13.3
Impact Factor
Theranostics 2017; 7(3):523-537. doi:10.7150/thno.17259 This issue Cite
Research Paper
Enhanced Blood Suspensibility and Laser-Activated Tumor-specific Drug Release of Theranostic Mesoporous Silica Nanoparticles by Functionalizing with Erythrocyte Membranes
1. State Key Laboratory of Drug Research & Center of Pharmaceutics, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 501 Haike Road, Shanghai 201203, China;
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. School of Pharmacy, Shenyang Pharmaceutical University, Shenyang, 110016, China.
Abstract
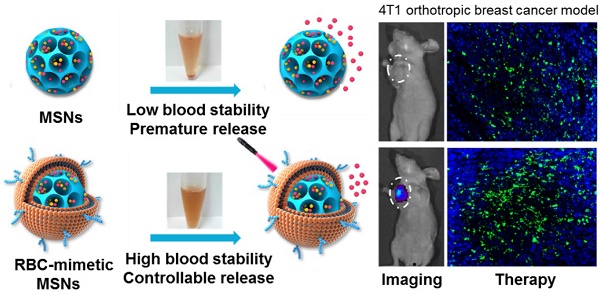
Mesoporous silica nanoparticles (MSNs), with their large surface area and tunable pore sizes, have been widely applied for anticancer therapeutic cargos delivery with a high loading capacity. However, easy aggregation in saline buffers and limited blood circulation lifetime hinder their delivery efficiency and the anticancer efficacy. Here, new multifunctional MSNs-supported red-blood-cell (RBC)-mimetic theranostic nanoparticles with long blood circulation, deep-red light-activated tumor imaging and drug release were reported. High loading capacities were achieved by camouflaging MSNs with RBC membrane to co-load an anticancer drug doxorubicin (Dox) (39.1 wt%) and a near-infrared photosensitizer chlorin e6 (Ce6) (21.1 wt%). The RBC membrane-coating protected drugs from leakage, and greatly improved the colloidal stability of MSNs, with negligible particle size change over two weeks. Upon an external laser stimuli, the RBC membrane could be destroyed, resulting in 10 times enhancement of Dox release. In a 4T1 breast cancer mouse model, the RBC-mimetic MSNs could realize in vivo tumor imaging with elongated tumor accumulation lifetime for over 24 h, and laser-activated tumor-specific Dox accumulation. The RBC-mimetic MSNs could integrate the Ce6-based photodynamic therapy and Dox-based chemotherapy, completely suppress the primary tumor growth and inhibit metastasis of breast cancer, which could provide a new strategy for optimization of MSNs and efficient anticancer drug delivery.
Keywords: Mesoporous silica nanoparticles, RBC-mimetic, Drug release, Fluorescence imaging, Metastasis.
 Global reach, higher impact
Global reach, higher impact