)3). 13C NMR (DMF-d7): δ 170.4, 168.6 (C=O), 157.1, 156.8, 156.5, 156.2, 155.5, 155.4, 155.3, 155.2, 155.0, 154.6, 154.4, 154.3, 154.2, 154.1, 153.5, 152.5, 152.1, 142.3, 140.0, 139.9, 139.6, 137.6, 137.5, 137.2, 135.0, 134.5, 131.7, 130.1, 129.4, 128.6, 128.4, 123.6, 123.5, 122.7, 122.5, 122.0, 120.4, 120.1, 120.0, 119.8, 119.7, 119.0, 118.9, 117.5 (Ar-C), 79.9, 78.7 (-OC(CH3)3), 72.3, 72.0 (OCH2), 48.4, 48.2, 47.6, 40.0, 39.8, 38.4, 36.8 (N-CH2), 32.7, 32.6 (Ar-C(CH3)3), 28.9, 28.8 (O-C(CH3)3). MS (MALDI-TOF) m/z 1053.438 [M-2Boc+H]+, calcd for C58H61N12O4Zn 1053.423. The blue solid (51.6 mg, 0.044 mmol) was dissolved in 1:1 CH2Cl2/TFA (6 mL) and stirred at 0 ºC for 3 h. The solvent was removed and the residue treated with 2N NaOH (10 mL) to afford a blue-greenish solid (38.2 mg, 89.2 %). 1H NMR (DMF-d7): δ 9.62-9.12 (m, 7H, Ar-H), 8.63-8.31 (m, 5H, Ar-H), 7.99-7.88 (m, 3H, Ar-H), 7.65-7.58 (m, 2H, Ar-H), 4.29-4.17 (m, 4H, CH2O), 4.01 (br, 4H, NH2), 3.63-3.55 (m, 6H, CH2NH), 3.38-3.35 (m, 2H, CH2NH), 1.81 (s, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.4, 168.6 ,168.5 (C=O), 161.2, 160.9, 160.6, 160.2, 157.1, 156.4, 155.9, 155.8, 155.6, 155.5, 154.9, 154.8, 154.7, 154.6,154.0, 153.8, 153.7, 153.6, 153.3, 152.7, 152.3, 142.8, 140.5, 140.3, 140.0, 138.0, 137.9, 137.6, 134.8, 134.3, 131.2, 130.5, 129.9, 128.1, 127.9, 123.4, 123.2, 122.9, 122.7, 122.5, 121.9, 120.3, 120.0, 119.8, 119.7, 119.6, 118.7, 117.4, 116.8 (Ar-C), 72.1, 71.8 (OCH2), 48.4, 46.0, 37.3, 36.7 (N-CH2), 32.7, 32.5 (Ar-C(CH3)3). MS (MALDI-TOF) m/z 1053.433 [M+H]+, calcd for C58H61N12O4Zn 1053.423. UV-vis (DMF): λmax (log ε) 350 (4.66), 612 (4.42), 680 (5.19) nm.
ZnPc 5b. A procedure similar to the one described above for ZnPc 5a was used: ZnPc 1b (40.0 mg, 0.04 mmol), DMF (0.5 mL), Et3N (5.2 mg, 0.051 mmol), HOBt (7.5 mg, 0.055 mmol), 1,4-bis-Boc-triazaheptane (15.5 mg, 0.051 mmol), and EDCI (9.8 mg, 0.051 mmol). The product was obtained as a blue solid (44.4 mg, 88.6%). 1H NMR (DMF-d7): δ 9.42-9.24 (m, 6H, Ar-H), 8.78 (br, 1H, NH), 8.40-8.20 (m, 4H, Ar-H), 8.10-8.04 (m, 2H, Ar-H), 7.89-7.79 (m, 1H, Ar-H), 7.64-7.59 (m, 2H, Ar-H), 6.82-6.76 (m, 1H, N-H), 4.38 (d, J = 6.92 MHz, 2H, CH2O), 4.29 (d, J = 9.68 MHz, 2H, CH2O), 3.47 (br, 4H, CH2NH), 3.38 (br, 2H, CH2NH), 3.25 (m, 2H, CH2NH), 1.87-1.83 (m, 27H, C(CH3)3), 1.48 (s, 9H, -OC(CH3)3), 1.41 (s, 18H, -OC(CH3)3). 13CNMR (DMF-d7): δ 170.6, 169.1 (C=O), 160.6, 160.5, 160.2, 160.1, 157.2, 156.6, 156.4, 154.8, 154.72, 154.65, 154.5, 154.3, 154.2, 154.1, 154.0, 153.9, 153.4, 152.8, 141.1, 139.8, 139.6, 137.4, 137.3, 137.2, 136.5, 136.2, 134.5, 134.3, 132.6, 129.8, 128.4, 128.3, 126.2, 125.0, 123.4, 123.3, 122.9, 122.0, 121.3, 121.1, 120.8, 120.7, 119.9, 119.7, 112.7, 111.5 (Ar-C), 80.0, 78.8 (-OC(CH3)3), 72.4, 72.1 (OCH2), 48.5, 48.3, 47.7, 40.1, 39.9, 38.7, 38.5, 36.8 (N-CH2), 32.7 (Ar-C(CH3)3), 29.0, 28.9 (O-C(CH3)3). MS (MALDI-TOF) m/z 1052.447 [M-2Boc]+, calcd for C58H60N12O4Zn 1052.415. The blue solid (44.4 mg, 0.035 mmol) was dissolved in 1:1 CH2Cl2/TFA (6 mL) and stirred at 0 ºC for 3 h. The solvent was removed and the residue treated with 2N NaOH (10 mL) to afford a blue-greenish solid (33.5 mg, 89.9 %). 1H NMR (DMF-d7): δ 9.55-9.24 (m, 7H, Ar-H), 8.97-8.89 (m, 1H, Ar-H), 8.40-8.11 (m, 4H, Ar-H), 8.07 - 8.00 (m, 1H, Ar-H), 7.92 - 7.90 (m, 1H, Ar-H), 7.65-7.50 (m, 2H, Ar-H), 4.37 (d, J = 6.88 MHz, 2H, CH2O), 4.26 (d, J = 4.24 MHz, 2H, CH2O), 3.47 (br, 2H, CH2NH), 3.03-2.91 (m, 2H, CH2NH), 1.82-1.79 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.4, 169.2 ,169.1 (C=O), 160.6, 160.5, 160.2, 159.7, 155.2, 155.1, 154.8, 154.7, 154.3, 154.1, 154.0, 153.4,153.2, 141.4, 140.0, 139.8, 137.7, 137.5, 134.9, 134.7, 128.3, 128.2, 125.0, 123.3, 123.2, 122.8, 121.2, 121.1,120.7, 119.9, 119.8, 119.3, 116.4, 112.7, 111.5 (Ar-C), 72.3, 72.1, 72.0 (OCH2), 49.1, 48.7, 47.5, 47.0, 39.5, 38.7, 36.7 (N-CH2), 32.6 (Ar-C(CH3)3). MS (MALDI-TOF) m/z 1052.411 [M]+, calcd for C58H60N12O4Zn 1052.415. UV-vis (DMF): λmax (log ε) 351 (4.73), 609 (4.43), 676 (5.19) nm.
ZnPc 6a. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 5a (20.0 mg, 0.021 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry THF (0.5 mL). The title Pc was obtained as a pale-green solid (14.7 mg, 53.3%). 1H NMR (DMF-d7): δ 9.56-9.06 (m, 6H, Ar-H), 8.36-8.24 (m, 4H, Ar-H), 7.96-7.80 (m, 4H, Ar-H), 7.58-7.51 (m, 2H, Ar-H), 4.34-4.13 (m, 8H, CH2O/N-CH2), 3.80-3.67 (m, 4H, N-CH2), 3.62-3.47 (m, 6H, N-CH3), 3.41-3.27 (m, 9H, N-CH3), 1.79-1.71 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.3, 168.7, 168.6, 168.4, 157.2, 156.7, 155.9, 155.8, 155.7, 155.6, 155.5, 155.43, 155.36, 155.1, 155.0, 154.9, 154.8, 154.7, 154.6, 154.5, 154.1, 154.0, 153.8, 153.7, 153.6, 153.5, 153.2, 152.7, 152.63, 152.55, 152.33, 152.27, 152.2, 142.8, 140.4, 140.3, 140.0, 137.9, 137.6, 134.7, 134.3, 133.4, 132.5, 131.8, 131.4, 130.6, 129.9, 128.3, 128.0, 123.4, 123.3, 123.2, 123.0, 122.6, 122.4, 120.5, 120.0, 119.9, 119.8, 119.7, 119.6, 119.5, 118.6, 117.3 (Ar-C), 72.0, 71.9, 71.8, 71.4 (CH2O), 66.9, 66.6, 64.9, 64.5, 58.6, 58.5, 57.1, 54.3, 52.40, 52.35 (N-CH3), 36.8, 36.7 (N-CH2) 32.7, 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1238.544 [M-I-CH3+2H]+, calcd for C62H71IN12O4Zn 1238.406. UV-Vis (DMF): λmax (log ε) 357 (4.72), 611 (4.46), 679 (5.23) nm
ZnPc 6b. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 5b (20.0 mg, 0.021 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry THF (0.5 mL). The title ZnPc was obtained as a blue-green solid (16.1 mg, 58.3%). 1H NMR (DMF-d7): δ 9.56-9.19 (m, 6H, Ar-H), 9.03-8.77 (m, 2H, Ar-H), 8.39-8.27 (m, 3H, Ar-H), 8.01-8.86 (m, 3H, Ar-H), 7.58-7.45 (m, 4H, Ar-H), 4.41-4.27 (m, 8H, CH2O/N-CH2), 3.92-3.82 (m, 4H, N-CH2), 3.53 (s, 6H, N-CH3), 3.49 (s, 9H, N-CH3), 1.86-1.72 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.4, 169.0, 168.6, 168.9, 160.5, 160.4, 160.1, 160.0, 155.9, 155.5, 155.3, 155.2, 155.1, 154.9, 154.8, 154.3, 154.0, 153.9, 153.8, 141.6, 140.2, 140.0, 137.8, 137.7, 137.6, 136.3, 136.0, 135.2, 135.0, 128.3, 128.12, 128.07, 125.1, 123.24, 123.15, 123.0, 122.8, 122.7, 121.3, 121.1, 120.6, 119.9, 119.8, 119.7, 112.8, 111.7 (Ar-C), 72.1, 71.9 (CH2O), 64.5, 58.7, 57.3, 54.5, 52.5 (N-CH3), 36.7 (N-CH2) 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1252.575 [M-I+2H]+, calcd for C63H73IN12O4Zn 1252.421. UV-Vis (DMF): λmax (log ε) 351 (4.83), 610 (4.55), 676 (5.31) nm.
ZnPc 7a. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 1a (50.0 mg, 0.05 mmol), DMF (0.8 mL), Et3N (6.2 mg, 0.061 mmol), HOBt (9.3 mg, 0.069 mmol), aspartic acid di-tert-butyl ester hydrochloride (18 mg, 0.064 mmol) and EDCI (12.3 mg, 0.064 mmol). The crude product was purified by column chromatography using CH2Cl2/methanol (98/2) for elution, giving the di-ester ZnPc as a blue solid (0.052 g, 87.1%). 1H NMR (DMF-d7): δ 10.09, 10.05 (s, 1H, N-H), 9.63-9.28 (m, 6H, Ar-H), 9.19-9.01 (m, 1H, Ar-H), 8.47-8.20 (m, 5H, Ar-H), 7.97-7.79 (m, 3H, N-H), 7.62-7.47 (m, 2H, N-H), 4.55-4.45 (m, 1H, N-H), 4.28-4.19 (m, 4H, CH2O), 2.96-2.67 (m, 3H, CH2), 1.81-1.77 (m, 27H, C(CH3)3), 1.45-1.36 (m, 18H, -OC(CH3)3). 13C NMR (DMF-d7): δ 170.9, 170.7, 170.4, 170.0, 168.7 (C=O), 156.8, 156.2, 155.6, 155.4, 154.5, 154.3, 154.1, 153.6, 152.5, 152.2, 142.3, 140.0,139.9, 139.6, 1137.5, 137.5, 137.2, 135.2, 134.6, 131.8, 130.2, 129.5, 128.8, 128.5, 123.7, 123.5, 123.3, 122.5, 122.3, 120.4, 120.1, 119.9, 119.7, 119.0, 117.6 (Ar-C), 82.4, 81.7 (-OC(CH3)3), 72.3, 71.9 (OCH2), 50.4, 38.4, 36.8 (N-CH2), 32.7, 32.6 (Ar-C(CH3)3), 28.4, 28.3 (O-C(CH3)3). MS (MALDI-TOF) m/z 1083.625 [M-2C4H9+H]+, calcd for C58H55N10O8Zn 1083.350. The product (0.052 g, 0.044 mmol) was dissolved in 1:1 CH2Cl2/TFA (8 mL) and stirred for 4 h at 0°C. The tert-butyl group was removed as described for ZnPc 5a to give the title compound as a blue solid (34.3 mg, 82.3 %). (MALDI-TOF) m/z 1195.180 [M+Na]+, calcd for C58H54N10NaO8Zn 1105.332. UV-vis (DMF): λmax (log ε) 349 (4.68), 612 (4.41), 679 (5.17) nm.
ZnPc 7b. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 1b (50.0 mg, 0.05 mmol), DMF (0.8 mL), Et3N (6.2 mg, 0.061 mmol), HOBt (9.3 mg, 0.069 mmol), aspartic acid di-tert-butyl ester hydrochloride (18 mg, 0.064 mmol) and EDCI (12.3 mg, 0.064 mmol). The protected ZnPc was obtained as a blue solid (0.056 g, 89%). 1H NMR (Acetone-d6): δ 9.86-9.68 (m, 1H, N-H), 9.37-8.57 (m, 6H, Ar-H), 8.38-7.96 (m, 6H, Ar-H), 7.92-7.56 (m, 4H, Ar-H), 4.74 (br, 1H, N-H), 4.25 (s, 4H, CH2O), 2.82 (s, 1H, CH), 2.66 (s, 2H, CH), 1.94-1.87 (m, 27H, C(CH3)3), 1.45 (s, 18H, -OC(CH3)3). 13C NMR (Acetone-d6): δ 170.6, 170.4, 169.99, 169.95, 168.3, 168.23, 168.17 (C=O), 160.0, 159.9, 159.8, 159.4, 159.3, 159.2, 155.3, 155.1, 154.6, 154.4, 153.2, 1153.1, 153.03, 152.97, 139.4, 139.1, 139.0, 136.8, 135.9, 135.8, 135.4, 127.7, 124.3, 122.8, 122.4, 121.8, 121.1, 121.0, 120.5, 120.4, 119.5, 112.8, 112.6, 111.5 (Ar-C), 82.4, 81.7 (-OC(CH3)3), 72.4, 71.9 (OCH2), 50.1, 50.0, 38.4, 36.6 (N-CH2), 32.6 (Ar-C(CH3)3), 28.4, 28.2 (O-C(CH3)3). MS (MALDI-TOF) m/z 1194.433 [M+H]+, 1138.332 [M-C4H9]+, 1083.257 [M-2C4H9+H]+, calcd for C66H70N10O8Zn 1194.467, C62H62N10O8Zn 1138.404, C58H55N10O8Zn 1083.350. The product (0.056 g, 0.047 mmol) was dissolved in 1:1 CH2Cl2/TFA (8 mL) and stirred for 4 h at 0°C. The solvent was removed and the residue treated with 2N NaOH (10 mL) to afford a blue solid (42.1 mg, 83.0 %). MS (MALDI-TOF) m/z 1082.7 [M]+, 1105.3315 [M+Na]+, calcd for C58H54N10O8Zn 1082.34, C58H54N10NaO8Zn 1105.7. UV-vis (DMF): λmax (log ε) 351 (4.83), 610 (4.51), 677 (5.28) nm.
ZnPc 8a. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 7a (18.4 mg, 0.017 mmol), DMF (0.5 mL), Et3N (6.2 mg, 0.061 mmol), HOBt (5.7 mg, 0.042 mmol), N-Boc-2,2′-(ethylenedioxy)diethylamine (9.4 mg, 0.038 mmol) and EDCI (6.5 mg, 0.042 mmol). The crude product was purified by column chromatography using CH2Cl2/methanol (95:5) for elution to give a blue solid (13.6 mg, 51.9 %). 1H NMR (DMF-d7): δ 9.59-9.08 (m, 6H, Ar-H), 8.50-8.27 (m, 5H, Ar-H), 7.99-7.74 (m, 5H, Ar-H), 7.61-7.53 (m, 2H, Ar-H), 6.65 (br, 1H, Ar-H), 4.77-4.71 (m, 1H, NHCH(CH2)CO), 4.28-4.16 (m, 4H, CH2O), 3.48-3.36 (m, 14H, CH2O), 3.31-3.16 (m, 8H, CH2O), 2.75-2.67 (m, 2H, CHCH2CO), 1.81-1.76 (m, 27H, C(CH3)3), 1.37 (s, 18H, OC(CH3)3). 13C NMR (DMF-d7): δ 171.90, 171.86, 171.35, 171.28, 170.3, 168.7, 168.6 (C=O), 157.1, 155.4, 155.3, 154.5, 153.6, 152.6, 142.4, 140.0, 137.6, 135.1, 134.6, 132.5, 131.8, 129.9, 128.7, 123.4, 122.6, 122.4, 120.1, 119.0, 117.6 (Ar-C), 78.7 (OC(CH3)3), 72.1, 71.9, 71.0, 70.95, 70.7, 70.4, 70.3 (CH2O), 51.1 (NHCH(CH2)CO), 41.2, 40.1, 38.4, 36.8 (CH2), 32.7, 32.6 (C(CH3)3), 28.9 (OC(CH3)3). MS (MALDI-TOF) m/z 1542.669 [M]+, calcd for C80H98N14O14Zn 1542.668. The protected ZnPc (13.1 mg, 8.5 mmol) was dissolved in a 1:1 mixture of CH2Cl2/TFA (4 mL) and stirred at 0°C for 3 h. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to afford a blue solid (10.5 mg, 92.3%). 1H NMR (DMF-d7): δ 9.60-9.10 (m, 7H, Ar-H), 8.60-8.29 (m, 5H, Ar-H), 7.95-7.87 (m, 2H, Ar-H), 7.56-7.51 (m, 2H, Ar-H), 4.56 (br, 1H, NH), 4.21 (d, 9.48 Hz, 2H, CH2O), 4.16 (d, 8.92 Hz, 2H, CH2O), 3.75-3.35 (m, 10H, CH2O), 3.16-2.95 (m, 9H, CH2O), 2.90-2.80 (m, 3H, CH2O), 2.90-2.80 (m, 3H, CH2O), 2.62-2.47 (m, 2H, CH2), 2.19 (br, 2H, NH2), 1.80-1.74 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.8, 171.3, 171.2, 170.2, 168.6, 168.5, 157.7, 157.4, 155.9, 155.6, 155.5, 155.0, 154.3, 154.2, 154.1, 154.0, 153.7, 152.4, 151.7, 142.4, 140.1, 139.8, 137.7, 137.3, 134.4, 134.1, 131.7, 130.8, 128.5, 123.7, 123.4, 123.1, 122.5, 122.3, 120.1, 120.0, 119.8, 117.6, 116.7 (Ar-C), 72.0, 71.8, 71.5, 71.0, 70.5, 70.4, 70.0 (N-CH), 51.2 (NHCH(CH2)CO), 40.9, 39.9, 39.8, 38.1, 36.8 (CH2), 32.7, 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1343.610 [M+H]+, calcd for C70H83N14O10Zn 1343.571. UV-vis (DMF): λmax (log ε) 349 (4.70), 612 (4.45), 680 (5.20) nm.
ZnPc 8b. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 7b (18.4 mg, 0.017 mmol), DMF (0.5 mL), Et3N (6.2 mg, 0.061 mmol), HOBt (5.7 mg, 0.042 mmol), N-Boc-2,2′-(ethylenedioxy)diethylamine (9.4 mg, 0.038 mmol) and EDCI (6.5 mg, 0.042 mmol). The ZnPc was obtained as a blue solid (13.3 mg, 50.8 %). 1H NMR (DMF-d7): δ 9.52-9.15 (m, 6H, Ar-H), 8.81-8.54 (m, 2H, Ar-H), 8.42-8.34 (m, 3H, Ar-H), 8.17-8.05 (m, 4H, Ar-H), 7.97-7.88 (m, 1H, Ar-H), 7.65-7.59 (m, 2H, Ar-H), 6.70 (br, 2H, Ar-H), 4.87 - 4.85 (m, 1H, N-H), 4.42 (d, 8.0 Hz, 2H, CH2O), 4.33 (d, 5.96 Hz, 2H, CH2O), 3.58-3.46 (m, 16H, CH2O), 3.42-3.37 (m, 4H, CH2NH), 3.25-3.19 (m, 4H, CH2NH), 2.83-2.78 (m, 2H, CH2CO), 1.86-1.83 (m, 27H, C(CH3)3), 1.40-1.36 (m, 18H, OC(CH3)3). 13C NMR (DMF-d7): δ 172.0, 171.4, 170.42, 170,40, 169.1, 169.0, 160.6, 160.5, 160.2, 160.1, 157.1, 154,73, 154.67, 154.4, 154.2, 154.0, 153.9, 153.4, 152.9, 141.2, 139.8, 139.6, 137.5, 137.3, 136.,5, 136.2, 134.6, 128.4, 125.1, 123.35, 123.25, 122.83, 122.79, 122.0, 121.3, 121.2, 121,1, 120.73, 120.67, 119.9, 1119.8, 112.8, 111.6 (Ar-C), 78.8 (OC(CH3)3), 72.2, 72.0, 71.1, 70.04, 70.01, 71.0, 70.8, 70.5, 70.4 (CH2O), 51.2 (NHCH(CH2)CO), 42.5, 41.2, 40.2, 38.5 (CH2), 32.6 (C(CH3)3), 28.9 (OC(CH3)3). MS (MALDI-TOF) m/z 1544.802 [M]+, calcd for C80H98N14O14Zn 1544.669. The protected ZnPc (13.1 mg, 8.5 mmol) was dissolved in a 1:1 a mixture of CH2Cl2/TFA (8 mL) and stirred at 0°C for 3 h. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to give the title product (10.2 mg, 89.7%). 1H NMR (DMF-d7): δ 9.54-9.23 (m, 7H, Ar-H), 9.00-8.77 (m, 1H, Ar-H), 8.39-8.29 (m, 3H, Ar-H), 8.09-7.94 (m, 3H, Ar-H), 7.47 - 7.42 (m, 2H, Ar-H), 4.66 (br, 1H, NH), 4.33 (d, 7.36 Hz, 2H, CH2O), 4.33 (d, 5.16 Hz, 2H, CH2O), 3.65-3.45 (m, 8H, CH2O), 3.25-3.14 (m, 8H, CH2O), 3.08-3.02 (m, 2H, CH2NH), 2.98-2.91 (m, 2H, CH2NH), 2.87-2.80 (m, 2H, CH2NH), 2.72-2.58 (m, 2H, CH2CO), 2.34 (br, 2H, NH2), 2.13 (br, 2H, NH2), 1.80-1.73 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.9, 171.5, 170.2, 170.17, 168.95, 168.87, 160.5, 160.0, 155.5, 155.4, 155.2, 155.1, 155.0, 154.8, 154.4, 154.1, 153.9, 153.3, 141.5, 140.0, 139.9, 137.7, 136.2, 135.2, 134.9, 132.5, 129.9, 128.5, 125.2, 123.3, 122.8, 122.7, 122.3, 122.0, 120.8, 120.1, 119.8, 113.3, 112.1 (Ar-C), 72.1, 72.0, 71.4, 71.1, 70.7, 70.52, 70.46, 70.3, 70.2, 70.1 (N-CH), 51.2 (NHCH(CH2)CO), 40.6, 40.2, 40.0, 39.9, 38.0, 36.8 (CH2), 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1343.588 [M+H]+, 1365.575 [M+Na]+, 1381.546 [M+K]+ calcd for C70H83N14O10Zn 1343.5708, C70H82NaN14O10Zn 1365.5528, C70H82KN14O10Zn 1381.5267. UV-vis (DMF): λmax (log ε) 352 (4.77), 611 (4.50), 677 (5.27) nm.
ZnPc 9a. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 8a (20.0 mg, 0.015 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry DMF (0.5 mL). The title compound was obtained as a blue solid (17.2 mg, 68.3%). 1H NMR (DMF-d7): δ 9.57-9.33 (m, 5H, Ar-H), 9.20-9.05 (m, 1H, Ar-H), 8.52-8.28 (m, 4H, Ar-H), 7.95-7.84 (m, 4H, Ar-H), 7.61-7.52 (m, 2H, Ar-H), 4.31-4.11 (m, 4H, OCH2), 3.93 (br, 2H, NH), 3.71-3.40 (m, 27H, OCH2/N-CH3), 3.33-3.29 (m, 10H, N-CH3), 1.80-1.76 (m, 27H, C(CH3)3). MS (MALDI-TOF) m/z 1427.749 [M-2I-H]+, calcd for C76H95N14O10Zn+ 1427.665. UV-Vis (DMF): λmax (log ε) 348 (4.48), 612 (4.23), 680 (5.01) nm.
ZnPc 9b. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 8b (20.0 mg, 0.015 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry DMF (0.5 mL). The title compound was obtained as a blue solid (16.2 mg, 64.3%). 1H NMR (DMF-d7): δ 9.56-9.27 (m, 7H, Ar-H), 9.06-8.83 (m, 2H, Ar-H), 8.36-8.26 (m, 4H, Ar-H), 8.13-8.06 (m, 2H, Ar-H), 7.92-7.81 (m, 1H, Ar-H), 7.52-7.44 (m, 2H, Ar-H), 4.38 (d, 8.0Hz, 2H), 4.28 (d, 6.0Hz, 2H), 3.94-3.90 (m, 5H, NH), 3.73-3.49 (m, 24H, OCH2), 3.34-3.23 (m, 18H, N-CH3), 1.78-1.64 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 172.2, 171.5, 170.5, 169.2 (C=O), 159.9, 159.1, 155.6, 155.1, 153.8, 141.8, 140.4, 140.2, 137.9, 136.3, 135.9, 135.2, 128.1, 124.9, 123.1, 122.9, 122.8, 121.0, 120.4, 119.7, 112.7, 111.6 (Ar-C), 72.2, 72.0, 71.0, 70.9, 70.8, 70.7, 70.4 (OCH2), 66.2, 65.65, 65.60 (NHCH2), 54.4 (N-CH3), 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1413.828 [M-2I-CH3]+, calcd for C75H93N14O10Zn+ 1413.649. UV-Vis (DMF): λmax (log ε) 350 (4.51), 610 (4.26), 677 (5.01) nm.
ZnPc 10a. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 7a (18.4 mg, 0.017 mmol), DMF (0.5 mL), Et3N (6.2 mg, 0.061 mmol), HOBt (5.7 mg, 0.042 mmol), N-Boc-ethylenediamine (6.1 mg, 0.038 mmol) and EDCI (6.5 mg, 0.042 mmol). The final reaction mixture was stirred for 4 days at room temperature. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to give a blue solid (13.4 mg, 57.8 %). 1H NMR (DMF-d7): δ 9.59-9.21 (m, 6H, Ar-H), 8.45-8.23 (m, 4H, Ar-H), 8.12-7.82 (m, 3H, Ar-H), 7.62-7.52 (m, 2H, Ar-H), 6.73-6.66 (m, 2H, Ar-H), 4.75-4.71 (m, 1H, NH), 4.28-4.18 (m, 4H, CH2O), 3.29-3.11 (m, 8H, CH2NH), 2.70-2.65 (m, 2H, CH2CO), 1.81-1.77 (m, 27H, C(CH3)3), 1.41-1.34 (m, 18H, C(CH3)3). 13C NMR (DMF-d7): δ 172.1, 172.0, 171.5, 171.4, 170.3, 168.7, 168.6, 157.2, 157.1, 155.6, 155.4, 155.2, 154.4, 154.1, 153.7, 153.6, 152.6, 152.3, 142.3, 139.9, 139.7, 137.6, 137.2, 135.1, 134.6, 131.8, 130.1, 129.4, 128.6, 128.5, 123.5, 122.6, 122.4, 122.0, 120.1, 119.7, 119.1, 119.0, 117.7 (Ar-C), 78.8 (OC(CH3)3), 72.2, 71.9 (CH2O), 51.3, 51.2 (NHCH(CH2)CO), 41.0, 40.5, 40.4, 38.8, 36.8 (CH2), 32.7, 32.6 (C(CH3)3), 28.9 (OC(CH3)3). MS (MALDI-TOF) m/z 1166.466 [M-2Boc]+, 1189.469 [M-2Boc+Na]+, 1205.444 [M-2Boc+K]+, calcd for C62H66N14O6Zn 1166.4581, C62H66NaN14O6Zn 1189.4479, C62H66KN14O6Zn 1206.4218 respectively. The protected ZnPc (13.1 mg, 0.0096 mmol) was dissolved in a 1:1 mixture of CH2Cl2/TFA (8 mL) at 0 °C for 3h. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to give a blue solid (10.1 mg, 90.1%). 1H NMR (THF-d8): δ 9.58-9.08 (m, 7H, Ar-H), 8.39-8.07 (m, 4H, Ar-H), 7.82-6.86 (m, 5H, Ar-H), 4.15-3.76 (m, 4H, CH2O), 3.57-3.20 (m, 8H, CH2NH), 2.28-2.13 (m, 2H, CH2CO), 1.80-1.72 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.2, 171.0, 169.6, 168.2, 157.7, 155.8, 155.7, 155.0, 154.4, 153.9, 153.5, 151.6, 142..4, 140.1,139.8, 139.5, 137.7, 137.2, 133.4, 131.9, 130.4, 128.5, 128.3, 123.9, 123.4, 122.2, 121.9, 120.8, 120.4, 119.9, 119.8, 117.1, 116.6 (Ar-C), 71.7, 71.6 (CH2NH), 50.7 (NHCH(CH2)CO), 40.7, 40.2, 36.8, 36.7 (CH2), 32.8, 32.7, 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1166.503 [M-2Boc]+, calcd for C62H66N14O6Zn 1166.4581. UV-vis (DMF): λmax (log ε) 349 (4.72), 613 (4.48), 680 (5.23) nm.
ZnPc 10b. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 7b (18.4 mg, 0.017 mmol), DMF (0.5 mL), Et3N (6.2 mg, 0.061 mmol), HOBt (5.7 mg, 0.042 mmol), N-Boc-ethylenediamine (6.1 mg, 0.038 mmol) and EDCI (6.5 mg, 0.042 mmol) After stirring for for 4 days at room temperature and purification the protected ZnPc was obtained as a blue solid (14.3 mg, 61.6 %). 1H NMR (DMF-d7): δ 9.52-9.14 (m, 6H, Ar-H), 8.80-8.33 (m, 4H, Ar-H), 8.20-8.10 (m, 3H, Ar-H), 7.65-7.61 (m, 2H, Ar-H), 6.79-6.76 (m, 2H, Ar-H) 4.83 (br, 1H, NH), 4.43 (d, 8.24 Hz, 2H, CH2O), (d, 5.76 Hz, 2H, CH2O), 3.39-3.25 (m, 4H, CH2NH), 3.24-3.16 (m, 4H, CH2NH), 2.82-2.77 (m, 2H, CH2CO), 1.95-1.84 (m, 27H, C(CH3)3), 1.41 (s, 18H, C(CH3)3). 13C NMR (DMF-d7): δ 172.1, 171.6, 170.4, 169.1, 169.0, 160.6, 160.5, 160.2, 160.1, 157.3, 157.2, 154.7, 154.4, 154.2, 154.0, 153.9, 152.9, 141.2, 139.8, 137.5, 137.3, 136.2, 134.5, 128.4, 128.23 125.0, 123.2, 122.83, 122.79, 122.0, 121.3, 121.1, 120.7, 119.9, 119.7, 112.7, 111.6 (Ar-C), 78.91, 78.87 (OC(CH3)3), 72.3, 72.0 (CH2O), 51.3 (NHCH(CH2)CO), 41.1, 40.6, 40.5, 38.8, 36.8 (CH2), 32.7 (C(CH3)3), 29.0 (OC(CH3)3). MS (MALDI-TOF) m/z 1166.471 [M-2Boc]+, 1189.475 [M-2Boc+Na]+, 1205.448 [M-2Boc+K]+, calcd for C62H66N14O6Zn 1166.4581, C62H66N14NaO6Zn 1189.4479, C62H66KN14O6Zn 1206.4218 respectively. The protected ZnPc (14.3 mg, 0.0105 mmol) was dissolved in a 1:1 mixture of CH2Cl2/TFA (8 mL) at 0 °C for 3h. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to afford the title ZnPc (11.1 mg, 91.2%). 1H NMR (THF-d8): δ 9.80-9.36 (m, 8H, Ar-H), 9.01-8.8.97 (m, 1H, Ar-H), 8.43-8.37 (m, 3H, Ar-H), 8.00-7.81 (m, 3H, Ar-H), 7.51 - 7.42 (m, 2H, Ar-H), 4.20-3.70 (m, 4H, CH2O), 3.47-3.10 (m, 8H, CH2NH), 2.08-1.93 (m, 2H, CH2CO), 1.75-1.65 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.8, 171.0, 170.4, 169.8, 168.8, 161.0, 160.4, 155.4, 154.9, 154.1, 141.6, 140.0, 137.6, 136.4, 135.9, 134.9, 134.6, 128.4, 125.2, 123.3, 122.7, 121.6, 121.1, 120.7, 119.8, 112.7, 111.3 (Ar-C), 72.0, 71.5 (CH2NH), 50.8 (NHCH(CH2)CO), 41.0, 40.1, 36.7 (CH2), 32.6, 32.3 (C(CH3)3). MS (MALDI-TOF) m/z 1166.534 [M-2Boc+2H]+, calcd for C62H66N14O6Zn 1166.4581. UV-vis (DMF): λmax (log ε) 352 (4.73), 610 (4.44), 677 (5.19) nm.
ZnPc 11a. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 10a (20.0 mg, 0.017 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry THF (0.5 mL). After work-up and purification, the title ZnPc was obtained (15.2 mg, 58.9%). 1H NMR (DMF-d7): δ 9.56-8.80 (m, 10H, Ar-H), 8.38-8.24 (m, 4H, Ar-H), 7.96-7.85 (m, 3H, Ar-H), 7.62-7.54 (m, 2H, Ar-H), 7.16 (d, 8.6Hz, 1H, Ar-H), 6.90 (d, 8.2Hz, 1H, Ar-H), 4.22-4.09 (m, 4H, OCH2), 3.38-3.29 (m, 8H, N-CH2), 3.11-3.02 (m, 18H, N-CH3), 1.92-1.73 (m, 27H, C(CH3)3). 13C NMR (d-DMF): δ 172.8, 172.7, 171.7, 170.8, 168.9, 168.8 (C=O), 157.9, 155.9, 155.7, 155.4, 154.9, 153.9, 153.8, 153.6, 144.2, 142.9, 140.4, 140.1, 138.0, 137.7, 134.7, 134.2, 128.7, 128.1, 123.4, 123.2, 122.8, 122.7, 119.8, 119.7, 119.5, 118.4, 117.3, 115.0 (Ar-C), 72.2, 71.9, 70.5, 65.4, 64.4 (N-CH2), 53.9, 53.6 (N-CH3), 32.7, 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1209.761 [M-3CH3-2I]+, calcd for C65H73N14O6Zn+ 1209.513. UV-Vis (DMF): λmax (log ε) 348 (4.65), 612 (4.42), 679 (5.19) nm.
ZnPc 11b. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 10b (20.0 mg, 0.017 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry DMF (0.5 mL). After work-up, the title ZnPc was obtained as a blue solid (17.4 mg, 67.4%). 1H NMR (DMF-d7): δ 9.56-9.27 (m, 7H, Ar-H), 9.04-8.92 (m, 1H, Ar-H), 8.65-8.50 (m, 3H, Ar-H), 8.39-8.31 (m, 3H, Ar-H), 7.92-7.90 (m, 1H, Ar-H), 7.54-7.47 (m, 2H, Ar-H), 4.47-4.31 (m, 4H, OCH2), 381-3.70 (m, 8H, CH2), 3.38-3.35 (m, 18H, N-CH3), 1.79-1.74 (m, 27H, C(CH3)3). 13C NMR (Acetone-d6): δ 172.6, 171.8, 170.9, 169.2, 155.5, 154.8, 154.0, 153.5, 153.0, 141.8, 140.3, 140.2, 137.7, 136.4, 136.0, 129.6, 128.8, 128.3, 126.2, 125.0, 123.2, 122.8, 121.1, 120.5, 119.6, 115.0, 112.8, 111.7 (Ar-C), 72.2, 72.1, 65.6 (N-CH2), 54.0 (N-CH3), 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1194.434 [M-4CH3-2I]+, calcd for C64H70N14O6Zn+ 1194.489. UV-Vis (DMF): λmax (log ε) 350 (4.55), 610 (4.25), 677 (4.99) nm.
Phthalonitrile 13a. A mixture of 3-nitrophthalonitrile 12a (2.0 g, 11.5 mmol) and tert-butyl 20-hydroxy-3,6,9,12,15,18-hexaoxaicosan-1-oate (4.99 g, 12.6 mmol) was dissolved in THF (15 mL). Potassium carbonate, K2CO3, (5.26 g, 40 mmol) was added into the solution in six portions after every 5 min and the reaction solution was heated to 65 ºC for 6 h (monitored by mass spectrometry). The solids were filtered off, the solvent evaporated, and the crude oil purified by silica column chromatography using CH2Cl2/methanol (199:1 → 98:2) for elution. The title compound was obtained as a brown-yellow oil (4.70 g, 78.3%). 1H NMR (d-CDCl3, 400 MHz): δ 7.64 (d, J = 8.1 Hz, 1H, Ar-H), 7.31 (d, J = 8.2 Hz, 1H, Ar-H), 4.27 (t, J = 4.4, 2H, OCH2), 3.96 (s, 2H, OCH2), 3.87 (t, 2H, J = 4.5, OCH2), 3.70-3.58 (m, 20H, OCH2), 1.41 (s, 9H, C(CH3)3). 13C NMR (CDCl3): δ 169.65 (CH2C(O)OtBu), 161.41, 134.71, 125.34, 117.55, 113.09, 104.91 (Ar-C), 116.77, 115.41, (CN), 81.49 (C(CH3), 71.11, 70.67, 70.54, 69.72, 69.21, 68.98 (OCH2), 28.09 (C(CH3)3). MS (MALDI-TOF) m/z 545.2232 [M+H+Na]+, calcd for, C18H36NaO9, 519.2257; m/z 545.2466 [M+H]+, calcd for, C26H37N2NaO9, 545.2475.
Phthalonitrile 13b. The same procedure as described above for 13a was used: 4-nitrophthalonitrile 12b (2.0 g, 11.5 mmol), tert-butyl 20-hydroxy-3,6,9,12,15,18-hexaoxaicosan-1-oate (4.99 g, 12.6 mmol), THF (15 mL) and K2CO3, (5.26 g, 40 mmol). The crude oil was purified by silica column chromatography using CH2Cl2/methanol (98:2) as eluting solvent system, giving a brown oil (4.87 g, 81.2%). 1H NMR (d-CDCl3, 400 MHz): δ 7.64 (d, J = 8.8 Hz, 1H, Ar-H), 7.24 (s, 1H, Ar-H), 7.18 (d, J = 8.8 Hz, 1H, Ar-H), 4.15 (t, J = 3.5, 2H, OCH2), 3.91 (s, 2H, OCH2), 3.79 (t, 2H, J = 3.7, OCH2), 3.60-3.50 (m, 20H, OCH2), 1.36 (s, 9H, C(CH3)3). 13C NMR (CDCl3): δ 169.48 (CH2C(O)OtBu), 161.95, 135.13, 119.81, 119.56, 117.01, 106.97 (Ar-C), 115.64, 115.20, (CN), 81.30 (C(CH3), 70.71, 70.50, 70.37, 70.29, 70.05 (OCH2), 28.12 (C(CH3)3). MS (MALDI-TOF) m/z 545.2473 [M+H+Na]+, calcd for, C26H37N2NaO9, 545.2475.
ZnPc 14a. A mixture of 4-tert-butylphthalonitrile (1.32 g, 7.16 mmol), phthalonitrile 13a (475.0 mg, 1.42 mmol) and zinc(II) acetate (500 mg, 2.73 mmol) was stirred in DMAE (35 mL). The solution was refluxed under the flow of argon and two drops of DBN were added. The reaction solution was refluxed at 145 °C for 5 h. The solvent was removed under reduced pressure and the residue purified by silica column chromatography using CH2Cl2/methanol (97:3) for elution. A second silica column chromatography was performed in chloroform, followed by chloroform/methanol (99:1 → 98:2) to afford a blue solid (273.6 mg, 16.9%). 1H NMR (DMF-d7): δ 9.63-8.92 (m, 6H, Ar-H), 8.47-8.05 (m, 3H, Ar-H), 7.85-7.62 (m, 3H, Ar-H), 4.95-4.88 (m, 2H, CH2O), 4.49-4.39 (m, 2H, CH2O), 4.16-4.00 (m, 3H, CH2O), 3.88-3.44 (m, 19H, CH2O), 1.88-1.82 (m, 27H, C(CH3)3), 1.45, 1.38, 1.36 (s, 9H, -OC(CH3)3). 13CNMR (DMF-d7): δ 170.73, 170.66 (C=O), 158.1, 157.8, 157.4, 157.3, 157.2, 154.9, 154.8, 154.7, 154.5, 154.3, 154.1, 153.8, 153.7, 141.9, 141.8, 140.1, 139.9, 139.8, 137.9, 137.5, 137.2, 136.7, 137.2, 134.0, 131.6, 131.30, 131.26, 130.5, 128.9, 128.4, 126.9, 126.7, 126.4, 123.6, 123.4, 123.3, 122.9, 120.2, 120.1, 119.8, 119.7, 118.7, 117.0, 116.3, 114.8, 114.7, 114.6, 104.6 (Ar-C), 81.73, 81.68 (-OC(CH3)3), 72.1, 71.9, 71.7, 71.5, 71.44, 71.36, 71.3, 71.2, 70.9, 70.4, 70.0, 69.9, 69.62, 69.56, 68.9 (OCH2), 52.1, 45.9, 36.8 (N-CH2), 32.8, 32.7 (Ar-C(CH3)3), 28.6 (O-C(CH3)3). MS (MALDI-TOF) m/z 1139.675 [M+H]+, calcd for C62H75N8O9Zn 1138.495. The protected ZnPc (273.6 mg, 0.240 mmol) was dissolved in a 1:1 mixture of CH2Cl2/TFA (10 mL) and the solution was stirred at 0 ºC for 3 h. After the reaction, the solvent was removed and the resulting residue was treated with 2 N NaOH (15 mL). The product was extracted using 5:1 CH2Cl2/methanol (20 mL × 4) and the organic phase was washed with water (20 mL × 2) and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to afford the title ZnPc as a blue solid (243.7 mg, 93.7 %). 1HNMR (DMF-d7): δ 9.59-9.11 (m, 6H, Ar-H), 8.40-8.34 (m, 3H, Ar-H), 8.19-7.70 (m, 3H, Ar-H), 5.00-4.94 (m, 2H, CH2O), 4.60-4.43 (m, 2H, CH2O), 4.19-4.06 (m, 2H, CH2O), 3.90-3.04 (m, 16H, CH2O), 1.83-1.78 (m, 27H, C(CH3)3). 13CNMR (DMF-d7): δ 170.6 (C=O), 157.5, 157.1, 156.8, 155.9, 155.3, 155.0, 154.5, 153.8, 153.6, 142.5, 140.5, 140.2, 140.0, 138.3, 137.9, 137.6, 136.2, 132.5, 131.4, 130.4, 128.9, 128.5, 128.1, 127.2, 127.0, 123.9, 123.6, 123.1, 122.8, 120.7, 120.0, 119.8, 119.7, 119.5, 116.5, 116.1, 113.9 (Ar-C), 72.5, 72.0, 71.7, 71.5, 71.3, 71.2, 71.0, 70.5, 70.2, 69.7, 69.6, 68.9, 68.6 (OCH2), 52.1, 46.2, 36.8, 36.7 (N-CH2), 32.7, 32.6 (Ar-C(CH3)3). MS (MALDI-TOF) m/z 1083.444 [M+H]+, calcd for C58H67N8O9Zn 1083.432. UV-vis (DMF): λmax (log ε) 345 (4.77), 613 (4.57), 681 (5.34) nm.
ZnPc 14b. A mixture of 4-tert-butylphthalonitrile (385 mg, 2.09 mmol), phthalonitrile 13b (273.4 mg, 0.69 mmol) and zinc(II) acetate (254.7 mg, 1.4 mmol) were stirred in DMAE (35 mL). The solution was refluxed under a flow of argon, and two drops of DBN were added into the reaction solution. The reaction was treated as ZnPc 14a above to afford blue solid (121.6 mg, 15.5%). 1HNMR (DMF-d7): δ 9.59-8.92 (m, 7H, Ar-H), 8.47-8.35 (m, 3H, Ar-H), 7.88-7.78 (m, 2H, Ar-H), 4.74-4.58 (m, 2H, CH2O), 4.19-4.00 (m, 4H, CH2O), 3.87-3.57 (m, 18H, CH2O), 1.83-1.75 (m, 27H, C(CH3)3), 1.42- 1.38 (s, 9H, -OC(CH3)3). 13CNMR (DMF-d7): δ 170.7 (C=O), 158.4, 158.1, 155.0, 154.9, 154.8, 154.6, 154.4, 154.2, 154.0, 142.8, 141.8, 140.0, 137.6, 133.5, 132.9, 132.5, 132.2, 131.7, 131.3, 130.4, 128.9, 128.4, 124.8, 123.9, 123.33, 123.25, 122.9, 120.8, 120.2, 119.9, 119.2, 118.9, 109.4, 107.4, 104.2 (Ar-C), 81.7 (-OC(CH3)3), 71.74, 71.69, 71.5, 71.4, 71.33, 71.30, 70.8, 70.2, 69.8, 69.61, 69.57, 69.0 (OCH2), 52.1, 47.4, 45.5, 36.8 (N-CH2), 32.6 (Ar-C(CH3)3), 28.5 (O-C(CH3)3). MS (MALDI-TOF) m/z 1138.454 [M]+, 1082.448.454 [M-C4H9]+, calcd for C62H74N8O9Zn 1138.487, C58H66N8O9Zn 1082.424. The blue solid (113.0 mg, 0.119 mmol) was dissolved in a mixture of CH2Cl2/TFA (4 mL/4 mL) and the solution was stirred at 0 ºC for 3 hours. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to afford a blue solid (94.7 mg, 93.7 %) was obtained. 1HNMR (DMF-d7): δ 9.59-9.09 (m, 7H, Ar-H), 8.36-8.24 (m, 3H, Ar-H), 7.89-7.70 (m, 2H, Ar-H), 4.56-4.46 (m, 2H, CH2O), 3.90-3.04 (m, 24H, CH2O), 1.83-1.78 (m, 27H, C(CH3)3). 13CNMR (DMF-d7): δ 171.8 (C=O), 159.6, 158.2, 157.9, 157.1, 155.2, 155.0, 154.9, 154.6, 154.1, 154.0, 153.8, 153.6, 142.2, 141.9, 140.2, 141.1, 140.0, 137.8, 137.6, 134.4, 133.4, 133.0, 132.5, 131.6, 131.4 130.8, 130.4, 128.9, 128.4, 128.3, 128.1, 128.0, 126.3, 124.7, 124.6, 123.9, 123.2, 122.8, 120.7, 120.0, 119.9, 119.8, 119.1, 115.0, 107.5, 107.4, 107.2 (Ar-C), 71.7, 71.6, 71.5, 71.4, 71.3, 71.2, 71.0, 70.7, 70.5, 70.1, 69.8, 69.5, 68.9 (OCH2), 52.1, 47.4, 46.1, 40.9, 36.8, 36.5 (N-CH2), 32.6 (Ar-C(CH3)3). MS (MALDI-TOF) m/z 1083.428 [M+H]+, calcd for C58H67N8O9Zn 1083.432. UV-vis (DMF): λmax (log ε) 351 (4.65), 609 (4.38), 676 (5.14) nm.
ZnPc 15a. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 14a (20.0 mg, 0.018 mmol), DMF (0.4 mL), Et3N (3.3 mg, 0.032 mmol), HOBt (4.7 mg, 0.035 mmol), 1,4-bis-Boc-triazaheptane (7.0 mg, 0.023 mmol) and EDCI (4.0 mg, 0.026 mmol). After work-up and purification the protected ZnPc was obtained as a blue solid (16.1 mg, 63.6%). 1H NMR (DMF-d7): δ 9.54-9.20 (m, 5H, Ar-H), 9.06-8.84 (m, 1H, Ar-H), 8.47-8.35 (m, 3H, Ar-H), 7.82-7.76 (m, 2H, Ar-H), 6.76-6.70 (m, 1H, Ar-H), 4.96-4.92 (m, 2H, COCH2O), 4.58-4.44 (m, 2H, CH2O), 4.19-4.10 (m, 1H, CH2O), 4.07-3.09 (m, 1H, CH2O), 3.88-3.72 (m, 5H, CH2O), 3.60-3.43 (m, 17H, CH2O), 3.34-3.28 (m, 6H, CH2NH), 3.20-3.18 (m, 2H, CH2NH), 1.86-1.82 (m, 27H, C(CH3)3), 1.46-1.39 (m, 18H, -OC(CH3)3). 13C NMR (DMF-d7): δ 170.7, 175.1, 156.4, 154.8, 154.0, 139.8, 137.5, 131.8, 128.5, 123.4, 119.8, 116.4, 114.9, 114.7 (Ar-C), 79.9, 78.7 (O-C(CH3)3), 72.0, 71.71, 71.66, 71.5, 71.4, 71.3, 71.2, 71.0, 70.4, 69.9 (CH2O), 48.4, 48.2, 44.3 40.1, 39.9, 38.4, 36.8 (N-CH2), 32.6 (Ar-C(CH3)3), 29.0, 28.8 (O-C(CH3)3). MS (MALDI-TOF) m/z 1367.708 [M]+, 1207.655 [M-2tBu+K]+; calcd for C72H93N11O12Zn 1367.630, C62H78KN11O8Zn 1207.496. The blue solid (16.1 mg, 0.0118 mmol) was dissolved in a 1:1 mixture of CH2Cl2/TFA (6 mL) and stirred at 0 ºC for 3 h. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to give a blue solid (12.5 mg, 91.0 %). 1H NMR (DMF-d7): δ 9.56-8.95 (m, 7H, Ar-H), 8.52-8.31 (m, 3H, Ar-H), 8.13-8.03 (m, 1H, Ar-H), 7.83-7.70 (m, 1H, Ar-H), 5.02-4.83 (m, 2H, COCH2O), 4.59-4.35 (m, 2H, CH2O), 4.19-4.00 (m, 2H, CH2O), 3.92-3.00 (m, 22H, CH2O), 1.79 (s, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.2, 170.9, 170.3, 157.3, 155.0, 154.4, 154.1, 154.0, 142.2, 140.3, 140.1, 138.0, 137.7, 131.6, 129.0, 128.3, 126.9, 123.6, 123.3, 119.8, 116.5, 115.0, 114.7 (Ar-C), 71.9, 71.6, 71.4, 71.3, 71.2, 71.1, 70.8, 70.6, 70.1, 69.6 (CH2O), 49.1, 48.0, 47.5, 47.0, 46.6, 46.3, 45.9, 45.8, 44.6, 44.3, 39.6, 38.8, 36.8 (N-CH2), 32.6 (Ar-C(CH3)3). MS (MALDI-TOF) m/z 1167.520 [M]+, calcd for C62H77N11O8Zn 1167.525. UV-vis (DMF): λmax (log ε) 346 (4.77), 615 (4.55), 683 (5.31) nm.
ZnPc 15b. A procedure similar to the one described above for ZnPc 3a was used: ZnPc 14b (20.0 mg, 0.018 mmol), DMF (0.4 mL), Et3N (3.2 mg, 0.032 mmol), HOBt (4.7 mg, 0.035 mmol), 1,4-bis-Boc-triazaheptane (7.0 mg, 0.023 mmol), and EDCI (4.0 mg, 0.026 mmol). The protected ZnPc was obtained as a blue solid (17.1 mg, 65.6%). 1H NMR (DMF-d7): δ 9.58-9.52 (m, 3H, Ar-H), 9.46-9.22 (m, 4H, Ar-H), 8.96-8.88 (m, 1H, Ar-H), 8.45-8.38 (m, 3H, Ar-H), 7.82-7.76 (m, 2H, Ar-H), 6.76-6.70 (m, 1H, Ar-H), 4.73-4.70 (m, 2H, COCH2O), 4.49-4.44 (m, 2H, CH2O), 3.89-3.82 (m, 4H, CH2O), 3.77-3.72 (m, 2H, CH2O), 3.69-3.58 (m, 14H, CH2O), 3.35-3.28 (m, 6H, CH2NH), 3.20-3.18 (m, 2H, CH2NH), 1.82-1.80 (m, 27H, C(CH3)3), 1.45-1.36 (m, 18H, -OC(CH3)3). 13CNMR (DMF-d7): δ 170.7, 162.2, 157.1, 156.5, 154.9, 154.8, 154.7, 154.3, 154.2, 141.7, 139.9, 137.5, 133.4, 132.8, 132.1, 131.4, 130.4, 128.4, 123.4, 120.8, 119.9, 119.8, 119.2, 115.0, 113.3, 107.4, 107.2 (Ar-C), 79.9, 78.7 (O-C(CH3)3), 71.74, 71.68, 71.51, 71.48, 71.41, 71.37, 71.34, 71.30, 71.1, 70.8, 69.6 (OCH2), 48.4, 48.2, 48.0, 47.6, 47.4, 46.1 40.9, 40.1, 39.9, 38.4, 36.8 (N-CH2), 32.6 (Ar-C(CH3)3), 29.0, 28.8 (O-C(CH3)3). MS (MALDI-TOF) m/z 1267.606 [M-tBu]+, calcd for C67H85N11O10Zn 1267.577. The blue solid (17.1 mg, 0.013 mmol) was dissolved in a 1:1 mixture of CH2Cl2/TFA (6 mL) and stirred at 0 ºC for 3 h. The solvent was removed under reduced pressure and the residue treated with 2N NaOH (10 mL) to obtain a blue solid (13.5 mg, 92.8 %). 1H NMR (DMF-d7): δ 9.60-9.25 (m, 7H, Ar-H), 9.15-8.91 (m, 1H, Ar-H), 8.52-8.31 (m, 3H, Ar-H), 7.89-7.73 (m, 1H, Ar-H), 4.74-4.63 (m, 2H, COCH2O), 4.19-4.05 (m, 3H, CH2O), 3.92-3.45 (m, 29H, CH2O), 3.29-3.10 (m, 6H, CH2NH) 1.80 (s, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.6, 162.1, 160.2, 159.7, 155.0, 154.6, 154.4, 154.1, 153.9, 141.9, 140.1, 137.7, 133.1, 128.2, 124.7, 123.3, 119.8, 119.1, 107.2 (Ar-C), 71.72, 71.67, 71.6, 71.4, 71.3, 71.2, 71.0, 70.7, 69.6 (OCH2), 49.0, 46.5, 38.4, 37.4, 36.7 (N-CH2), 32.6 (Ar-C(CH3)3). MS (MALDI-TOF) m/z 1167.531 [M]+, calcd for C62H77N11O8Zn 1167.525. UV-vis (DMF): λmax (log ε) 352 (4.90), 611 (4.61), 678 (5.35) nm.
ZnPc 16a. A procedure similar to the one described above for ZnPc 4a was used: ZnPc 15a (20.0 mg, 0.017 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry DMF (0.5 mL). The title ZnPc was obtained (17.4 mg, 67.4%). 1H NMR (DMF-d7): δ 9.57-9.34 (m, 6H, Ar-H), 9.13-8.99 (m, 1H, Ar-H), 8.38-8.18 (m, 3H, Ar-H), 8.20-8.09 (m, 1H, Ar-H), 7.88-7.72 (m, 1H, Ar-H), 5.03-4.94 (m, 2H, COCH2), 4.65-4.36 (m, 2H, CH2O),4.15-3.51 (m, 28H, CH2O/N-CH2/N-CH3), 3.43 (s, 9H, N-CH3), 1.83-1.79 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.7, 170.5 (C=O), 157.5, 157.4, 155.1, 155.0, 154.8, 154.5, 154.1, 154.0, 142.3, 142.2, 142.1, 140.4, 140.0, 138.0, 137.7, 131.7, 128.4, 126.9, 123.6, 123.3, 119.9, 119.8, 116.5, 115.0, 114.8 (Ar-C), 72.1, 72.0, 71.8, 71.7, 71.4, 71.2, 71.0, 70.7, 70.5, 70.2 (CH2O), 64.5, 54.5, 53.5, 52.5, 52.2, 51.4, 48.2 (N-CH3), 43.3, 43.2, 36.8 (N-CH2) 32.7, 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1404.705 [M-I+2H+K]+; calcd for C67H90IKN11O8Zn+ 1404.495. UV-Vis (DMF): λmax (log ε) 347 (4.75), 615 (4.51), 682 (5.28) nm.
ZnPc 16b. A similar procedure to the one described above for ZnPc 4a was used: ZnPc 15b (20.0 mg, 0.017 mmol), DIPA (0.015 mL, 0.107 mmol), CH3I (0.2 mL) and dry DMF (0.5 mL). The title ZnPc was obtained as a blue solid (16.2 mg, 62.8%). 1H NMR (DMF-d7): δ 9.57-9.34 (m, 7H, Ar-H), 9.03-8.99 (m, 1H, Ar-H), 8.38-8.18 (m, 4H, Ar-H), 7.84-7.82 (m, 1H, N-H), 4.80-4.70 (m, 2H, COCH2), 4.35 (br, 2H, CH2O),4.15-3.51 (m, 28H, CH2O/N-CH2/N-CH3), 3.37 (s, 9H, N-CH3), 1.85-1.79 (m, 27H, C(CH3)3). 13C NMR (DMF-d7): δ 171.7 (C=O), 162.0, 155.0, 154.5, 154.3, 153.9, 151.2, 142.1, 140.2, 137.7, 133.2, 128.3, 128.1, 124.6, 123.2, 119.7, 119.0, 107.5, 107.3, 107.1 (Ar-C), 71.8, 71.7, 71.4, 71.3, 71.2, 71.1, 71.0, 70.7, 69.6 (CH2O), 64.3, 58.6, 57.2, 55.9, 54.3, 52.4, 47.9 (N-CH3), 43.2, 36.7 (N-CH2) 32.6 (C(CH3)3). MS (MALDI-TOF) m/z 1493.721 [M]+, 1404.681 [M-I-H+K]+; calcd for C67H89I2N11O8Zn + 1493.428, C67H88IKN11O8Zn+ 1404.479. UV-Vis (DMF): λmax (log ε) 351 (4.81), 610 (4.50), 677 (5.26) nm.
Atomic Force Microscopy (AFM)
A model 5500 or 5420 scanning probe microscope from Agilent (Chandler, AZ) was used for tapping-mode atomic force microscopy experiments. Picoview imaging software was used for data acquisition and analysis. Data files were processed using Gwyddion open source software, which is freely available on the internet and supported by the Czech Metrology Institute [37]. Rectangular tapping-mode probes with an average frequency of 164 kHz with a highly reflective aluminum backside coating were purchased from MikroMasch (Estonia) for imaging samples in ambient conditions.
For the AFM sample preparation, Pc samples were dissolved in 10 mL of DMF to yield solutions of 100 µM concentration. An aliquot of 10 µL of the prepared solutions was deposited onto freshly cleaved mica(0001) and dried for 4 days before imaging.
Cell studies
Human carcinoma HEp2 cells were maintained in a 50:50 mixture of DMEM:AMEM (Invitrogen) supplemented with 5% FBS (Invitrogen), Primocin antibiotic (Invitrogen) and 5% CO2 at 37 oC. The cells were subcultured twice weekly to maintain subconfluent stocks. The 4th to 15th passage cells were used for all the experiments.
Time-Dependent Cellular Uptake: The HEp2 cells were incubated at 7500 cells per well in a Costar 96-well plate and allowed to grow for 48 h. The stock solutions for each ZnPc were prepared at 32 mM in DMSO and diluted to give 20 µM in medium (a 2X stock). Further dilution into the 96-well plate achieved a final concentration of 10 µM with a maximum DMSO concentration of 1%. Uptake was allowed to continue for 0, 1, 2, 4, 8, 12 and 24 h, when it was terminated by removing the loading medium and washing the wells with PBS. Each Pc concentration was determined from standard curves using intrinsic fluorescence, measured with a BMG FLUOstar plate reader equipped with a 355 nm excitation and a 650 nm emission filter. Using a CyQuant cell proliferation assay (Invitrogen) as per manufacture's instruction, cells were measured and the uptake expressed in terms of nM compound concentration per cell.
Dark Cytotoxicity: The HEp2 cells were plated as described above and the compounds diluted into medium to give a final concentration of 400 µM. By preparing two-fold serial dilutions to 50 µM, the cells were incubated overnight and the cell toxicity measured using Promega's Cell Titer Blue Viability assay as per manufacturer's instructions. Untreated cells were considered 100% viable while cells treated with 0.2% saponin were considered to be 0% viable. The IC50 values were then determined from dose-response curves obtained using GraphPad Prism software.
Phototoxicity: The cells were plated as described above with ZnPc concentrations ranging from 6.25-100 µM. After overnight loading, new medium containing 50 mM HEPES pH 7.2 was introduced, replacing the former. The cells were then exposed to a NewPort light system equipped with a 175 W halogen lamp for 20 min, filtered through a water filter to provide approximately 1.5 J/cm2 light dose. The culture was kept on a 5 oC Echotherm chilling/heating plate (Torrey Pines Scientific, Inc.) to keep the cells cool. After exposure to light, the plate was incubated overnight and the cell viability measured as described above for the dark cytotoxicity.
Microscopy: The cells were incubated in a glass bottom 6-well plate (MatTek) and allowed to grow for 48 h. They were exposed to 10 µM of each compound for 6 h. Organelle tracers (from Invitrogen) were used at the following concentrations: LysoSensor Green 50 nM, MitoTracker Green 250 nM, ER Tracker Blue/white 100 nM, and BODIPY FL C5 ceramide 1 µM. The tracers were diluted in medium and the cells incubated concurrently with ZnPc and tracers for 30 min before washing 3 times with PBS. Microscopy images were acquired using a Leica DMRXA microscope with a 40× NA 0.8dip objective lens and DAPI, GFP and Texas Red filter cubes (Chroma Technologies).
Results and Discussion
Syntheses
The di-cationic ZnPcs 4a,b, 6a,b, 9a,b, 11a,b, and 16a,b were synthesized as shown in Schemes 1 and 2 (Figure A and Figure B), from 3- or 4-nitrophthalonitrile (12a,b). The ZnPcs 1a,b and 2a,b were prepared according to procedures previously reported [35,36]. In brief, the nitrophthalonitrile reacted with p-N-Boc-aminophenol in DMF at 80 ºC under basic conditions to give the corresponding p-N-Boc-aminophenoxy)phthalonitriles, which were heated at 140 ºC in dimethylaminoethanol (DMAE) and in the presence of zinc(II) acetate, 3 equiv. 4-tert-butylphthalonitrile and a catalytic amount of 1,5-diazabicyclo(4.3.0)non-5-ene (DBN), giving the Boc-protected α- or β-substituted A3B-type ZnPcs in 15-20% yields [35]. Deprotection of the Boc groups using TFA followed by reaction with diglycolic anhydride gave ZnPcs 1a,b, which were conjugated with commercially available tert-butyl-12-amino-4,7,10-trioxadodecanoate, using 1-hydroxybenzotriazole (HOBt), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDCI) and triethylamine (TEA) in DMF to produce pegylated ZnPcs 2a,b after deprotection with TFA [36,38]. Reaction of ZnPcs 1a,b and 2a,b with 1,4-bis(N-Boc)-triazaheptane under similar coupling conditions, followed by TFA-mediated Boc-deprotection gave ZnPcs 5a,b and 3a,b, respectively, in 72-79% yields. Quaternization of the amino groups using excess methyl iodide and diisopropylamine (DIPA) in DMF [35] gave the corresponding di-cationic ZnPcs 6a,b and 4a,b in 53-68% yields. The branched ZnPcs 7a,b were prepared via reaction of 1a,b with di-tert-butyl ester protected L-aspartic acid in DMF, using TEA and 2-(1H-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) and HOBt, followed by deprotection with TFA [39]. The dicarboxylate terminated ZnPcs 7a,b were coupled to N-Boc-2,2′-(ethylenedioxy)diethylamine or N-Boc-ethylenediamine using TEA, HOBt and EDCI, giving ZnPcs 8a,b and 10a,b respectively, which were quaternized as described above, affording the di-cationic ZnPcs 9a,b and 11a,b in 59-68% yields (Scheme 1/Figure A).
Phthalonitriles 13a,b were obtained from reaction of tert-butyl 20-hydroxy-3,6,9,12,15,18-hexaoxaicosan-1-oate, prepared in 38% yield following a published procedure [40], with 3- or 4-nitrophthalonitrile in THF at 65 ºC, in the presence of K2CO3 [35] (Scheme 2/Figure B). Cyclotetramerization of phthalonitriles 13a,b with excess 4-tert-butylphthalonitrile in the presence of zinc(II) acetate and a catalytic amount of DBN in DMAE gave the corresponding A3B-type ZnPcs 14a,b in about 12% yield, after deprotection of the tert-butyl group using TFA at room temperature. Conjugation of the pegylated ZnPcs 14a,b with 1,4-bis(N-Boc)-triazaheptane using TEA, HOBt and EDCI, followed by cleavage of the Boc group gave ZnPcs 15a,b in 58-61% yields. Quaternization as described above gave the corresponding di-cationic ZnPcs 16a,b in 63-67% yields.
(Scheme 1) Synthesis of dicationic ZnPcs 4, 6, 9 and 11. Reaction conditions: (a) tert-butyl-12-amino-4,7,10-trioxadodecanoate, TEA, HOBt, EDCI, DMF (77-82%); (b) TFA, CH2Cl2. 0 oC, 3 h (82-92%); (c) 1,4-bis(N-Boc)-triazaheptane, TEA, HOBt, EDCI, DMF (77-89%); (d) CH3I, DIPA, DMF (59-68%); (e) L-aspartic acid di(tert-butyl) ester, TEA, HATU, DMF (87-89%); (f) N-Boc-2,2′-(ethylenedioxy)diethylamine, TEA, HOBt, EDCI, DMF (51-52%); (g) N-Boc-ethylenediamine, TEA, HOBt, EDCI, DMF (58-62%).
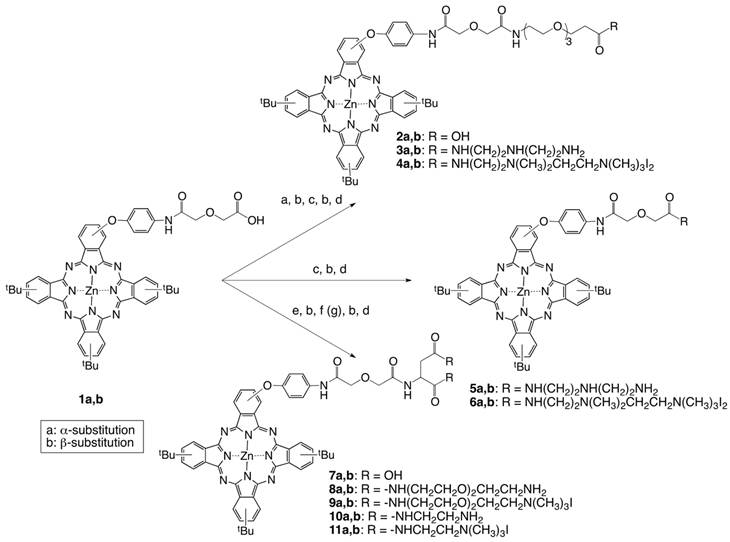
(Scheme 2) Synthesis of pegylated ZnPc 16. Reaction conditions: (a) tert-butyl-20-hydroxy-3,6,9,12,15,18-hexaoxaicosan-1-oate, K2CO3, THF, 65 oC, 6 h (78%); (b) 4-tert-butylphthalonitrile, Zn(OAc)2, DMAE, 145 oC, 5 h (16-17%); (c) TFA, CH2Cl2, 0 oC, 3 h (91-94 %); (d) 1,4-bis(N-Boc)-triazaheptane, TEA, HOBt, EDCI, DMF (58-61%); (e) CH3I, DIPA, DMF, r.t., 2 d (59-67%).
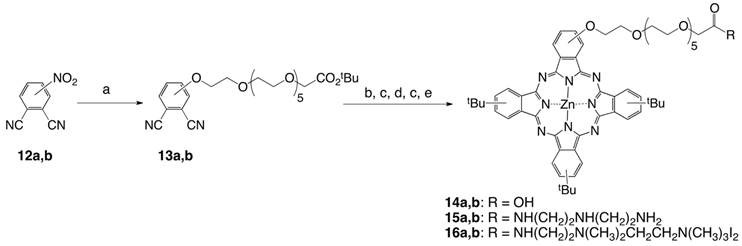
Side-by-side comparison of sample aggregation on surfaces of mica(0001). Topography images of Pcs (A) 6a, (B) 2b, and (C) 16a, with corresponding cursor profiles. The frame sizes are 2 × 2 µm2, acquired in ambient air using tapping-mode AFM.
All di-cationic ZnPcs 4a,b, 6a,b, 9a,b, 11a,b, and 16a,b are soluble in polar organic solvents, such as DMF, THF and methanol, but none of the cationic Pcs was water soluble; they remained aggregated in water, even upon sonication. To compare how the samples self-assemble into aggregate structures, AFM studies were accomplished using tapping-mode for samples prepared on mica surfaces. Topography views are shown in Figure 1 for samples of Pcs 6a, 2b, and 16a. Considerable differences are apparent for the heights and surface coverage of the samples. The largest aggregates were revealed for the non-pegylated Pc 6a, with an average height of 9.6 ± 0.5 nm (mean ± standard error, n = 93). Most of the clusters (70%) ranged from 8 to 14 nm in height. The smallest clusters were observed for Pc 16a containing a longer PEG chain than 2b, measuring 1.0 ± 0.15 nm (n = 52). The average size of surface deposits for Pc 2b measured 1.1 ± 0.1 nm, with most of the clusters measuring 0.6 to 1.2 nm in height (77%, n = 227). The sizes were measured using height information obtained from individual cursor profiles within the images. The lateral dimensions of surface features depend closely on the AFM probe geometry and are not as reliable for estimating sizes. Comparing the samples using a t-test, the mean values for 16a and 2b indicate that the surface structures are essentially the same height. However, the size of the aggregates of Pc 6a is significantly larger than those of either Pcs 2b or 16a. The sizes of aggregates follow the trend: Pc 6a >> Pc 2b > Pc 16a.
Nevertheless, all Pcs remained in aqueous solution upon dilution from concentrated Pc stocks in DMF or DMSO into PBS (final DMF or DMSO concentration of 1%) at 10 µM concentrations, which were used for the cellular uptake and microscopy experiments (vide infra). Since the cationic ZnPcs undergo N-demethylation upon storage for over one week at room temperature, as we have previously observed [35], all cationic ZnPcs were characterized and investigated within 2-3 days of their purification.
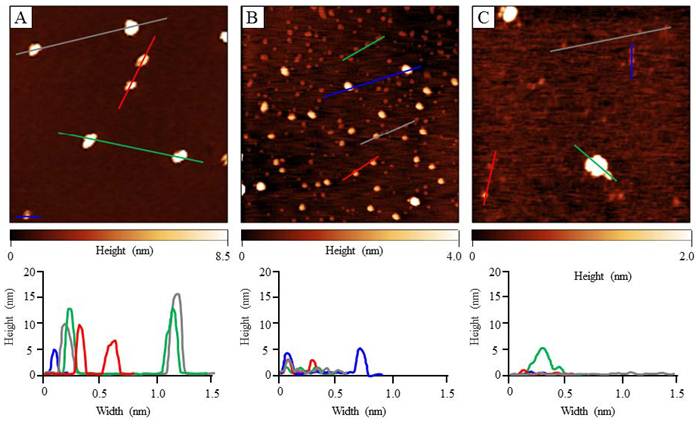
The spectroscopic properties of the di-cationic ZnPcs 4a,b, 6a,b, 9a,b, 11a,b, 16a,b and their amine precursors in DMF are summarized in Table 1 and shown in Figure 2. No aggregation of the Pcs was apparent up to 10 μM concentrations in DMF [42]. All ZnPcs showed strong Q absorptions and emissions in the near-IR between 679-686 nm in DMF, with small Stokes' shifts between 2-4 nm, as is characteristic of this type of macrocycle [35,41-43]. All the ZnPcs show a Soret absorption between 330 - 360 nm, a strong Q band between 677-683 nm and a vibrational band at around 620 nm, that strictly follow the Lambert-Beer law. The ZnPcs had fluorescence quantum yields in the range 0.09-0.23 in DMF, as expected for this type of compound [35,41-43].
Spectroscopic Properties of ZnPcs in DMF at room temperature.
| ZnPc | Absorption λmax (nm) | Emissiona λmax (nm) | Stokes' Shift (nm) | log ε (M-1cm-1) | фFb |
|---|---|---|---|---|---|
| 3a | 680 | 684 | 4 | 5.52 | 0.23 |
| 3b | 677 | 680 | 3 | 5.28 | 0.15 |
| 4a | 679 | 683 | 4 | 5.32 | 0.15 |
| 4b | 677 | 680 | 3 | 5.30 | 0.14 |
| 5a | 680 | 684 | 4 | 5.19 | 0.19 |
| 5b | 677 | 679 | 2 | 5.19 | 0.20 |
| 6a | 679 | 682 | 3 | 5.23 | 0.21 |
| 6b | 676 | 679 | 3 | 5.31 | 0.15 |
| 7a | 679 | 682 | 3 | 5.17 | 0.13 |
| 7b | 677 | 680 | 3 | 5.28 | 0.10 |
| 8a | 680 | 683 | 3 | 5.20 | 0.14 |
| 8b | 677 | 681 | 4 | 5.27 | 0.11 |
| 9a | 680 | 683 | 3 | 5.01 | 0.14 |
| 9b | 677 | 680 | 3 | 5.01 | 0.11 |
| 10a | 680 | 683 | 3 | 5.23 | 0.13 |
| 10b | 677 | 680 | 3 | 5.19 | 0.12 |
| 11a | 679 | 682 | 3 | 5.19 | 0.09 |
| 11b | 677 | 680 | 3 | 4.99 | 0.11 |
| 14a | 681 | 684 | 3 | 5.34 | 0.19 |
| 14b | 676 | 680 | 4 | 5.14 | 0.19 |
| 15a | 683 | 686 | 3 | 5.31 | 0.21 |
| 15b | 678 | 681 | 3 | 5.35 | 0.18 |
| 16a | 682 | 685 | 3 | 5.28 | 0.18 |
| 16b | 677 | 680 | 3 | 5.26 | 0.17 |
a Excitation at 640 nm. b Calculated using ZnPc (Фf = 0.17) as the standard in DMF.
(a) Absorption and (b) fluorescence spectra for Pc 4a (red), 4b (green), 6a (black), 6b (brown), 9a (grey), 9b (purple), 11a (light green), 11b (orange), 16b (blue), 16a (pink), at 8.0 and 0.5-0.8 μM concentrations, respectively, in DMF.
Biological Evaluation
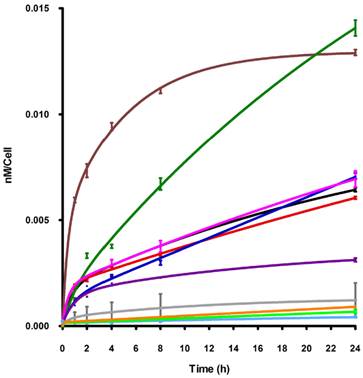
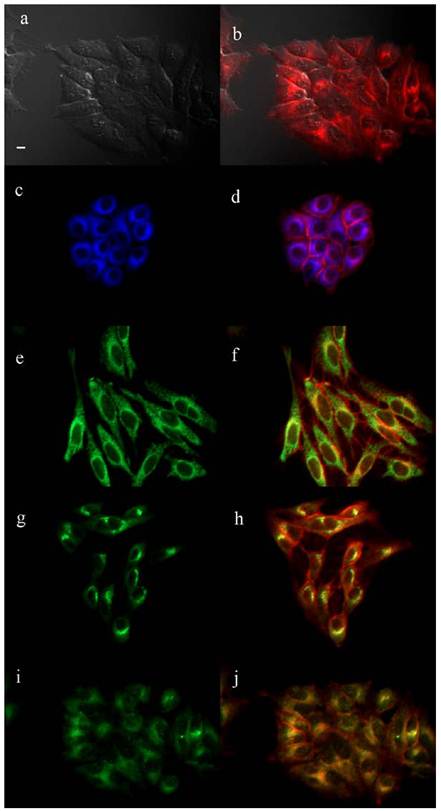
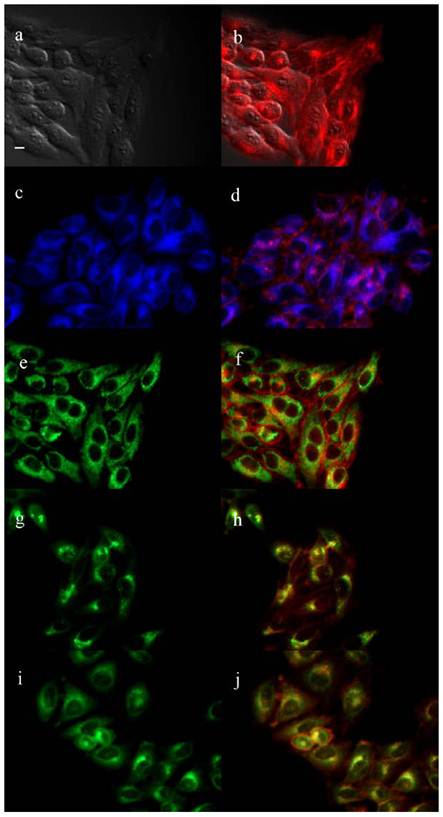
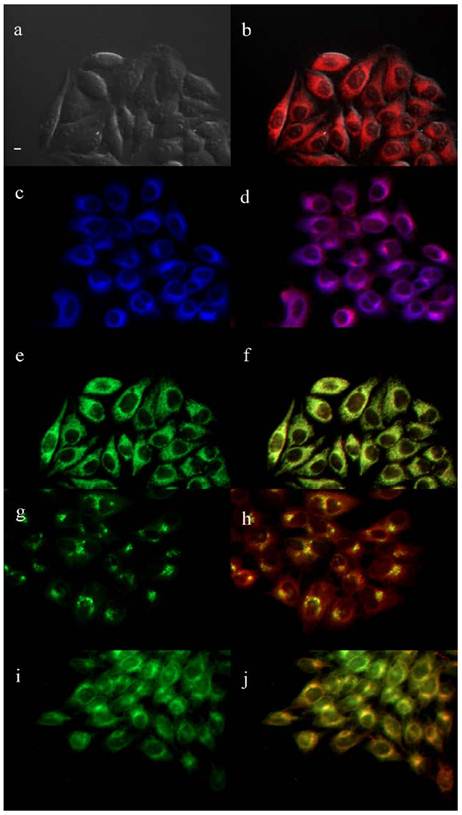
The biological properties of the cationic ZnPcs including time-dependent cellular uptake, cytotoxicity and intracellular localization, were investigated in human carcinoma HEp2 cells. The cytotoxicity was evaluated using Promega's Cell Titer Blue viability assay, as we have previously reported [28,35,36,39,44] at concentrations up to 400 μM for each di-cationic ZnPc and for the pegylated, neutral ZnPc 2b (see Supplementary Material: Figure S9). The time-dependent uptake into HEp2 cells was investigated at a concentration of 10 μM of each Pc up to 24 h (Figure 3). The subcellular sites of localization were observed by fluorescence microscopy, 6 h after exposure of HEp2 cells to each ZnPc (see Figures 4-6 and Supporting Information, Supplementary Material: Figures S1-S8). Co-localization experiments were conducted using the organelle specific fluorescent tracers: ER Tracker Blue/White (endoplasmic reticulum, ER), MitoTracker Green (mitochondria), BODIPY Ceramide (Golgi) and LysoSensor Green (lysosomes). Table 2 summarizes the results obtained from these studies. Our results show that all the α-substituted ZnPcs are much more toxic than the corresponding β-substituted ZnPcs, both in the dark and upon exposure to approx. 1.5 J/cm2 light dose. We had previously observed that the cytotoxicity of cationic Pcs depends on the substitution at the macrocycle periphery, and that the α-substituted compounds tend to be more toxic than the corresponding β-substituted Pcs [35]. This might be due to their increased electron density of the ring and distinct conformations, as shown in Supplementary Material: Figure S21; the β-substituted di-cationic ZnPcs tend to adopt more extended conformations than the α-substituted analogues. All ZnPcs had very low toxicity in the dark (IC50 > 180 μM), in particular the branched dicationic Pcs 9a,b and 11a,b which showed remarkable low dark toxicity (IC50 > 400 μM), maybe due to their very low uptake into cells (see Figure 3). Upon irradiation with low light dose (~1.5 J/cm2) all β-substituted ZnPcs were non-toxic up to 100 μM concentrations, with the exception of 11b, which was moderately phototoxic (IC50 = 47 μM). The most phototoxic compounds were the α-substituted ZnPcs 4a, 6a, 9a, 11a and 16a, with determined IC50 values (calculated from dose-response curves, see Supporting Information) of 10.7, 14.8, 28.8, 12.7 and 8.7 μM, respectively. Of this series, Pcs 4a, 11a and 16a are the most promising for PDT applications due to their high ratio (>25) of dark/photo cytotoxicity and high phototoxicity. It is interesting to note that the most phototoxic Pcs (4a and 16a) contain a PEG group, the two positive charges in close proximity (separated only by an ethylene group), and both localize in the cell ER. It is possible that pegylation of Pc macrocycles favors intracellular localization in the ER, as we have previously observed [38], whereas the positive charges might favor localization at the plasma membrane and subcellularly in mitochondria and lysosomes [44-47]. On the other hand, the β-substituted ZcPcs 6b and 4b accumulated within HEp2 cells to a much higher extent than all other Pcs. The cellular uptake of the non-pegylated Pc 6b was the fastest of all ZnPcs, although it slowed down to a plateau 8 h after exposure, while Pc 4b continued to accumulate within cells up to the 24 h period investigated, probably as a result of its PEG group. Furthermore, the di-cationic Pcs bearing the two charges in close proximity on the same chain, showed increased uptake compared with the neutral Pc-PEG 2b, as well as to the cationic Pcs 9a,b and 11a,b bearing the charges on two side branches. All cationic ZnPcs localized in multiple sites within the cell, with exception of 9b which was mainly found in lysosomes (Table 2). In addition, ZnPcs 4a, 6a, 11a and 16b were observed at the plasma membrane. The most phototoxic compounds 4a, 11a and 16a all localized within the ER, an important organelle that regulates protein synthesis and stress responses, potentially leading to PDT-induced cell apoptosis [48,49]. Our results show that the intracellular localization of the ZnPcs, rather than the extent of their cellular uptake, and their charge distribution mainly determine their photodynamic activity, probably as a result of their different interactions with intracellular components.
Cytotoxicity and intracellular sites of localization for ZnPcs in HEp2 cells.
| ZnPc | Dark toxicity (IC50, µM) | Phototoxicity (IC50, µM) | Ratio | Major (++) and minor (+) sites of localization |
|---|---|---|---|---|
| 2b | 397.3 | >100.0 | >4.0 | Mitochondria(++), ER(+), Golgi(+) |
| 4a | 351.7 | 10.7 | 32.9 | ER(++), Golgi (+),Lysosomes(+) |
| 4b | 280.0 | >100.0 | >2.8 | Mitochondria(++), ER(+), Golgi(++) |
| 6a | 179.9 | 14.8 | 12.2 | Golgi(++), Lysosomes(+) |
| 6b | 346.3 | >100 | >3.5 | Mitochondria(++), Golgi(+), Lysosomes(++) |
| 9a | >400.0 | 28.8 | >13.9 | Lysosomes(++), ER(+), Golgi(+) |
| 9b | >400.0 | >100.0 | >4.0 | Lysosomes(++) |
| 11a | >400.0 | 12.7 | >31.5 | Lysosomes(++), ER(+), Golgi(+) |
| 11b | >400.0 | >100.0 | >4.0 | Mitochondria(++), Golgi(+) |
| 16a | 220.1 | 8.7 | 25.3 | Mitochondria(++), ER(++), Lysosomes(+) |
| 16b | 249.7 | 47.0 | 5.3 | ER(++), Lysosomes (+) |
Time-dependent uptake of ZnPcs 2b (purple), 4a (red), 4b (green), 6a (black), 6b (brown), 9a (grey), 9b (light blue), 11a (light green), 11b (orange), 16a (pink), 16b (blue) at 10 μM in HEp2 cells.
Subcellular localization of ZnPc 4a in HEp2 cells at 10 μM for 6 h. (a) Phase contrast, (b) Overlay of 4a fluorescence and phase contrast, (c) ER Tracker Blue/White fluorescence, (e) MitoTracker green fluorescence, (g) BODIPY Ceramide fluorescence, (i) LysoSensor green fluorescence, and (d, f, h, j) overlays of organelle tracers with 4a fluorescence. Scale bar: 10 μm.
Subcellular localization of ZnPc 11a in HEp2 cells at 10 μM for 6 h. (a) Phase contrast, (b) Overlay of 11a fluorescence and phase contrast, (c) ER Tracker Blue/White fluorescence, (e) MitoTracker green fluorescence, (g) BODIPY Ceramide, fluorescence (i) LysoSensor green fluorescence, and (d, f, h, j) overlays of organelle tracers with 11a fluorescence. Scale bar: 10 μm.
Subcellular localization of ZnPc 16a in HEp2 cells at 10 μM for 6 h. (a) Phase contrast, (b) Overlay of 16a fluorescence and phase contrast, (c) ER Tracker Blue/White fluorescence, (e) MitoTracker green fluorescence, (g) BODIPY Ceramide fluorescence, (i) LysoSensor green fluorescence, and (d, f, h, j) overlays of organelle tracers with 16a fluorescence. Scale bar: 10 μm.
Conclusions
A series of ten amphiphilic dicationic ZnPcs were synthesized and their cellular properties were investigated in human carcinoma HEp2 cells. All Pcs are A3B-type and were prepared by statistical condensation of two different phthalonitriles. All α-substituted Pcs were found to be significantly more phototoxic (> 5-fold) than the corresponding β-substituted Pcs, probably due to their increased electron density of the ring and distinct macrocyclic conformation. ZnPc 16a bearing the longer PEG group, showed the highest phototoxicity of this series (IC50 = 8.7 µM at 1.5 J/cm2), followed by 4a (IC50 = 10.7 µM); this may be a result of their preferred localization in the cell ER and favorable interaction with important biological targets. Furthermore, our results suggest that a PEG group, as well as the presence of the positive charges in close proximity, tend to increase phototoxicity. On the other hand, the β-substituted ZcPcs 6b and 4b accumulated the most within HEp2 cells, maybe due to their more extended conformations compared with the α-substituted ZcPcs. We conclude that the charge localization on the Pc macrocycle (α-substitution and close proximity of charges) and the intracellular distribution of the cationic ZnPcs, rather than the extent of their cellular uptake, mainly determine their photodynamic activity. In the absence of a PEG group, the di-cationic ZnPcs tend to form large aggregates, as visualized by atomic force microscopy.
Supplementary Material
Phototoxicity and dark toxicity plots, microscopy images, UV-Vis spectra and ChemBio3D conformations.
Acknowledgements
This work was supported by the National Institutes of Health, grant number R21 CA139385. JCG acknowledges support from the National Science Foundation (DMR- 1006336).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Leznoff CC, Lever ABP. Phthalocyanines: Properties and Applications. VCH: Weinheim. 1996:1-4
2. Ben-Hur E, Chan W-S. In The Porphyrin Handbook: Phthalocyanines in photobiology and their medical applications. Kadish KM, Smith KM, Guilard R. Eds. Academic Press: Boston. 2003;19:1-35
3. Erk P, Hengelsberg H. In The Porphyrin Handbook: Phthalocyanines dyes and pigments. Kadish KM, Smith KM, Guilard R. Eds. Academic Press: Boston. 2003;19:106-146
4. Jori G, Coppellotti O. Inactivation of pathogenic microorganisms by photodynamic techniques: mechanistic aspects and perspective applications. Anti-Infective Agents in Medicinal Chemistry. 2007;6(2):119-131
5. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889-905
6. Pandey RK. Recent advances in photodynamic therapy. J. Porphyrins Phthalocyanines. 2000;4:368-373
7. Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497-508
8. Huang Z. A review of progress in clinical photodynamic therapy. Tech Cancer Res Treatment. 2005;4:283-293
9. Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hanneman KK, Hsia AH, Oleinick NL, Colussi VC, Cooper KD. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a Phase 1 clinical trial. Lasers Surg Med. 2010;42(10):728-735
10. Kinsella TJ, Baron ED, Colussi VC, Cooper KD, Hoppel CL, Ingalls ST, Kenney ME, Li X, Oleinick NL, Stevens SR, Remick SC. Preliminary clinical and pharmacologic investigation of photodynamic therapy with the silicon phthalocyanine photosensitizer Pc 4 for primary or metastatic cutaneous cancers. Front Oncol. 2011;1(14):1-6
11. Sokolov VV, Chissov VI, Yakubovskya RI, Aristarkhova EI, Filonenko EV, Belous TA, Vorozhtsov GN, Zharkova NN, Smirnov VV, Zhitkova MB. Photodynamic therapy (PDT) of malignant tumors by photosensitizer Photosens: Results of 45 clinical cases. Proceedings of SPIE (Photochemistry: Photodynamic Therapy and Other Modalities). 1996;2625:281-287
12. Gijsens A, Derycke A, Missiaen L, De Vos D, Huwyler J, Eberle A, de Witte P. Targeting of the photocytotoxic compound AlPcS4 to hela cells by transferrin conjugated peg-liposomes. Int J Cancer. 2002;101:78-85
13. Arnida Nishiyama N, Kanayama N Jang W-D, Yamasaki Y Kataoka K. PEGylated gene nanocarriers based on block catiomers bearing ethylenediamine repeating units directed to remarkable enhancement of photochemical transfection. J Controlled Release. 2006;115(2):208-215
14. Suzuki T, Oishi M, Nagasaki Y. Photochemical and photobiological studies of zinc phthalocyanine incorporated into PEGylated nanogels for photodynamic therapy. Journal of Photopolymer Science and Technology. 2009;22(4):547-550
15. Durmus M, Ayhan MM, Gürek AG, Ahsen V. Peripherally alpha(α)-substituted novel phthalocyanines. Dyes and Pigments. 2008;77:570-577
16. Bai M, Lo P-C, Ye J, Wu C, Fong W-P, Ng Dennis KP. Facile synthesis of pegylated zinc(II) phthalocyanines via transesterification and their in vitro photodynamic activities. Organic & Biomolecular Chemistry. 2011;9(20):7028-7032
17. Li H, Fronczek FR, Vicente MGH. Pegylated phthalocyanines: synthesis and spectroscopic properties. Tetrahedron Lett. 2011;52:6675-6678
18. Hamblin MR, Miller JL, Rizvi I, Loew HG, Hasan T. Pegylation of charged polymer-photosensitizer conjugates: effects on photodynamic therapy. Br J Cancer. 2003;89:937-943
19. Rovers JP, Saarnak AE, de Jode M, Sterenborg HJ, Terpstra OT, Grahn MF. Biodistribution and bioactivity of tetra-pegylated meta-tetra(hydroxyphenyl)chlorin compared to native meta-tetra(hydroxyphenyl)chlorin in a rat liver tumor model. Photochem Photobiol. 2000;71:211-217
20. Krueger T, Altermatt HJ, Mettler D, Scholl B, Magnusson L, Ris HB. Experimental photodynamic therapy for malignant pleural mesothelioma with pegylated mTHPC. Lasers Surg Med. 2003;32:61-68
21. Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1-8
22. Savellano MD, Hasan T. Targeting cells that overexpress the epidermal growth factor receptor with polyethylene glycolated BPD verteporfin photosensitizer immunoconjugates. Photochem Photobiol. 2003;77:431-439
23. Ichikawa K, Hikita T, Maeda N, Takeuchi Y, Namba Y, Oku N. PEGylation of liposome decreases the susceptibility of liposomal drug in cancer photodynamic therapy. Biol Pharm Bull. 2004;27:443-444
24. Wöhrle D, Iskander N, Graschew G, Sinn H, Friedrich EA, Maier-Borst W, Stern J, Schlag P. Synthesis of positively charged phthalocyanines and their activity in the Photodynamic Therapy of Cancer Cells. Photochem Photobiol. 1990;51:351-356
25. Ball DJ, Mayhew S, Wood SR, Griffiths J, Vernon DI, Brown SB. A comparative study of the cellular uptake and photodynamic efficacy of three novel zinc phthalocyanines of differing charge. Photochem Photobiol. 1999;69:390-396
26. Duan W, Lo P-C, Duan L, Fong W-P, Ng Dennis KP. Preparation and in vitro photodynamic activity of amphiphilic zinc(II) phthalocyanines substituted with 2-(dimethylamino)ethylthio moieties and their N-alkylated derivatives. Bioorganic Medicinal Chemistry. 2010;18:2672-2677
27. Dummin H, Cernay T, Zimmermann HW. Selective photosensitization of mitochondria in HeLa cells by cationic Zn(II) phthalocyanines with lipophilic side-chains. J Photochem Photobiol B: Biol. 1997;37:219-229
28. Li H, Jensen TJ, Fronczek FR, Vicente MGH. Syntheses and Properties of a Series of Cationic Water-Soluble Phthalocyanines. J Med Chem. 2008;51(3):502-511
29. Marino J, Garcia MCV, Dicelio LE, Roguin LP, Awruch J. Photodynamic effects of isosteric water-soluble phthalocyanines on human nasopharynx KB carcinoma cells. Eur J Med Chem. 2010;45(9):4129-4139
30. Chen Z, Zhou S, Chen J, Deng Y, Luo Z, Chen H, Hamblin MR, Huang M. Pentalysine β-Carbonylphthalocyanine Zinc: an Effective Tumor-Targeting Photosensitizer for Photodynamic Therapy. Chem Med Chem. 2010;5(6):890-898
31. Durmus M, Ahsen V. Water-soluble cationic gallium(III) and indium(III) phthalocyanines for photodynamic therapy. Journal of Inorganic Biochemistry. 2010;104(3):297-309
32. Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both Gram-negative and Gram-positive bacteria. J Photochem Photobiol B: Biol. 1996;32:159-164
33. Mantareva V, Kussovski V, Angelov I, Wohrle D, Dimitrov R, Popova E, Dimitrov S. Non-aggregated Ga(III)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms. Photochem Photobiol Sci. 2011;10:91-102
34. Tempesti TC, Alvarez MG, Durantini EN. Synthesis and photodynamic properties of amphiphilic A3B-phthalocyanine derivatives bearing N-heterocycles as potential cationic phototherapeutic agents. Dyes and Pigments. 2011;91:6-12
35. Ongarora BG, Hu X, Li H, Fronczek FR, Vicente MGH. Syntheses and properties of trimethylaminophenoxy-substituted Zn(II)-phthalocyanines. Med Chem Commun. 2012;3:179-194
36. Ongarora BG, Fontenot KR, Hu X, Sehgal I, Satyanarayana-Jois SD, Vicente MGH. Phthalocyanine-Peptide Conjugates for Epidermal Growth Factor Receptor Targeting. J Med Chem. 2012;55:3725-3738
37. Nečas DKP. Gwyddion 2.25. Brno, Czech Republic: Czech Metrology Institute. 2009
38. Sibrian-Vazquez M, Jensen TJ, Vicente MGH. Synthesis and cellular studies of PEG-functionalized meso-tetraphenylporphyrins. J Photochem Photobiol B: Biol. 2007;86:9-21
39. Sehgal I, Sibrian-Vazquez M, Vicente MGH. Photoinduced Cytotoxicity and Biodistribution of Prostate Cancer Cell-Targeted Porphyrins. J Med Chem. 2008;51:6014-6020
40. Warnecke A, Kratz F. Maleimide-oligo(ethylene glycol) Derivatives of Camptothecin as Albumin-Binding Prodrugs: Synthesis and Antitumor Efficacy. Bioconjugate Chemistry. 2003;14:377-387
41. Hofman J-W, van Zeeland F, Turker S, Talsma H, Lambrechts SAG, Sakharov DV, Hennink WE, van Nostrum CF. Peripheral and Axial Substitution of Phthalocyanines with Solketal Groups: Synthesis and In Vitro Evaluation for Photodynamic Therapy. J Med Chem. 2007;50:1485-1494
42. Zorlu Y, Dumoulin F, Durmuş M, Ahsen V. Comparative studies of photophysical and photochemical properties of solketal substituted platinum(II) and zinc(II) phthalocyanine sets. Tetrahedron. 2010;66(17):3248-3258
43. Masilela N, Nyokong T. The synthesis and photophysical properties of water soluble tetrasulfonated, octacarboxylated and quaternised 2,(3)-tetra-(2 pyridiloxy) Ga phthalocyanines. Dyes and Pigments. 2010;84(3):242-248
44. Jensen TJ, Vicente MGH, Luguya R, Norton J, Fronczek FR, Smith KM. Effect of overall charge and charge distribution on cellular uptake, distribution and phototoxicity of cationic porphyrins in HEp2 cells. J Photochemistry and Photobiology B: Biology. 2010;100(2):100-111
45. Kessel D, Luguya R, Vicente MGH. Localization and photodynamic efficacy of two cationic porphyrins varying in charge distribution. Photochem. Photobiol. 2003;78:431-435
46. Sibrian-Vazquez M, Jensen TJ, Fronczek FR, Hammer RP, Vicente MGH. Synthesis and characterization of positively charged porphyrin-peptide conjugates. Bioconjugate Chem. 2005;16(4):852-863
47. Sibrian-Vazquez M, Ortiz J, Nesterova IV, Fernández-Lázaro F, Sastre-Santos A, Soper SA, Vicente MGH. Synthesis and properties of cell-targeted Zn(II)-phthalocyanine-peptide conjugates. Bioconjugate Chem. 2007;18:410-420
48. Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE. Coupling Endoplasmic Reticulum Stress to the Cell Death Program. J. Biol. Chem. 2001;276(36):33869-33874
49. Groenendyk J, Michalak M. Endoplasmic Reticulum Quality Control and Apotosis. Acta Biochim. Pol. 2005;52:381-395
Author contact
![]() Corresponding author: Maria da Graça H. Vicente, PhD, Department of Chemistry, Louisiana State University, Baton Rouge, LA, 70803; vicenteedu.
Corresponding author: Maria da Graça H. Vicente, PhD, Department of Chemistry, Louisiana State University, Baton Rouge, LA, 70803; vicenteedu.
 Global reach, higher impact
Global reach, higher impact