13.3
Impact Factor
Theranostics 2016; 6(7):969-985. doi:10.7150/thno.14856 This issue Cite
Review
Click Chemistry-Mediated Nanosensors for Biochemical Assays
Beijing Engineering Research Center for BioNanotechnology and CAS Key Lab for Biological Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for NanoScience and Technology, Beijing 100190, China.
*These authors equally contribute to this work.
Received 2015-12-30; Accepted 2016-2-11; Published 2016-4-28
Abstract
Click chemistry combined with functional nanoparticles have drawn increasing attention in biochemical assays because they are promising in developing biosensors with effective signal transformation/amplification and straightforward signal readout for clinical diagnostic assays. In this review, we focus on the latest advances of biochemical assays based on Cu (I)-catalyzed 1, 3-dipolar cycloaddition of azides and alkynes (CuAAC)-mediated nanosensors, as well as the functionalization of nanoprobes based on click chemistry. Nanoprobes including gold nanoparticles, quantum dots, magnetic nanoparticles and carbon nanomaterials are covered. We discuss the advantages of click chemistry-mediated nanosensors for biochemical assays, and give perspectives on the development of click chemistry-mediated approaches for clinical diagnosis and other biomedical applications.
Keywords: Click chemistry, nanosensor, bio-conjugation, signal amplification system.
1. Introduction
To develop biosensing methods that allow high sensitivity and convenient operation is of great significance for clinical diagnosis, prognosis and the monitor of treatment1-4. For example, detection of biomarkers at low concentrations can realize early diagnosis that helps enhance the survival rate of the patients5-7. With the development of nanotechnology, a wide range of sensors based on functional nanoparticles have emerged with great sensitivity, simple operation and low cost 8-11. Such sensors have shown great promise for point-of-care testing12-14. Nanoscale materials including gold nanoparticles (Au NPs)15-17, magnetic nanoparticles (MNPs)14, 18-21, quantum dots (QDs)22-24 and carbon nanomaterials24-26 have emerged as excellent probes for biochemical assays owing to their unique physical and chemical properties such as large surface-to-volume ratio27, excellent luminescent28, electrical29, 30, magnetic31, 32 and plasmon resonance properties16. For typical nanosensors, nanomaterials should be functionalized by conjugating biomolecules on their surface to prepare nanoprobes that enable target recognition, effective signal amplification and straightforward signal readout33-35. The strategy for the surface modification of NPs determines the analytical performance of nanosensors36-39, and effective bio-conjugation strategy for biomolecules and NPs essentially dictates the effectiveness of a nanosensor. Thus it is crucial to control the biomolecules that are conjugated to the functional NPs and to control NP/biomolecule ratio, the activity of the biomolecules, and the steric hindrance of the bioconjugate35, 38. These controls are especially critical in preparing multifunctional NPs-biomolecule conjugates for effective signal amplification and convenient signal readout in biochemical assays39, 40. In addition, an effective signal transformation system is also very important to the nanosensor, because a suitable signal transformation system can improve the detection efficiency and reduce the cost. For example, naked-eye detection strategy based on gold nanoparticles for the signal readout has shown great advantages in the testing of infectious diseases in Africa because of its convenient signal readout and low cost1, 15. Thus it is very important to develop effective strategies for the surface modification and bio-conjugation in nanosensors for biosensing applications.
Click chemistry provides an excellent platform for biomedical applications especially for the effective signal translation41, 42, surface modification43-45 and bio-conjugation of nanomaterials46-50. The concept of “click chemistry” was first proposed in 200145. This new type of organic reaction is selective, orthogonal to most known reactions and yields no side products with high efficiency at room temperature in aqueous solvents 51-53. Compared to conventional reactions, high selectivity and yields are the most prominent advantages of “click chemistry”, which provide control and flexibility for manipulating biological systems with high selectivity and speediness in bio-reaction48-50. In addition, because the reaction ligands of click chemistry are small molecules, biomacromolecules (antibodies, enzymes) or NPs can easily conjugate to these reaction ligands, which is suitable for broad applications in labeling. 48, 54, 55 and signal amplification system 46, 56 that allow bio-conjugation of biomolecules and NPs43, 47, 57. More importantly, the bio-conjugation process using click chemistry does not perturb the activity of the biomolecules owing to the small size of the ligands in click chemistry54, 58, 59. There are four kinds of click chemistry reactions that are mainly used in biochemical assays or biomedical applications, namely cycloaddition reaction (Cu (I)-catalyzed 1,3-dipolar cycloaddition of azides and alkynes, and copper-free cycloadditions), nucleophilic ring-opening reaction, non-aldol carbonylation reaction and carbon-carbon multiple bonds addition reaction40,41,45. Among all the reactions, Cu(I)-catalyzed 1, 3-dipolar cycloaddition of azides and alkynes (CuAAC) is the most popular and widely used reaction 50, 51(Scheme 1A). In the past ten years, CuAAC has been widely adopted as the signal transformation system in nanosensors for detecting many types of targets, which has provided a new platform in the field of biochemical analysis. Meanwhile, the bioorthogonal click chemistry reactions prove to be effective bio-conjugation strategies to construct nanoprobes for biosensing application62, 63. For example, the copper-free cycloaddition reaction between 1,2,4,5-tetrazines (Tzs) and trans-cyclooctene (TCO) (Scheme 1B), have recently gained significant research interests in imaging, drug delivery, clinical diagnosis because of its high reaction rate and low-toxicity46,47. Compared with traditional bio-conjugation methods, click chemistry could not only simplify the procedure, but also better control the conjugation process with shortened time and enhanced chemo-selectivity55, 64, 65. Thus, click chemistry is not only a simple, fast, broadly applicable bio-conjugation strategy that can improve the analytical performances of nanosensors in bio-analysis, but also an attractive tool to develop new nanosensors that can greatly broaden the applications of nanosensors for biochemical analysis35,66,67.
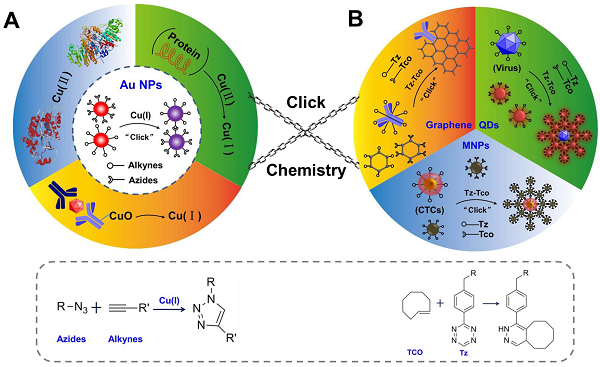
The scheme of click chemistry-mediated nanosensors for biochemical assays. (A) CuAAC combined with Au NPs for detection of Cu (II) and other targets, in which CuAAC is used for signal transformation. The “R-N=N=N-R” represents the ligands of azides, and R-C≡C-R represents the ligands of alkynes (B) Three types of nanosensors based on nanoparticles (quantum dots, QDs; magnetic nanoparticles, MNPs; and graphene) and click chemistry for detection of circulating tumor cells (CTC), pathogens, cancer biomarkers and imaging applications. Click chemistries are employed to functionalize the surface of NPs or used as bio-conjugation strategy. “Tz” presents one ligands of 1,2,4,5-tetrazines, and “TCO” presents another ligands of trans-cyclooctene.
In the past decade, many reviews have systematically introduced works on how the click chemistry is used as a powerful tool to modify the surface of NPs or used as effective bio-conjugation strategies to bind biomolecules to the NPs67, 68. However, few articles reviewed the topic of using the detection of the components of click chemistry in combination with nanosensors for biochemical assays. In this review, we first introduce the CuAAC-mediated nanosensors for detection of many types of targets, via detection of the components of CuAAC (Scheme 1A), and then we describe how to combine NPs (QDs, MNPs and graphene) with click chemistry to construct effective nanoprobes and readout systems in nanosensors for bio-sensing (Scheme 1B). Finally, we discuss the advantages of click chemistry and give perspectives on its further development in nanosensors. The main aim of this review is to introduce the advantages of click chemistry and demonstrate how to construct effective nanosensors based on click chemistry and NPs, for improvement of efficiency in clinical assays.
2. Biochemical assays via detection of the components of CuAAC
CuAAC is an efficient and selective reaction that can occur over a broad pH range in aqueous solutions65, 66, 69. Without a catalyst, the reaction between azides and alkynes only proceeds at high temperatures. However, when catalyzed by Cu (I), the reaction proceeds at an accelerated rate at room temperature. The concentration of Cu (I) determines the degree of the click reaction. Based on this property, many nanosensors were developed for detection of Cu (I) and related targets70, 71. Among these nanosensors, CuAAC-meditated Au NPs-implemented approaches are widely recognized that combine the selectivity of CuAAC and the excellent optical properties of Au NPs41, 72, 73. Au NPs have high extinction coefficients and distance-dependent optical properties which can be used to design colorimetric sensors for biological and chemical analyses16, 74-76. For example, the state of change of Au NPs (from dispersed state to aggregated state) can result in the color change of Au NPs (from red to blue)77, 78. The colorimetric sensors based on Au NPs and CuAAC have three advantages79-81: (1) the convenient signal readout which is very important to point-of-care testing; (2) high sensitivity and specificity, which is a key factor to the early diagnosis such as the detection of infectious disease; (3) equipment-free, which has potential applications in the resource-limited settings. In this section, we focus on the progress of CuACC-mediated Au NPs-implemented nanosensors for bio-analysis.
2.1. Detection of Cu
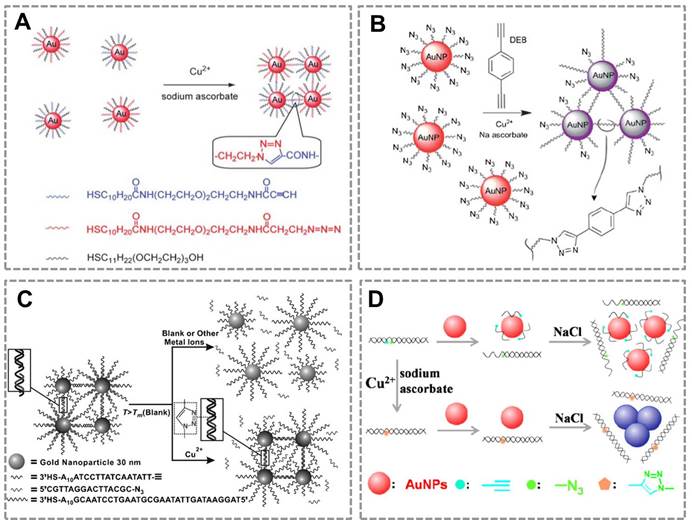
Copper is an essential trace element in the human body and plays an important role in various biological processes82, 83. However, long-term exposure to excess Cu(II) is highly toxic to organisms and the human body. Monitoring the concentration of Cu (II) in human body and environmental samples is becoming more and more important84. Based on the localized surface plasmon resonance (LSPR) of Au NPs and the high selectivity of CuAAC, our group first combined CuAAC with Au NPs to develop a nanosensor for detecting Cu (II)42. Au NPs were modified with azide and alkyne groups by the ligand exchange reaction, and CuAAC reaction can crosslink the azide-Au NPs and alkyne-Au NPs to cause their aggregation. This aggregation results in the color change of Au NPs (from red to blue), and the degree of aggregation is related to the concentration of Cu (I). This assay can be employed for Cu(II) detection by reducing Cu(II) into Cu(I) (Figure 1A). A similar work has reported the detection of Cu (II) by using the dialkyne cross-linker. The advantage of this method is that, the dialkyne cross-linker is used as a “bridge” to conjugate adjacent azide-AuNPs by CuAAC without the chemical synthesis of alkyne-AuNPs (Figure 1B)70. A colorimetric method for the detection of Cu (II) is also reported based on densely functionalized DNA-AuNP conjugates and CuAAC85. This approach uses the oligonucleotides as a template to align the alkyne and azide groups for optimal reactivity which can greatly shorten the assay time. In addition, the sharp melting properties of the DNA-Au NPs allow researchers to distinguish subtle differences in melting temperature that allows for Cu (II) quantification (Figure 1C). A colorimetric biosensor for Cu (II) detection based on the “alkyne-azide” clickable DNA probe and unmodified Au NPs 86 was also developed (Figure 1D). This nanosensor can sensitively and specifically detect Cu (II) with a limit of detection of 250 nM and a linear range of 0.5-10 mM. More importantly, this method is simple and economic without dual-labeling of the DNA probe and the modification of Au NPs.
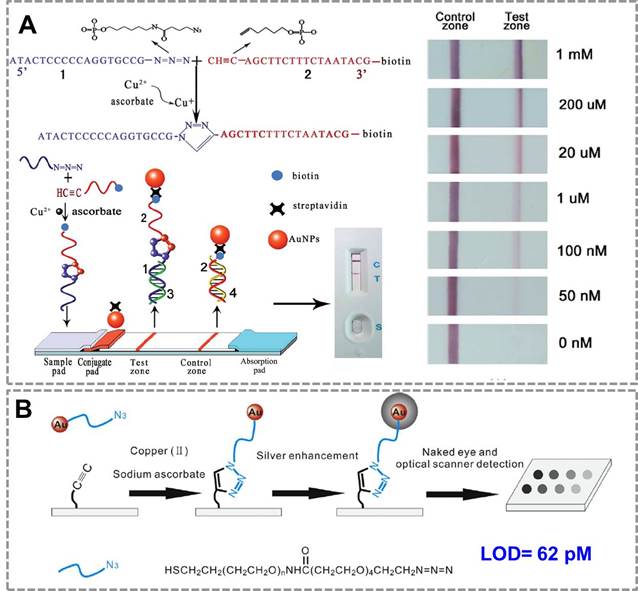
For point-of-care applications, it is important to develop surface-based assays for detection of Cu(II) to simplify the assaying process. A lateral flow device for the rapid detection of Cu(II) based on CuAAC has been constructed 87(Figure 2A). In the presence of sodium ascorbate, Cu (II) was reduced to Cu (I) which could catalyze the cycloaddition between azide-DNA and alkyne/biotin-DNA in aqueous solution. The ligated DNA product could then be immobilized onto the test zone of the lateral flow biosensor to form a red band which could be easily read by the naked eye. Compared with conventional methods, this biosensor is simpler to operate and more cost-effective to use, thus has great potential in point-of-care diagnosis and environmental monitoring. To achieve a high sensitivity for detection of Cu (II), a silver enhancement signal amplification strategy is employed to achieve highly sensitive detection of copper ions in aqueous solution88(Figure 2B). Based on Cu(I)-catalyzed 'click' reaction between azide-functionalized Au NPs and alkyne-modified glass slide, this method enables Cu(II) detection down to 62 pM by the commonly used flatbed scanner, which is 2-3 orders of magnitude lower than those in previous reports. Thus, CuAAC-mediated Au nanosensor has provided a convenient and sensitive method for detection of Cu (II). Many of these existing assays have focused on detection of copper in environmental samples; we expect them to be used in many clinical settings in the future (direct or indirect assays, as outlined below).
CuAAC-mediated Au NPs-implemented nanosensors for detection of Cu(II) in solution-based assay. (A) Azide-and alkyne-functionalized Au NPs can be triggered to aggregate in the presence of Cu (I) by CuAAC, and the degree of color change of AuNPs is related to the concentration of Cu(II). (B) Schematic depiction of the copper-triggered aggregation of AuNPs for Cu (II) detection. (C) The colorimetric method for detection of Cu (I) based on densely functionalized DNA-Au NP conjugates and CuAAC. (D) The unmodified Au NPs combines with “alkyne-azide” clickable DNA probe for detection of Cu (II). Adapted with permission from [42, 70, 85, 86].
2.2. Assays for the reducing agents
Because a reducing agent is required to reduce Cu (II) to Cu(I) for CuAAC, CuAAC-mediated Au NPs-implemented nanosensors were developed to detect reducing agents that can reduce Cu (II) into Cu (I). Our group has developed a colorimetric sensor for detection of the total proteins using CuAAC-mediated approach 73(Figure 3A). In this work, protein as a reducing agent can reduce Cu(II) to Cu(I), and the amount of Cu(I) generated in the redox reaction depends on the concentration of the total proteins in sample, because of the reducing nature of the peptide bond. Combined with the CuAAC, it results in the color change of functionalized Au NPs (azide/alkyne-Au NPs) that relates to the amount of proteins in the sample. This approach, with a detection range from 30 to 2500 μg mL-1, has a broader linear range than conventional methods such as bicinchoninic acid assays. We have reported the detection of organophosphate pesticides (OPs) using CuAAC and CuO NPs (Figure 3B) 89. In this method, Cu(I) required as the catalyst for CuAAC is released from CuO NPs by CH3COOH which is enzymatically produced from the acetylcholine esterase- catalyzed hydrolysis of acetylthiocholine in the presence of sodium ascorbate. OPs inhibit the activity of AChE, which subsequently blocks the production of CH3COOH needed for the release of Cu(I)10. Thus, the concentration of OPs in samples relates to the amount of Cu(I), which can be employed for the detection of OPs. Reducing agents such as ascorbic acid 90(Figure 3C) and NO (g) 91 (Figure 3D) can be detected based on Au NPs and CuAAC, demonstrating that CuAAC-mediated Au NPs-implemented nanosensors have a wide range of applications. In the CuAAC-mediated approaches, these targets (NO (g) or ascorbic acid) can react with Cu (II) to yield Cu (I), which can highly efficiently catalyze the click reaction between azide-functionalized Au NPs and alkyne-modified Au NPs. The degree of color change of Au NPs relates to the amount of NO (g) or ascorbic acid, which can be employed for the sensitive detection of these reducing agents.
2.3 CuAAC-based immunoassays
To further extend the applications of CuAAC-mediated approach, we developed a series of immunoassays based on Au NPs and CuAAC for the detection of other targets. Our group reported a colorimetric immunoassay based on detecting Cu (II) released from copper monoxide nanoparticle (CuO NPs)-labeled antibodies 92 (Figure 4A). In this type of immunoassays, CuO NPs are dissolved by acid to release Cu (II) and further reduced to Cu (I) which catalyzes the click reaction between azide- and alkyne-modified AuNP. The concentration of target in the sample relates to the amount of CuO NPs in the immunoassay, and the degree of aggregation and thus the color change of Au NPs depend on the amount of Cu(I) released from CuO NPs. This method can detect human immunodeficiency virus (HIV) antibody in real serum samples without equipment, and the accuracy rate is 100%. The high selectivity and sensitivity of the immunoassay result from the highly selective and sensitive nature of the Cu(I)-catalyzed reaction. This new immunoassay may therefore be useful for many applications, including clinical assays in resource-poor settings.
CuAAC-mediated Au NPs-implemented nanosensors for detection of Cu(II) in surface-based assay. (A) The lateral flow test based on “clickable” DNA and Au NPs for detection of Cu (II). (B) A naked-eye biosensor based on silver enhancement signal amplification strategy and CuAAC for highly sensitive detection of Cu (II). Adapted with permission from [87, 88].
CuAAC-mediated Au NPs-implemented nanosensors for detection of reducing agents. (A) CuAAC-mediated nanosensor for detection of the whole protein in which azide-Au NPs and alkyne-Au NPs are triggered to aggregate by CuAAC with the protein and Cu (II). (B) Naked-eye-based colorimetric detection of OPs using CuAAC (OPs: organophosphate pesticides; Au: gold nanoparticles; AChE: Acetylcholineesterase; ATCl: acetylthiocholine; CuO: CuO nanoparticles). (C) CuAAC-mediated nanosensor for detection of the ascorbic acid which can reduce Cu (II) into Cu(I). (D) CuAAC-mediated nanosensor for detection of NO(g) which can reduce Cu(II) to Cu(I) in the redox reaction. Adapted with permission from [73, 89, 90, 91].
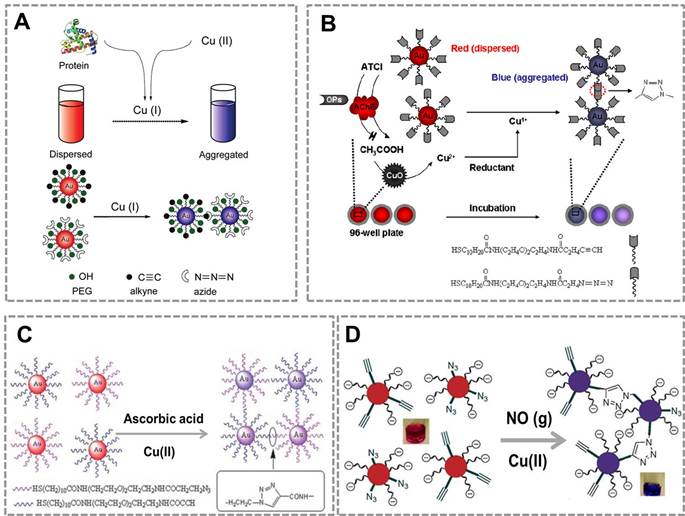
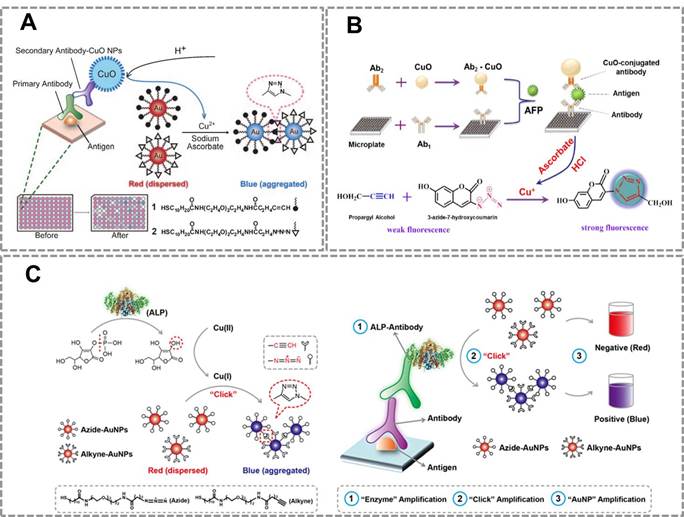
This strategy has also been successfully used for developing a novel fluorescent immunosensor for detection of alpha-fetoprotein (AFP) in human samples (Figure 4B) 93.The CuO NPs modified on the sandwich structure can be dissolved to Cu (I) ions which triggered the click reaction between the weak fluorescent compound (3-azido-7-hydroxycoumarin) and propargyl alcohol to form a strong fluorescent compound. Compared with traditional ELISA methods, this method has two advantages: (1) The CuO NPs labeled antibodies instead of enzyme as the signal producer, which is much cheaper than the biological enzyme. (2)The CuO NPs are insusceptible to change of external environments, pH value and inhibitors of enzymes. These results show that CuAAC-mediated immunoassay is an attractive platform to detect the low concentration of targets in clinical samples with high sensitivity and low cost. However, a main problem in this strategy is that we have to modify the antibody with CuO NP, which affects the activity of the antibodies. To address this problem of attenuated antibody activity by CuO NP-modification, our group developed an immunoassay that realizes sensitive detection using commercially available, optimized antibodies. This straightforward naked-eye readout employs CuAAC reaction and alkaline phosphatase (ALP)-lableled antibody (Figure 4C) 15. The commonly used ALP-labeled secondary antibody rather than CuO NPs-labeled secondary antibody is used that accommodates conventional immunoassays. Under ALP-catalyzed dephosphorylation process, ascorbic acid-phosphate can be transformed into ascorbic acid which reduces Cu(II) to Cu(I). CuAAC reaction results in the aggregation of Au NPs, and the color change of Au NPs relates to the amount of ALP-labeled secondary antibodies. This CuAAC-based immunoassay allows naked-eye detection of 80 ng mL-1 rabbit antihuman IgG, while the conventional immunoassay required a concentration of 100 ng mL-1 for the naked-eye detection. This assay has been successfully used to analyze the serum of patients who suffer from Mycoplasma pneumonia (MP). Results from 20 clinical samples show that this nanosensor enables a naked-eye diagnosis of MP infection (100%), whereas conventional ELISA is not capable of distinguishing with the naked eye in some cases (only 50% can be discriminated). The high sensitivity of this sensor owes to the three-round signal amplification: “enzyme” amplification, “click” amplification and “Au NP” amplification. Compared to the conventional colorimetric sensors for detection of Cu(II) or related targets, CuAAC-mediated Au NPs-implemented nanosensors have three advantages: (1) Convenient signal readout using the color change of Au NPs, which is suitable for point-of-care detection. (2) The high stability of Au NPs and high selectivity of CuAAC, which ensures the repeatability and accuracy; (3) High sensitivity due to the signal amplification of click chemistry. In addition, our group has also tried to develop CuACC-mediated immunoassay based on horseradish peroxidase (HRP), which is a widely used labeling enzyme in conventional immunoassays. As we attempted to generalize CuAAC-based approach for another type of widely used HRP-labelled antibody, we unexpectedly found that Cu(I) as the catalyst in CuAAC can severely inhibit the activity of HRP, thus HRP is not suitable for CuAAC-based assays94.
3. Functionalization of nanoprobes based on click chemistry
In the nanosensors, one critical problem is how to effectively conjugate the biomolecules to the surface of NPs to prepare the functionalize nanoprobes 37, 39, 95. The analytical performances of the nanosensor mainly depends on the quality of the nanoprobes53, 96, 97. Conventional bio-conjugation strategies mainly include the cross-linking reaction between amines and carboxylic acids using carbodiimide activation (e.g., 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, or EDC), and biotin-avidin bio-recognition. The carbodiimide chemistry has two disadvantages: (1) The reactive o-acylisourea intermediate formed through the activation of carboxylic acids is prone to hydrolysis which affects the chemical stability of the bio-conjugates. (2) The over-activation of carboxyl groups on the NPs can affect the colloidal stability and lead to the aggregation of NPs. Although biotin-avidin recognition is widely used in nanosensors, it suffers from indiscriminate labeling of nucleophilic residues (e.g., lysines and cysteines) on the targeting ligand. In addition, avidin as a protein is hard to be conjugated to antibody or other proteins, thus it is not convenient to conjugate the antibody and an enzyme using biotin-avidin recognition. How to rationally design high-quality, stable bio-conjugates that integrate the activities of biomolecules and the useful properties of NPs is still challenging for biochemical assays and other biomedical applications50, 53, 98.
CuAAC-mediated Au NPs-implemented nanosensors for immunoassays. (A) Immunoassay based on the CuO-labled antibody, CuAAC and Au NPs as the signal readout. CuO NPs are dissolved to release copper ions that trigger the aggregation of the Au NPs via CuAAC. (B) The scheme of the fluorescence sensor for detection of alpha fetoprotein (AFP) based on CuAAC and CuO NPs. (C) Colorimetric immunoassay based on ALP-triggered CuAAC between azide/alkyne functionalized Au NPs. Adapted with permission from [92, 93, 15].
Click chemistry provides an attractive platform to modify the surface of NPs and can be an excellent bio-conjugate strategy36, 99, 100. CuAAC is efficient, selective, and proceeds over a broad pH range in aqueous solutions at room temperature, which is suitable to specifically modify or conjugate molecules to the surface of NPs as well biomolecules in ambient conditions60, 61, 101. Bioorthogonal click chemistry102-104, for example, the cycloaddition between 1,2,4,5-tetrazines (Tz) and trans-cyclooctene (TCO) has been used as an useful bio-conjugation tool in nanosensors that recently gained significant research interests because of its rapid reaction kinetics, low-toxicity and controllability (Scheme 1) 46, 105. Compared with traditional bio-conjugation methods, click chemistry could not only simplify the procedure, but also better control the conjugation process with shortened time and enhanced chemo-selectivity. In this section, we focus on the functionalization of several types of nanoprobes (QDs, MNPs and carbon nanomaterials) using click chemistry, along with the progress of the nanosensors based on these nanoprobes for biomedical applications.
3.1 Functionalization of quantum dots nanoprobes
Quantum dots (QDs) are highly fluorescent inorganic semiconductor nanocrystals with a diameter ranging from 2 to 10 nm106-108. They have excellent optical properties: depending on the size of the QDs, a single light source can produce different colors of emission; a feature particularly suitable for multplexed analysis109, 110. Due to their unique optical and physicochemical properties, QDs hold great promise as fluorescent probes in clinical diagnosis, pathological analysis, cell/virus targeting, in vivo imaging, and so on110-112. Biological and medical applications require QDs that are highly water-soluble, low toxicity, biocompatible, and functionalized with biomolecules107, 113, 114. Efficient strategies for surface functionalization and bio-conjugation still remain a serious challenge in the application of QDs. Because of their rapid reaction kinetics and biocompatibility, the bioorthogonal click reactions can be an effective bio-conjugation platform for biomedical applications in biological systems (cell, virus or protein) with high specificity and high sensitivity35, 115-117. QDs modified with enzymes or antibodies by the bioorthogonal click reaction enable targeted imaging of the receptor on the surface of cells/viruses37, 118. The ligands of click chemistry, such as Tz or TCO can be easily conjugated onto the surface of the QDs to prepare the nanoprobes without compromising the fluorescence property of QDs. In addition, QDs functionalized by the bioorthogonal click reactions enable real-time labeling and multiplex sensing due to the rapidness and stability of click chemistry and multiple colors of QDs 37, 115, 119.
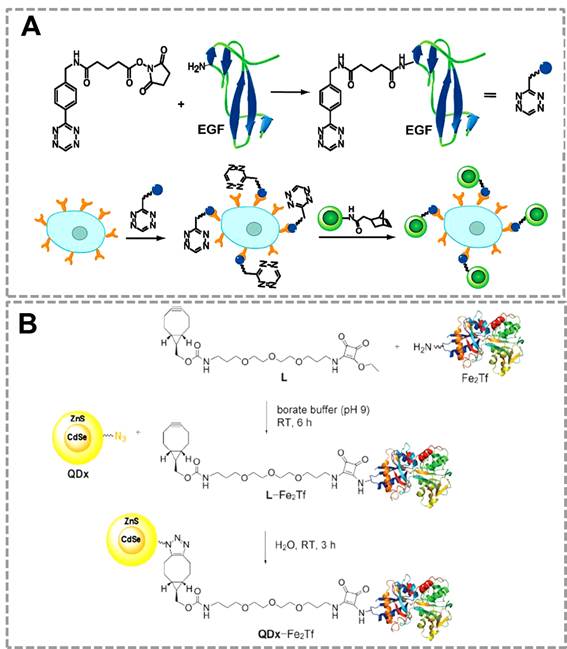
QDs have been used as an efficient fluorescent probe for imaging of lung cancer cells by using the bioorthogonal click reaction between norbornene and Tz 35(Figure 5A). In this strategy, QDs modified with norborneneand and epidermal growth factor (EGF) was functionalized with Tz, and the norbornene-QDs will selectively attach to the Tz-EGF due to the bioorthogonal click reaction. Tz-EGF can bind with EGF reporter (EGFR) which is a biomarker that is overexpressed in lung cancer cells. Thus, norbornene-QDs can be applied for the in vivo imaging of cancer cells by click reaction between norbornene and tetrazine cycloaddition. Another related work also successfully used click chemistry-mediated QDs to image the transferrin-receptor (TfR) expressing tumor cells 116(Figure 5B). In this strategy, QDs and the iron-binding glycoprotein transferrin (Fe2Tf, which is involved in cellular iron-acquisition) were first modified with azide and cyclooctyne functional groups respectively. Azide-modified CdSe/ZnS QDs can specifically recognize the cyclooctyne-modified Fe2Tf through click chemistry, which can be used as fluorescent probes for labeling the TfR expressing cells.
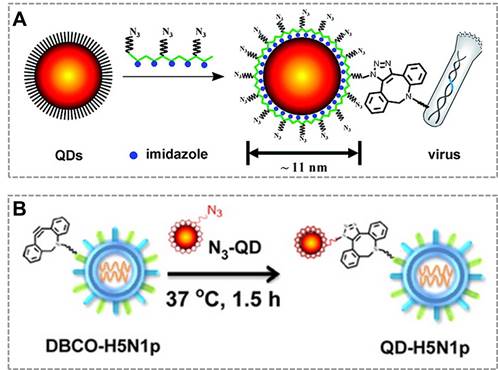
Combined with click chemistry, QDs can be also used as fluorescent probes to visualize and track an individual virus particle, which is important for investigating viral infection routes and characterizing the dynamic interactions between viruses and target cells. The new class of multifunctional multidentate-imidazole polymer ligands is synthesized by a one-step reaction to prepare functionalized QDs with great stability and controllable size (from 5 nm to 11 nm according to different colors of QDs). More importantly, the click functional groups are biologically unique, stable, inert, and small enough that do not affect the fluorescent property of QDs37. Based on this clickable surface modification strategy, a general method for efficient labeling of living viruses with QDs probes was developed, which can be an attractive strategy to monitor the virus in host cells120 (Figure 6A). They also employed bioorthogonal click reaction to conjugate the virus (H5N1p) and near-infrared (NIR)-emitting QDs to prepare the QDs-virus conjugate, and this conjugate showed bright and sustained fluorescent signals in mouse lung tissues that allows researchers to visualize respiratory viral infection noninvasively in real-time. Hence, virus labeling with NIR QDs provides a simple, reliable, and quantitative strategy for tracking respiratory viral infection and antiviral drug screening121 (Figure 6B). These studies suggest that click chemistry provides an effective tool to functionalize the surface of QDs, which broaden the applications of QDs in bio-sensing and in vivo imaging.
Click chemistry-mediated functionalization of quantum dots to label cancer cells. (A) Tetrazine-functionalized EGF can bind to the surface of cancer cells that overexpressed EGFR, and the nonbornene-coated QDs (green circles) can react with tetrazine-modified EGF to form the QDs conjugates for targeted imaging. (B) Cyclooctyne-modified Fe2Tf has been attached to azide-modified water-soluble CdSe/ZnS QDs for monitoring the uptake of fluorescent QD-transferrin conjugates in transferrin-receptors (TfR) expressing tumor cells. Adapted with permission from [35, 116].
Click chemistry-mediated functionalization of quantum dots. (A) Schematic illustration of the one-step synthesis of the azido-derivatized multidentate-imidazole polymer ligands (N3-PMAH) along with the structures of QDs protected by the multidentate ligands, and the general strategy for the specific labeling of viruses with QDs via strain-promoted metal-free click chemistry. (B) Bioorthogonal labeling of H5N1p with NIR QDs and particle sizes of QDs, H5N1p, and QD-labeled H5N1p (QD-H5N1p). Adapted with permission from [120, 121].
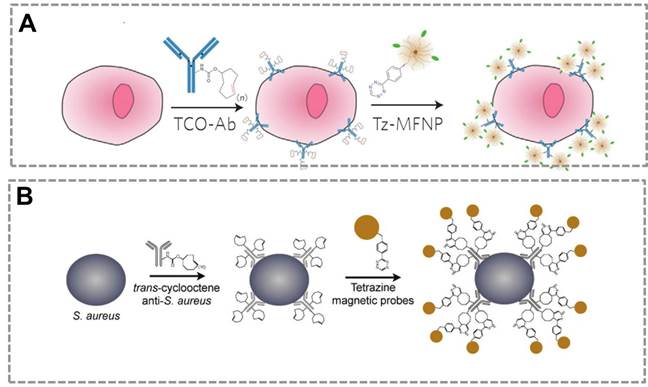
Click chemistry-mediated functionalization of SMNPs. (A) The magnetic relaxation switch (MRS) sensor based on SMNPs and a two -step bioorthogonal reaction strategy for diagnosis of tumor cells. (B) Detection of pathogenic bacterium in blood samples based on SMNPs and layer-by-layer “bioorthogonal reaction” signal amplification strategy. Adapted with permission from [46, 142].
3.2 Functionalization of magnetic nanoprobes
Superparamagnetic nanoparticles (SMNPs, the size is from 2 -50 nm) can directionally move under an external magnetic field due to their superparamagnetic properties, and SMNPs can be synthesized to have controlled physical size, good colloidal dispersion and excellent biocompatibility19, 36, 122. SMNPs have recently drawn increasing research interests and have been widely used in the magnetic separation/enrichment 17, magnetic resonance imaging (MRI)123-125, drug delivery126-128, magnetic sensor 14, 129 and so forth. By conjugating functional ligands onto their surface, SMNPs can not only be used as an ideal carrier that can specifically capture targets from complicated samples, but can also serve as magnetic signal probes in the magnetic sensor 32, 130. To develop effective bio-conjugation strategies for these applications, the surface modification of SMNPs are critical131, 132. Many previous works have suggested that the bioorthogonal click reaction is a straightforward tool to functionalize the surface of SMNPs by conjugating the recognition elements (antibody, aptamer and receptor) or signal probes (enzyme) to the SMNPs to realize and effective signal amplification and convenient signal readout133-136. The surface modification of SMNPs using click chemistry strategy does not affect the stability of SMNPs. It is straightforward to prepare two types of SMNPs, each modified with one of the ligands of click chemistry. These functionalized SMNPs can amplify the signal of low-concentration target through the “layer-by-layer” assembly, which can greatly improve the magnetic signal (Figure 7).
Many previous works show that bioorthogonal click chemistry has provided a powerful tool to improve the sensitivity of the magnetic relaxation switching (MRS) sensors20, 63, 137. MRS sensors that depend on target-induced aggregation (or disaggregation) of SMNPs are used to detect a wide range of targets, because they are homogeneous and light-independent, which allow one-step detection 138-141. However, the sensitivity of conventional MRS sensor is limited when detecting the trace concentration of targets in complex samples, such as detection of circulating tumor cells (CTC) in human whole blood samples. An MRS sensor was developed that combines SMNPs (20-30 nm) and bioorthogonal reactions to specifically detect tumor cells with enhanced sensitivity46 (Figure 7A). In this strategy, Tz-SMNPs and TCO-Ab bio-conjugates are prepared. TCO-Ab can selectively bind to the tumor cells and form the “cell-Ab-TCO” conjugate. One Ab molecule can bind about 30 molecules of TCO to form the TCO-Ab conjugate, which enhances the sensitivity of the sensor. The Tz-MNPs can specifically bind to the “TCO-Ab-cell” conjugate by the Tz/TCO cycloaddition bioorthogonal reaction, and transverse relaxation time (T2) is used as the magnetic signal readout. This click reaction allows for higher SMNPs binding to targets and results in the significantly improved detection sensitivity in the MRS-based sensor. The two-step bioorthogonal reaction can largely enhance the sensitivity than that of one-step bioorthogonal reaction, which suggests the bioorthogonal reaction is an effective strategy for signal amplification in biochemical assays. In bioorthogonal reaction system, it is easy to achieve multiple signal amplification through layer-by-layer bio-conjugation strategy. This result shows that the Tz/TCO cycloaddition reaction is better than biotin-streptavidin recognition for signal amplification. The ligands of click chemistry are small molecules which are suitable to modify NPs or biomolecules, and this bio-conjugation can be controlled to satisfy the need of signal amplification and readout. In contrast, in the avidin-biotin system, the large molecular size of avidin (6 nm, 67 kDa) could potentially mask adjacent biotin sites. In addition, biotin must associate within a deep cleft inside the avidin protein, which could physically or spatially constrain certain binding configurations. By contrast, Tz is a small molecule that can interact with TCO (also a small molecule) on the surface of the antibody without physical restriction on neighboring TCO sites. The small molecule-based bioorthogonal chemistry allows nanoparticles to pack more ligands densely onto the antibody scaffolds, yielding greater signal amplification. Thus, bioorthogonal reaction is a more effective bio-conjugation method for biochemical analysis, especially for detection of targets (CTC, biomarkers, pathogen, virus) in complex sample at low-concentrations.
A similar method was also reported to detect pathogens combining the SMNPs with the Tz/TCO cycloaddition. It was demonstrated that the use of bioorthogonal chemistry for magnetically labeling specific pathogens enables subsequent pathogen detection by the T2 signal142 (Figure 7B). Based on these previous works, multiple steps of alternating orthogonal chemistry could be used as an effective amplification platform with higher sensitivity by up to 1-2 orders of magnitude over the two-step bioorthogonal reaction strategy143. The components, SMNPs-TCO, SMNPs-Tz and Ab-TCO, can form the “Ab-TCO-Tz-SMNPs-Tz-TCO-SMNPs” bio-conjugate. Ab-TCO can specifically recognize the cell to form the “TCO-Ab-cell” complex, and SMNPs-Tz will bind the “TCO-Ab-cell” to form an initial SMNP layer. This primary labeling can be then amplified through alternating applications of SMNPs-TCO and SMNPs-Tz to form multiple SMNPs layers, which can greatly improve the sensitivity. More importantly, the SMNPs that bind to the cells could also be released, and these SMNPs can be used to generate T2 signals. The improvement in sensitivity was achieved by both the significantly reduced background noise arising from nonspecific binding, and the increased SMNPs layers through multiple rounds of orthogonal amplification. Many other studies also demonstrate that the combination of the MRS and click chemistry have been successfully used to detect extremely trace targets (pathogens, small harmful molecules), which suggest that bioorthogonal reaction strategy can effectively improve the performance of MRS-based sensors.
Magnetic beads (MBs) whose size exceeds 100 nm can be rapidly separated in the magnetic field. These MBs not only can be used as magnetic separators but also can be used as carriers for loading biomolecules due to their large specific surface areas where antibodies and enzymes can be simultaneously conjugated through click chemistry17, 144. Previous works reported the controllable and repeatable modification of antibody on the surface of MBs by click chemistry, and MBs of 1 µm can conjugate 104 to 105 protein molecules (antibody or enzyme) which can be used as carriers for signal amplification in biochemical assays27. One previous work suggested that CuAAC can be used as a tool to modify the surface of MBs, and the functionalized MBs can be simultaneously conjugated with an antibody and an enzyme145. In this method, azide-functionalized Fe3O4@SiO2 (silica shell coated Fe3O4 core) was prepared by the functionalization of silica shell with azide. Alkyne-functionalized antibody and horseradish peroxidase were coupled to azide-functionalized Fe3O4@SiO2 by click reaction as nanoprobes for assays. This antibody-Fe3O4@SiO2-HRP conjugate was used in the sandwich immunoreaction to detect Microcystin-LR (Microcystin-LR is a serious toxin in drinking water) with high sensitivity and good selectivity. This work demonstrated that click chemistry-mediated strategy is a useful tool for functionalization of the MBs to prepare nanoprobes.
Another work reported that MBs of 1000 nm can also simultaneously conjugate acetylcholinesterase (AChE) and antibody (Ab2) using step-by-step “clickable” bio-conjugation144. In this bio-conjugation strategy, a number of AChE molecules were firstly conjugated to the surface of MBs, and this MB-AChE conjugate was modified with NHS-azide molecules. Ab2- dibenzocyclooctyl (Ab2-DBCO) was prepared which can bind to the surface of MB-AChE-azide conjugate by the click reaction between the DBCO and azide. Using this Ab2-MB-AChE conjugate, a colorimetric assay for pathogen detection was developed based on AChE-catalyzed hydrolysis reaction with ultrahigh sensitivity. The Ab2-MB-AChE conjugate has three advantages: (1) a large amount of AChE molecules can be conjugated to the surface of MBs because MBs have large specific surface areas; (2) Ab2 and AChE can be conjugated to different sites of MBs by click chemistry, which avoids the steric hindrance that exist in the immune-reaction. (3) This conjugate can realize magnetic separation and signal amplification in one step, which will simplify the whole analysis.
3.3 Functionalization of carbon nanomaterials
Carbon nanomaterials, such as carbon nanotubes (CNTs) and graphene, have attracted enormous interest in the field of biosensors due to their high chemical stability, relatively inert electrochemistry, and extraordinary electron transport properties146-149. To develop biosensors, the surface of CNTs or graphene should be first modified to improve its biocompatibility or provide functional groups for further applications. Developing strategies for reproducible functionalization of graphene without adversely altering its chemical structure and electronic properties is of key importance150. Click chemistry provides an approach that uses only the most practical and reliable chemical reactions to introduce diverse structures bearing a wide variety of functional groups151-153. It provide an elegant protocol to prepare carbon-based functional materials. This section covers recent progress of CuAAC-mediated functionalization of carbon nanoparticles.
Graphene has drawn increasing attention in the field of biosensing due to its extraordinary catalytic/electronic properties26, 154. To further improve its application in the biosensors, many researchers have used the CuAAC to modify the surface of graphene. Azide and alkyne groups are simultaneously functionalized on the surface of graphene oxide (Click2 GO)155. Angiopep-2, a blood-brain barrier targeting peptide was conjugated to the Click2 GO via click chemistry, followed by a sequential CuAAC to conjugate bis-azide polyethylene glycol (Figure 8A). One particular advantage of click chemistry for GO functionalization is that click reaction in aqueous solution provides a possible way to offer maximum exposure of GO surface to the reactant. Another work reported a versatile approach for noncovalent functionalization of the basal plane of graphene based on π-π interaction and CuAAC(Figure 8B)156.
Click chemistry-mediated functionalization of carbon nanomaterials. (A) Schematic illustration of sequential functionalisation of GO using two-step CuAAC click reactions. (B) Schematic representation for conjugation of graphene onto Au surface via π-π interaction and CuAAC click reaction. (C) Illustration for the process of the bio-conjugation between the azide-SWNTs and alkyne-anti-IgG antibody by CuAAC. The anti-IgG antiobody-functionalized SWNTs conjugation can be used as signal recognition system in the immunosensor. (D) Schematic illustration of bio-conjugation of Fe3O4 NPs to the surface of multi-carbon nanotube nanomaterials by CuAAC. Azide-Fe3O4 can bind to the surface of alkyne- multi-carbon nanotube nanomaterials to prepare multifunctional nanoparobes, which has great potential in the fields of in vivo imaging and in vitro diagnosstics. Adapted with permission from [155, 156, 150, 157].
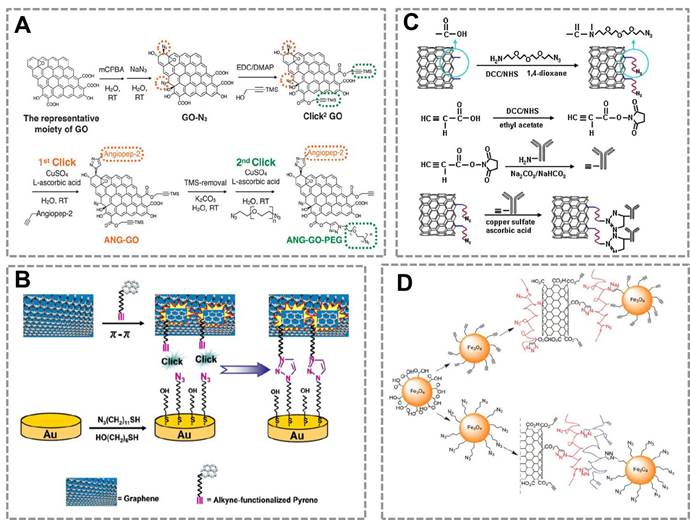
This method developed a strategy for reproducible functionalization of the inert graphene without adversely altering its structure and electronic properties, which is very important to broaden the amplication of GO for biochemical assays. Thus, click chemistry may provide a new strategy to modify the GO to improve its solubility and affect little with its electronic properties.
Single walled carbon nanotubes (SWNTs) can combine with click chemistry to act as a carrier in immunoassay (Figure 8 C)150. In this strategy, the alkyne-modified antibody was conjugated to the surface of azide-decorated SWNTs by click reaction, and the conjugate showed excellent dispersion and kept good bioactivity in water. This strategy provides a stable immobilization method and a platform for detecting analytes. CuACC is also used to conjugate different types of nanomaterials. For example, a previous work suggested that Fe3O4 NPs can be conjugated to the surface of multi-walled carbon nanotubes to form multifunctional NPs that can be used in biosensing, in vivo imaging or drug delivery (Figure 8D)157. These works show that CuAAC is a powerful strategy for surface modification and bio-conjugation of the nanosensors.
4. Discussion
In current studies, CuAAC-mediated nanosensors mainly focus on solution-based assays. For point-of-care applications, it is of greater significance to develop surface-based assays. Surface-based assays are more convenient and simpler than solution-based assays because they typically do not involve manipulation/washing with different types of fluids. Further work should pay more attention to developing many types of surface-based assays based on CuAAC-mediated nanosensors. For example, gold lateral flow test is a popular surface-based assay that has been widely used in clinical diagnosis.
In addition, current CuAAC-mediated nanosensors mainly adopt Au NPs for the signal readout. However, Au NPs-based method has limitations that the dispersion and aggregation of Au NPs are easily affected by the complex samples. It is expected that other NPs, such as magnetic nanoparticles combined with click chemistry can be used as the magnetic nanoprobes which can overcome this problem. Many works have showed that the state of MNPs will affect the transverse relaxation time (T2) of surrounding water molecules. The ΔT2 value (the change of T2) relates to the degree of aggregation of MNPs, and this degree of aggregation depends on the amount of target in sample, which can be used as the magnetic signal readout in the MRS-based sensor. Thus it is possible to combine MNPs with CuAAC to develop magnetic nanosensors. Furthermore, the “layer-by-layer” bioorthogonal reaction can be implemented on the functionalized MNPs for signal amplification. Compared with the optical readout, magnetic signal is independent of optical signal, thus it is little affected by the biological matrix such as cells, virus or pathogenic bacteria. Thus the magnetic nanosensors based on MNPs and click chemistry will be a potential direction of exploration in future studies.
In a few last years, many efforts have been made to accelerate the applications of bioorthogonal click chemistry mediated magnetic relaxation switch (MRS) sensor for point of care diagnosis in clincis and hospitals. On the one hand, some novel analytical strategies have been adopted in click chemistry mediated MRS sensor to improve the analytical performance of MRS sensor. For example, a previous work employing multimarker strategy based on click chemistry mediated MRS sensor to detect individual circulating tumor cells (CTCs) directly in whole blood without the need for primary purification158. The multimarker strategy can greatly improve the detection accuracy for detecting CTCs, which could potentially benefit a broad range of applications in clinical oncology. On the other hand, simple sample pre-treatment device and portable signal readout system have been developed to broaden the applications of click chemistry mediated nanosensors in the field of clinical diagnosis. Among these portable devices, the miniaturized nuclear magnetic resonance (μNMR) system is a classical representative. A series of μNMR system have been developed as portable signal readout device for MRS sensor, and this system combines microfluidic chips that can largely simplify the whole analysis of MRS sensor159. In this μNMR system, the whole immunoreaction and signal amplification using “layer-by layer” bioorthogonal reaction can be finished in the microfluidic chips, and then the magnetic signal (T2) can be collected by portable μNMR. Based on the μNMR system, click chemistry mediated MRS sensor has successfully used to detect the circulating tumor cells (CTCs) in whole blood samples, which has great potential in the point of care diagnosis in the clinics and hospitals. MRS sensor using μNMR may also provide an attractive tool for detecting cancer or infection biomarkers in real serum samples.
Click chemistry also has potential in the multiplex sensing or imaging applications. In in vitro diagnosis, multiplex detection of biomarkers is becoming more and more important because it can improve the detection accuracy and reduce the amount of samples as well as the whole cost. In in vivo imaging, methods that enable the simultaneous monitoring of multiple biomolecules (proteins, cell or genes) or small molecules are needed. Bioorthogonal reaction provides such a platform for simultaneous labeling and imaging of multiple targets in biological environments39, 105. Researchers have developed two bioorthogonal and mutually orthogonal reaction pairs using tetrazine-TCO and azide-cyclooctyne cycloaddition reactions, which showed that these two click reactions can be used at the same time in cells and provide precise control of desired reaction products. These are technologies potentially useful for multiplexed assays. Besides, NPs such as QDs are ideal multiple signal probes which have great potential in the detection of multiple targets or simultaneous tracking of multiple elements within a single system. Bioorthogonal click chemistry has provided a practicable tool to conjugate the biomolecules with such probes for multiplex detection of biomarkers and imaging.
Although nanosensors based on click chemistry have be proved to a powerful platform in the fields of clinical diagnosis, they also face some challenges when detecting targets in real samples. For example, the whole analysis cost will increase when using click chemistry strategy because the cost of ligands of click chemistry is higher than conventional coupling reagents, such as EDC. In addition, “layer-by-layer” bioorthogonal reaction strategy is adopted to improve the sensitivity to detect the trace target in the real samples, however, the nonspecific adsorption will increase when using “layer-by-layer” bioorthogonal reaction strategy, and the whole analysis time also will longer than that using one step of bioorthogonal reaction.
Conclusion and outlook
In colusion, click chemistry is a powerful tool for biochemical assays, as well as an effective bio-conjugation strategy to functionalize the nanoprobes in the nanosensors. Combined with click chemistry, NPs can be used as effective signal tags or signal amplification system in the biochemical assays. Future work should spare efforts in: (1) Development of more effective types of click chemistry to improve the analytical performances of NPs-based sensing platforms, as well as to utilize multiple bioorthogonal chemistries simultaneously or in concert with classic chemistries for multiplex detection; (2) Development of more convenient method for the functionalization of nanoprobes using click chemistry, for example, the one-step synthesis and surface modification of nanoprobes; (3) Development of more convenient and operable sensors such as lateral flow devices for biochemical assays combined with click chemistry, which will reduce the cost of the nanosensors and shorten the analysis time, for point-of-care testings. Combined with emerging techniques such as microfluidics and nanotechnology, click chemistry-mediated approaches hold great promise for biochemical assays and other related biomedical applications.
Acknowledgements
We thank the National Natural Science Foundation of China (81361140345, 21505027, 51373043, 21535001), the Chinese Academy of Sciences (XDA09030305), the Ministry of Health (2012ZX10001-008), and the CAS/SAFEA International Partnership Program for Creative Research Teams.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sun JS, Xianyu YL, Jiang XY. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem Soc Rev. 2014;43:6239-53
2. Swierczewska M, Liu G, Lee S, Chen XY. High-sensitivity nanosensors for biomarker detection. Chem Soc Rev. 2012;41:2641-55
3. Chen WW, Li QZ, Zheng WS, Hu F, Zhang GX, Wang Z. et al. Identification of Bacteria in Water by a Fluorescent Array. Angew Chem Int Edit. 2014;53:13734-9
4. Yen SK, Padmanabhan P, Selvan ST. Multifunctional Iron Oxide Nanoparticles for Diagnostics, Therapy and Macromolecule Delivery. Theranostics. 2013;3:975-92
5. Tekin HC, Gijs MAM. Ultrasensitive protein detection: a case for microfluidic magnetic bead-based assays. Lab Chip. 2013;13:4711-39
6. Lee J, Kang HJ, Lee YS, Heo H, Gu HN, Cho S. et al. A self-assembling magnetic resonance beacon for the detection of microRNA-1. Chem Commun. 2015;51:7199-202
7. Zhang Y, Guo YM, Xianyu YL, Chen WW, Zhao YY, Jiang XY. Nanomaterials for Ultrasensitive Protein Detection. Adv Mater. 2013;25:3802-19
8. Lei JP, Ju HX. Signal amplification using functional nanomaterials for biosensing. Chem Soc Rev. 2012;41:2122-34
9. Chen Y, Xianyu Y, Wang Y, Zhang X, Cha R, Sun J. et al. One-Step Detection of Pathogens and Viruses: Combining Magnetic Relaxation Switching and Magnetic Separation. ACS Nano. 2015;9:3184-91
10. Liu DB, Chen WW, Wei JH, Li XB, Wang Z, Jiang XY. A Highly Sensitive, Dual-Readout Assay Based on Gold Nanoparticles for Organophosphorus and Carbamate Pesticides. Anal Chem. 2012;84:4185-91
11. Chen H, Zhen Z, Todd T, Chu PK, Xie J. Nanoparticles for improving cancer diagnosis. Mat Sci Eng R. 2013;74:35-69
12. de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol. 2012;7:821-4
13. Gao ZQ, Xu MD, Hou L, Chen GN, Tang DP. Magnetic Bead-Based Reverse Colorimetric Immunoassay Strategy for Sensing Biomolecules. Anal Chem. 2013;85:6945-52
14. Wang W, Wang Y, Tu L, Klein T, Feng Y, Li Q. et al. Magnetic Detection of Mercuric Ion Using Giant Magnetoresistance-Based Biosensing System. Anal Chem. 2014;86:3712-6
15. Xianyu YL, Wang Z, Jiang XY. A Plasmonic Nanosensor for Immunoassay via Enzyme-Triggered Click Chemistry. ACS Nano. 2014;8:12741-7
16. Springer T, Ermini ML, Spackova B, Jablonku J, Homola J. Enhancing Sensitivity of Surface Plasmon Resonance Biosensors by Functionalized Gold Nanoparticles: Size Matters. Anal Chem. 2014;86:10350-6
17. Liu DB, Yang J, Wang HF, Wang ZL, Huang XL, Wang ZT. et al. Glucose Oxidase-Catalyzed Growth of Gold Nanoparticles Enables Quantitative Detection of Attomolar Cancer Biomarkers. Anal Chem. 2014;86:5800-6
18. Chung HJ, Castro CM, Im H, Lee H, Weissleder R. A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nat Nanotechnol. 2013;8:369-75
19. Tang L, Casas J, Venkataramasubramani M. Magnetic Nanoparticle Mediated Enhancement of Localized Surface Plasmon Resonance for Ultrasensitive Bioanalytical Assay in Human Blood Plasma. Anal Chem. 2013;85:1431-9
20. Tassa C, Shaw SY, Weissleder R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Accounts Chem Res. 2011;44:842-52
21. Li L, Jiang W, Luo K, Song HM, Lan F, Wu Y. et al. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics. 2013;3:595-615
22. Gu YP, Cui R, Zhang ZL, Xie ZX, Pang DW. Ultrasmall Near-Infrared Ag2Se Quantum Dots with Tunable Fluorescence for in Vivo Imaging. J Am Chem Soc. 2012;134:79-82
23. Yi Y, Zhu G, Liu C, Huang Y, Zhang Y, Li H. et al. A Label-Free Silicon Quantum Dots-Based Photoluminescence Sensor for Ultrasensitive Detection of Pesticides. Anal Chem. 2013;85:11464-70
24. Lou J, Liu S, Tu W, Dai Z. Graphene Quantums Dots Combined with Endonuclease Cleavage and Bidentate Chelation for Highly Sensitive Electrochemiluminescent DNA Biosensing. Anal Chem. 2015;87:1145-51
25. Zeng X, Ma S, Bao J, Tu W, Dai Z. Using Graphene-Based Plasmonic Nanocomposites to Quench Energy from Quantum Dots for Signal-On Photoelectrochemical Aptasensing. Anal Chem. 2013;85:11720-4
26. Chou SS, De M, Luo J, Rotello VM, Huang J, Dravid VP. Nanoscale Graphene Oxide (nGO) as Artificial Receptors: Implications for Biomolecular Interactions and Sensing. J Am Chem Soc. 2012;134:16725-33
27. Mani V, Wasalathanthri DP, Joshi AA, Kumar CV, Rusling JF. Highly Efficient Binding of Paramagnetic Beads Bioconjugated with 100 000 or More Antibodies to Protein-Coated Surfaces. Anal Chem. 2012;84:10485-91
28. Wang Z, Li X, Song Y, Li L, Shi W, Ma H. An Upconversion Luminescence Nanoprobe for the Ultrasensitive Detection of Hyaluronidase. Anal Chem. 2015;87:5816-23
29. Huang J, Ng AL, Piao Y, Chen C-F, Green AA, Sun C-F. et al. Covalently Functionalized Double-Walled Carbon Nanotubes Combine High Sensitivity and Selectivity in the Electrical Detection of Small Molecules. J Am Chem Soc. 2013;135:2306-12
30. Liu X, Wang F, Aizen R, Yehezkeli O, Willner I. Graphene Oxide/Nucleic-Acid-Stabilized Silver Nanoclusters: Functional Hybrid Materials for Optical Aptamer Sensing and Multiplexed Analysis of Pathogenic DNAs. J Am Chem Soc. 2013;135:11832-9
31. Munge BS, Coffey AL, Doucette JM, Somba BK, Malhotra R, Patel V. et al. Nanostructured Immunosensor for Attomolar Detection of Cancer Biomarker Interleukin-8 Using Massively Labeled Superparamagnetic Particles. Angew Chem Int Edit. 2011;50:7915-8
32. Krishnan S, Mani V, Wasalathanthri D, Kumar CV, Rusling JF. Attomolar Detection of a Cancer Biomarker Protein in Serum by Surface Plasmon Resonance Using Superparamagnetic Particle Labels. Angew Chem Int Edit. 2011;50:1175-8
33. Brennan JL, Hatzakis NS, Tshikhudo TR, Dirvianskyte N, Razumas V, Patkar S. et al. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjugate Chem. 2006;17:1373-5
34. Cutler JI, Zheng D, Xu XY, Giljohann DA, Mirkin CA. Polyvalent Oligonucleotide Iron Oxide Nanoparticle "Click" Conjugates. Nano Lett. 2010;10:1477-80
35. Han HS, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. Development of a Bioorthogonal and Highly Efficient Conjugation Method for Quantum Dots Using Tetrazine-Norbornene Cycloaddition. J Am Chem Soc. 2010;132:7838-39
36. N'Guyen TTT, Duong HTT, Basuki J, Montembault V, Pascual S, Guibert C. et al. Functional Iron Oxide Magnetic Nanoparticles with Hyperthermia-Induced Drug Release Ability by Using a Combination of Orthogonal Click Reactions. Angew Chem Int Edit. 2013;52:14152-6
37. Bilan R, Fleury F, Nabiey I, Sukhanova A. Quantum Dot Surface Chemistry and Functionalization for Cell Targeting and Imaging. Bioconjugate Chem. 2015;26:609-24
38. Kotagiri N, Li ZY, Xu XX, Mondal S, Nehorai A, Achilefu S. Antibody Quantum Dot Conjugates Developed via Copper-Free Click Chemistry for Rapid Analysis of Biological Samples Using a Microfluidic Microsphere Array System. Bioconjugate Chem. 2014;25:1272-81
39. Li XR, Guo J, Asong J, Wolfert MA, Boons GJ. Multifunctional Surface Modification of Gold-Stabilized Nanoparticles by Bioorthogonal Reactions. J Am Chem Soc. 2011;133:11147-53
40. Yang KS, Budin G, Tassa C, Kister O, Weissleder R. Bioorthogonal Approach to Identify Unsuspected Drug Targets in Live Cells. Angew Chem Int Edit. 2013;52:10593-7
41. Gole A, Murphy CJ. Azide-derivatized gold nanorods: Functional materials for "Click" chemistry. Langmuir. 2008;24:266-72
42. Zhou Y, Wang SX, Zhang K, Jiang XY. Visual detection of copper(II) by azide- and alkyne-functionalized gold nanoparticles using click chemistry. Angew Chem Int Edit. 2008;47:7454-6
43. Das M, Bandyopadhyay D, Mishra D, Datir S, Dhak P, Jain S. et al. "Clickable", Trifunctional Magnetite Nanoparticles and Their Chemoselective Biofunctionalization. Bioconjugate Chem. 2015;26:1981-81
44. Li N, Binder WH. Click-chemistry for nanoparticle-modification. J Mater Chem. 2011;21:16717-34
45. Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Edit. 2001;40:2004-21
46. Haun JB, Devaraj NK, Hilderbrand SA, Lee H, Weissleder R. Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nat Nanotechnol. 2010;5:660-5
47. Yang KS, Budin G, Reiner T, Vinegoni C, Weissleder R. Bioorthogonal Imaging of Aurora Kinase A in Live Cells. Angew Chem Int Edit. 2012;51:6598-603
48. Chen X, Li F, Wu Y-W. Chemical labeling of intracellular proteins via affinity conjugation and strain-promoted cycloadditions in live cells. Chem Commun. 2015;51:16537-40
49. Hao Z, Hong S, Chen X, Chen PR. Introducing Bioorthogonal Functionalities into Proteins in Living Cells. Accounts Chem Res. 2011;44:742-51
50. Wang J, Cheng B, Li J, Zhang Z, Hong W, Chen X. et al. Chemical Remodeling of Cell-Surface Sialic Acids through a Palladium-Triggered Bioorthogonal Elimination Reaction. Angew Chem Int Edit. 2015;54:5364-8
51. Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B. et al. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew Chem Int Edit. 2004;43:3928-32
52. Speers AE, Adam GC, Cravatt BF. Activity-based protein profiling in vivo using a copper(I)-catalyzed azide-alkyne 3+2 cycloaddition. J Am Chem Soc. 2003;125:4686-7
53. Yang M, Chen PR. Progress in the Bioorthogonal Labeling Reactions. Acta Chim Sinica. 2015;73:783-92
54. Hoffmann J-E, Plass T, Nikic I, Aramburu IV, Koehler C, Gillandt H. et al. Highly Stable trans-Cyclooctene Amino Acids for Live-Cell Labeling. Chem-Eur J. 2015;21:12266-70
55. Walper SA, Turner KB, Medintz IL. Enzymatic bioconjugation of nanoparticles: developing specificity and control. Current Opinion in Biotechnology. 2015;34:232-41
56. Polito L, Monti D, Caneva E, Delnevo E, Russo G, Prosperi D. One-step bioengineering of magnetic nanoparticles via a surface diazo transfer/azide-alkyne click reaction sequence. Chem Commun. 2008:621-3
57. Uttamapinant C, Tangpeerachaikul A, Grecian S, Clarke S, Singh U, Slade P. et al. Fast, Cell-Compatible Click Chemistry with Copper-Chelating Azides for Biomolecular Labeling. Angew Chem Int Edit. 2012;51:5852-6
58. Blizzard RJ, Backus DR, Brown W, Bazewicz CG, Li Y, Mehl RA. Ideal Bioorthogonal Reactions Using A Site-Specifically Encoded Tetrazine Amino Acid. J Am Chem Soc. 2015;137:10044-7
59. Neef AB, Pernot L, Schreier VN, Scapozza L, Luedtke NW. A Bioorthogonal Chemical Reporter of Viral Infection. Angew Chem Int Edit. 2015;54:7911-4
60. Sun EY, Josephson L, Weissleder R. "Clickable" nanoparticles for targeted imaging. Mol Imaging. 2006;5:122-8
61. von Maltzahn G, Ren Y, Park JH, Min DH, Kotamraju VR, Jayakumar J. et al. In vivo tumor cell targeting with "Click" nanoparticles. Bioconjugate Chem. 2008;19:1570-8
62. Elias DR, Cheng ZL, Tsourkas A. An Intein-Mediated Site-Specific Click Conjugation Strategy for Improved Tumor Targeting of Nanoparticle Systems. Small. 2010;6:2460-8
63. Haun JB, Devaraj NK, Marinelli BS, Lee H, Weissleder R. Probing Intracellular Biomarkers and Mediators of Cell Activation Using Nanosensors and Bioorthogonal Chemistry. ACS Nano. 2011;5:3204-13
64. van Duijnhoven SMJ, Rossin R, van den Bosch SM, Wheatcroft MP, Hudson PJ, Robillard MS. Diabody Pretargeting with Click Chemistry In Vivo. J Nucl Med. 2015;56:1422-8
65. Wieneke R, Raulf A, Kollmannsperger A, Heilemann M, Tampe R. SLAP: Small Labeling Pair for Single-Molecule Super-Resolution Imaging. Angew Chem Int Edit. 2015;54:10216-9
66. Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3+2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2005;127:11196-96
67. Boisselier E, Salmon L, Ruiz J, Astruc D. How to very efficiently functionalize gold nanoparticles by "click'' chemistry. Chem Commun. 2008:5788-90
68. Rahim MK, Kota R, Lee S, Haun JB. Bioorthogonal chemistries for nanomaterial conjugation and targeting. Nanotechnol Rev. 2013;2:215-27
69. Becer CR, Hoogenboom R, Schubert US. Click Chemistry beyond Metal-Catalyzed Cycloaddition. Angew Chem Int Edit. 2009;48:4900-8
70. Hua C, Zhang WH, De Almeida SRM, Ciampi S, Gloria D, Liu G. et al. A novel route to copper(II) detection using 'click' chemistry-induced aggregation of gold nanoparticles. Analyst. 2012;137:82-6
71. Zhang YF, Li BX, Xu CL. Visual detection of ascorbic acid via alkyne-azide click reaction using gold nanoparticles as a colorimetric probe. Analyst. 2010;135:1579-84
72. Fischler M, Sologubenko A, Mayer J, Clever G, Burley G, Gierlich J. et al. Chain-like assembly of gold nanoparticles on artificial DNA templates via 'click chemistry'. Chem Commun. 2008:169-71
73. Zhu K, Zhang Y, He S, Chen WW, Shen JZ, Wang Z. et al. Quantification of Proteins by Functionalized Gold Nanoparticles Using Click Chemistry. Anal Chem. 2012;84:4267-70
74. Zhou W, Gao X, Liu D, Chen X. Gold Nanoparticles for In Vitro Diagnostics. Chemical Reviews. 2015;115:10575-636
75. Chen WW, Cao FJ, Zheng WS, Tian Y, Xianyu YL, Xu P. et al. Detection of the nanomolar level of total Cr[(III) and (VI)] by functionalized gold nanoparticles and a smartphone with the assistance of theoretical calculation models. Nanoscale. 2015;7:2042-9
76. Xianyu Y, Sun J, Li Y, Tian Y, Wang Z, Jiang X. An ultrasensitive, non-enzymatic glucose assay via gold nanorod-assisted generation of silver nanoparticles. Nanoscale. 2013;5:6303-6
77. Xianyu YL, Xie YZY, Wang NX, Wang Z, Jiang XY. A Dispersion-Dominated Chromogenic Strategy for Colorimetric Sensing of Glutathione at the Nanomolar Level Using Gold Nanoparticles. Small. 2015;11:5510-4
78. Xianyu Y, Jiang X. Nanoscale materials and approaches for optical glucose assays. Current Opinion in Chemical Engineering. 2014;4:144-51
79. Xianyu Y, Chen Y, Jiang X. Horseradish Peroxidase-Mediated, Iodide-Catalyzed Cascade Reaction for Plasmonic Immunoassays. Anal Chem. 2015;87:10688-92
80. Xianyu Y, Wang Z, Sun J, Wang X, Jiang X. Colorimetric Logic Gates through Molecular Recognition and Plasmonic Nanoparticles. Small. 2014;10:4833-8
81. Xianyu Y, Xie Y, Wang N, Wang Z, Jiang X. A Dispersion-Dominated Chromogenic Strategy for Colorimetric Sensing of Glutathione at the Nanomolar Level Using Gold Nanoparticles. Small. 2015;11:5510-4
82. Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317-37
83. Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26:268-98
84. Pramanik D, Ghosh C, Dey SG. Heme-Cu Bound A beta Peptides: Spectroscopic Characterization, Reactivity, and Relevance to Alzheimer's Disease. J Am Chem Soc. 2011;133:15545-52
85. Xu X, Daniel WL, Wei W, Mirkin CA. Colorimetric Cu2+ Detection Using DNA-Modified Gold-Nanoparticle Aggregates as Probes and Click Chemistry. Small. 2010;6:623-6
86. Shen Q, Li W, Tang S, Hu Y, Nie Z, Huang Y. et al. A simple "clickable" biosensor for colorimetric detection of copper(II) ions based on unmodified gold nanoparticles. Biosens Bioelectron. 2013;41:663-8
87. Wang D, Ge C, Wang L, Xing X, Zeng L. A simple lateral flow biosensor for the rapid detection of copper(II) ions based on click chemistry. Rsc Advances. 2015;5:75722-7
88. Zhang Z, Li W, Zhao Q, Cheng M, Xu L, Fang X. Highly sensitive visual detection of copper (II) using water-soluble azide-functionalized gold nanoparticles and silver enhancement. Biosens Bioelectron. 2014;59:40-4
89. Fu G, Chen W, Yue X, Jiang X. Highly sensitive colorimetric detection of organophosphate pesticides using copper catalyzed click chemistry. Talanta. 2013;103:110-5
90. Zhang Y, Li B, Xu C. Visual detection of ascorbic acid via alkyne-azide click reaction using gold nanoparticles as a colorimetric probe. Analyst. 2010;135:1579-84
91. Marti A, Costero AM, Gavina P, Parra M. Selective colorimetric NO(g) detection based on the use of modified gold nanoparticles using click chemistry. Chem Commun. 2015;51:3077-9
92. Qu WS, Liu YY, Liu DB, Wang Z, Jiang XY. Copper-Mediated Amplification Allows Readout of Immunoassays by the Naked Eye. Angew Chem Int Edit. 2011;50:3442-5
93. Xie Q, Weng X, Lu L, Lin Z, Xu X, Fu C. A sensitive fluorescent sensor for quantification of alpha-fetoprotein based on immunosorbent assay and click chemistry. Biosens Bioelectron. 2016;77:46-50
94. Xianyu YL, Zhu K, Chen WW, Wang XF, Zhao HM, Sun JS. et al. Enzymatic Assay for Cu(II) with Horseradish Peroxidase and Its Application in Colorimetric Logic Gate. Anal Chem. 2013;85:7029-32
95. Zheng MM, Zheng L, Zhang PY, Li JB, Zhang Y. Development of Bioorthogonal Reactions and Their Applications in Bioconjugation. Molecules. 2015;20:3190-205
96. Sorgenfrei S, Chiu CY, Gonzalez RL, Yu YJ, Kim P, Nuckolls C. et al. Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor. Nat Nanotechnol. 2011;6:125-31
97. Das J, Aziz MA, Yang H. A nanocatalyst-based assay for proteins: DNA-free ultrasensitive electrochemical detection using catalytic reduction of p-nitrophenol by gold-nanoparticle labels. J Am Chem Soc. 2006;128:16022-3
98. Hassertt R, Pagel M, Ming Z, Haupl T, Abel B, Braun K. et al. Biocompatible Silicon Surfaces through Orthogonal Click Chemistries and a High Affinity Silicon Oxide Binding Peptide. Bioconjugate Chemistry. 2012;23:2129-37
99. Boyce M, Bertozzi CR. Bringing chemistry to life. Nat Methods. 2011;8:638-42
100. Ramil CP, Lin Q. Bioorthogonal chemistry: strategies and recent developments. Chem Commun. 2013;49:11007-22
101. Thorek DLJ, Elias DR, Tsourkas A. Comparative Analysis of Nanoparticle-Antibody Conjugations: Carbodiimide versus Click Chemistry. Mol Imaging. 2009;8:221-9
102. Kamber DN, Liang Y, Blizzard RJ, Liu F, Mehl RA, Houk KN. et al. 1,2,4-Triazines Are Versatile Bioorthogonal Reagents. J Am Chem Soc. 2015;137:8388-91
103. Matikonda SS, Orsi DL, Staudacher V, Jenkins IA, Fiedler F, Chen J. et al. Bioorthogonal prodrug activation driven by a strain-promoted 1,3-dipolar cycloaddition. Chem Sci. 2015;6:1212-8
104. Rossin R, van den Bosch SM, ten Hoeve W, Carvelli M, Versteegen RM, Lub J. et al. Highly Reactive trans-Cyclooctene Tags with Improved Stability for Diels-Alder Chemistry in Living Systems. Bioconjugate Chem. 2013;24:1210-7
105. Karver MR, Weissleder R, Hilderbrand SA. Bioorthogonal Reaction Pairs Enable Simultaneous, Selective, Multi-Target Imaging. Angew Chem Int Edit. 2012;51:920-2
106. Li Z, Yao W, Kong L, Zhao Y, Li L. General Method for the Synthesis of Ultrastable Core/Shell Quantum Dots by Aluminum Doping. J Am Chem Soc. 2015;137:12430-3
107. Wang W, Ji X, Kapur A, Zhang C, Mattoussi H. A Multifunctional Polymer Combining the Imidazole and Zwitterion Motifs as a Biocompatible Compact Coating for Quantum Dots. J Am Chem Soc. 2015;137:14158-72
108. Zhan N, Palui G, Safi M, Ji X, Mattoussi H. Multidentate Zwitterionic Ligands Provide Compact and Highly Biocompatible Quantum Dots. J Am Chem Soc. 2013;135:13786-95
109. Bi X, Adriani G, Xu Y, Chakrabortty S, Pastorin G, Ho HK. et al. Gene Detection in Complex Biological Media Using Semiconductor Nanorods within an Integrated Microfluidic Device. Anal Chem. 2015;87:10292-8
110. Wang J-J, Jiang Y-Z, Lin Y, Wen L, Lv C, Zhang Z-L. et al. Simultaneous Point-of-Care Detection of Enterovirus 71 and Coxsackievirus B3. Anal Chem. 2015;87:11105-12
111. Liu X, Jiang H, Fang Y, Zhao W, Wang N, Zang G. Quantum Dots Based Potential-Resolution Dual-Targets Electrochemiluminescent Immunosensor for Subtype of Tumor Marker and Its Serological Evaluation. Anal Chem. 2015;87:9163-9
112. Zhao P, He K, Han Y, Zhang Z, Yu M, Wang H. et al. Near-Infrared Dual-Emission Quantum Dots-Gold Nanoclusters Nanohybrid via Co-Template Synthesis for Ratiometric Fluorescent Detection and Bioimaging of Ascorbic Acid In Vitro and In Vivo. Anal Chem. 2015;87:9998-10005
113. Piyeteau L, Ong T-C, Rossini AJ, Emsley L, Coperet C, Kovalenko MV. Structure of Colloidal Quantum Dots from Dynamic Nuclear Polarization Surface Enhanced NMR Spectroscopy. J Am Chem Soc. 2015;137:13964-71
114. Wang W, Kapur A, Ji X, Safi M, Palui G, Palomo V. et al. Photoligation of an Amphiphilic Polymer with Mixed Coordination Provides Compact and Reactive Quantum Dots. J Am Chem Soc. 2015;137:5438-51
115. Blanco-Canosa JB, Wu M, Susumu K, Petryayeva E, Jennings TL, Dawson PE. et al. Recent progress in the bioconjugation of quantum dots. Coordin Chem Rev. 2014;263:101-37
116. Schieber C, Bestetti A, Lim JP, Ryan AD, Tich-Lam N, Eldridge R. et al. Conjugation of Transferrin to Azide-Modified CdSe/ZnS Core-Shell Quantum Dots using Cyclooctyne Click Chemistry. Angew Chem Int Edit. 2012;51:10523-7
117. Schmidtke C, Kreuziger A-M, Alpers D, Jacobsen A, Leshch Y, Eggers R. et al. Glycoconjugated Amphiphilic Polymers via Click-Chemistry for the Encapsulation of Quantum Dots. Langmuir. 2013;29:12593-600
118. Bernardin A, Cazet A, Guyon L, Delannoy P, Vinet F, Bonnaffe D. et al. Copper-Free Click Chemistry for Highly Luminescent Quantum Dot Conjugates: Application to in Vivo Metabolic Imaging. Bioconjugate Chem. 2010;21:583-8
119. Karakoti AS, Shukla R, Shanker R, Singh S. Surface functionalization of quantum dots for biological applications. Adv Colloid Interfac. 2015;215:28-45
120. Zhang P, Liu S, Gao D, Hu D, Gong P, Sheng Z. et al. Click-Functionalized Compact Quantum Dots Protected by Multidentate-Imidazole Ligands: Conjugation-Ready Nanotags for Living-Virus Labeling and Imaging. J Am Chem Soc. 2012;134:8388-91
121. Pan H, Zhang PF, Gao DY, Zhang YJ, Li P, Liu LL. et al. Noninvasive Visualization of Respiratory Viral Infection Using Bioorthogonal Conjugated Near-Infrared-Emitting Quantum Dots. ACS Nano. 2014;8:5468-77
122. Malhotra R, Patel V, Chikkaveeraiah BV, Munge BS, Cheong SC, Zain RB. et al. Ultrasensitive Detection of Cancer Biomarkers in the Clinic by Use of a Nanostructured Microfluidic Array. Anal Chem. 2012;84:6249-55
123. Banerjee SR, Ngen EJ, Rotz MW, Kakkad S, Lisok A, Pracitto R. et al. Synthesis and Evaluation of Gd-III-Based Magnetic Resonance Contrast Agents for Molecular Imaging of Prostate-Specific Membrane Antigen. Angew Chem Int Edit. 2015;54:10778-82
124. Nakamura T, Matsushita H, Sugihara F, Yoshioka Y, Mizukami S, Kikuchi K. Activatable F-19 MRI Nanoparticle Probes for the Detection of Reducing Environments. Angew Chem Int Edit. 2015;54:1007-10
125. Tsitovich PB, Spernyak JA, Morrow JR. A Redox-Activated MRI Contrast Agent that Switches Between Paramagnetic and Diamagnetic States. Angew Chem Int Edit. 2013;52:13997-4000
126. Lee SB, Kim HL, Jeong H-J, Lim ST, Sohn M-H, Kim DW. Mesoporous Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal Reaction in a Living Body. Angew Chem Int Edit. 2013;52:10549-52
127. Yang X-C, Samanta B, Agasti SS, Jeong Y, Zhu Z-J, Rana S. et al. Drug Delivery Using Nanoparticle-Stabilized Nanocapsules. Angew Chem Int Edit. 2011;50:477-81
128. Yoo D, Jeong H, Noh S-H, Lee J-H, Cheon J. Magnetically Triggered Dual Functional Nanoparticles for Resistance-Free Apoptotic Hyperthermia. Angew Chem Int Edit. 2013;52:13047-51
129. Liong M, Tassa C, Shaw SY, Lee H, Weissleder R. Multiplexed Magnetic Labeling Amplification Using Oligonucleotide Hybridization. Adv Mater. 2011;23:H254-57
130. Chung HJ, Reiner T, Budin G, Min C, Liong M, Issadore D. et al. Ubiquitous Detection of Gram-Positive Bacteria with Bioorthogonal Magnetofluorescent Nanoparticles. ACS Nano. 2011;5:8834-41
131. Devaraj NK, Weissleder R. Biomedical Applications of Tetrazine Cycloadditions. Accounts Chem Res. 2011;44:816-27
132. Karver MR, Weissleder R, Hilderbrand SA. Synthesis and Evaluation of a Series of 1,2,4,5-Tetrazines for Bioorthogonal Conjugation. Bioconjugate Chem. 2011;22:2263-70
133. Castro CM, Ghazani AA, Chung J, Shao H, Issadore D, Yoon T-J. et al. Miniaturized nuclear magnetic resonance platform for detection and profiling of circulating tumor cells. Lab Chip. 2014;14:14-23
134. Issadore D, Chung HJ, Chung J, Budin G, Weissleder R, Lee H. mu Hall Chip for Sensitive Detection of Bacteria. Adv Healthc Mater. 2013;2:1224-8
135. Muluneh M, Issadore D. Microchip-based detection of magnetically labeled cancer biomarkers. Adv Drug Deliver Rev. 2014;66:101-9
136. Rahim MK, Kota R, Haun JB. Enhancing Reactivity for Bioorthogonal Pretargeting by Unmasking Antibody-Conjugated trans-Cyclooctenes. Bioconjugate Chem. 2015;26:352-60
137. Ullal AV, Reiner T, Yang KS, Gorbatov R, Min C, Issadore D. et al. Nanoparticle-Mediated Measurement of Target-Drug Binding in Cancer Cells. ACS Nano. 2011;5:9216-24
138. Bamrungsap S, Chen T, Shukoor MI, Chen Z, Sefah K, Chen Y. et al. Pattern Recognition of Cancer Cells Using Aptamer-Conjugated Magnetic Nanoparticles. ACS Nano. 2012;6:3974-81
139. Min C, Shao HL, Liong M, Yoon TJ, Weissleder R, Lee H. Mechanism of Magnetic Relaxation Switching Sensing. ACS Nano. 2012;6:6821-8
140. Chen YP, Zou MQ, Qi C, Xie M-X, Wang D-N, Wang Y-F. et al. Immunosensor based on magnetic relaxation switch and biotin-streptavidin system for the detection of Kanamycin in milk. Biosens Bioelectron. 2013;39:112-7
141. Chen YP, Zou MQ, Wang DN, Li YL, Xue Q, Xie MX. et al. An immunosensor based on magnetic relaxation switch and polystyrene microparticle-induced immune multivalency enrichment system for the detection of Pantoea stewartii subsp. Stewartii. Biosens Bioelectron. 2013;43:6-11
142. Liong M, Fernandez-Suarez M, Issadore D, Min C, Tassa C, Reiner T. et al. Specific Pathogen Detection Using Bioorthogonal Chemistry and Diagnostic Magnetic Resonance. Bioconjugate Chem. 2011;22:2390-4
143. Peterson VM, Castro CM, Lee H, Weissleder R. Orthogonal Amplification of Nanoparticles for Improved Diagnostic Sensing. ACS Nano. 2012;6:3506-13
144. Liu DB, Wang ZT, Jin A, Huang XL, Sun XL, Wang F. et al. Acetylcholinesterase-Catalyzed Hydrolysis Allows Ultrasensitive Detection of Pathogens with the Naked Eye. Angew Chem Int Edit. 2013;52:14065-9
145. Ge SG, Liu WY, Ge L, Yan M, Yan JX, Huang JD. et al. In situ assembly of porous Au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for Microcystin-LR immunoassay. Biosens Bioelectron. 2013;49:111-7
146. Jung JH, Cheon DS, Liu F, Lee KB, Seo TS. A Graphene Oxide Based Immuno-biosensor for Pathogen Detection. Angew Chem Int Edit. 2010;49:5708-11
147. Morales-Narvaez E, Hassan A-R, Merkoci A. Graphene Oxide as a Pathogen-Revealing Agent: Sensing with a Digital-Like Response. Angew Chem Int Edit. 2013;52:13779-83
148. Liu S, Zhang X, Luo W, Wang Z, Guo X, Steigerwald ML. et al. Single-Molecule Detection of Proteins Using Aptamer-Functionalized Molecular Electronic Devices. Angew Chem Int Edit. 2011;50:2496-502
149. Liu SF, Petty AR, Sazama GT, Swager TM. Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew Chem Int Edit. 2015;54:6554-7
150. Qi HL, Ling C, Huang R, Qiu XY, Li SG, Gao Q. et al. Functionalization of single-walled carbon nanotubes with protein by click chemistry as sensing platform for sensitized electrochemical immunoassay. Electrochim Acta. 2012;63:76-82
151. Bravo I, Garcia-Mendiola T, Revenga-Parra M, Pariente F, Lorenzo E. Diazonium salt click chemistry based multiwall carbon nanotube electrocatalytic platforms. Sensor Actuat B-Chem. 2015;211:559-68
152. Fedeli S, Paoli P, Brandi A, Venturini L, Giambastiani G, Tuci G. et al. Azido-Substituted BODIPY Dyes for the Production of Fluorescent Carbon Nanotubes. Chem-Eur J. 2015;21:15349-53
153. Cui L, Zhu B-W, Qu S, He X-P, Chen G-R. "Clicked" galactosyl anthraquinone on graphene electrodes for the label-free impedance detection of live cancer cells. Dyes Pigments. 2015;121:312-5
154. Cui SM, Mao S, Lu GH, Chen JH. Graphene Coupled with Nanocrystals: Opportunities and Challenges for Energy and Sensing Applications. J Phys Chem Lett. 2013;4:2441-54
155. Mei KC, Rubio N, Costa PM, Kafa H, Abbate V, Festy F. et al. Synthesis of double-clickable functionalised graphene oxide for biological applications. Chem Commun. 2015;51:14981-4
156. Shen X, Liu Y, Pang Y, Yao W. Conjugation of graphene on Au surface by pi-pi interaction and click chemistry. Electrochem Commun. 2013;30:13-6
157. Islam MR, Bach LG, Nga TT, Lim KT. Covalent ligation of gold coated iron nanoparticles to the multi-walled carbon nanotubes employing click chemistry. J Alloy Compd. 2013;561:201-5
158. Ghazani A A, Castro CM, Gorbatov R, Lee H, Weissleder R. Sensitive and Direct Detection of Circulating Tumor Cells by Multimarker μ-Nuclear Magnetic Resonance. Neoplasia. 2012;14:388-95
159. Castro C M, Ghazani AA, Chung J, Shao H, Issadore D, Yoon T J, Weissleder R, Lee H. Miniaturized nuclear magnetic resonance platform for detection and profiling of circulating tumor cells. Lab Chip. 2014;14:14-23
Author contact
![]() Corresponding author: Xingyu Jiang, E-mail address: xingyujiangcn.
Corresponding author: Xingyu Jiang, E-mail address: xingyujiangcn.
 Global reach, higher impact
Global reach, higher impact