13.3
Impact Factor
Theranostics 2017; 7(10):2593-2605. doi:10.7150/thno.19894 This issue Cite
Research Paper
Self-Assembly DNA Polyplex Vaccine inside Dissolving Microneedles for High-Potency Intradermal Vaccination
1. Institute of Medical Science and Technology, National Sun Yat-sen University, Kaohsiung 80424, Taiwan;
2. Department of Biotechnology, Kaohsiung Medical University, Kaohsiung 80708, Taiwan;
3. Doctoral Degree Program in Marine Biotechnology, National Sun Yat-sen University, Kaohsiung 80424, Taiwan;
4. Department of Neurosurgery, Linkou Chang Gung Memorial Hospital, Taoyuan 33305, Taiwan. School of Medicine, Chang Gung University, Taoyuan 33302, Taiwan;
5. Department of Neurosurgery, Chang Gung Memorial Hospital, Keelung 20401, Taiwan
School of Medicine, Chang Gung University, Taoyuan 33302, Taiwan.
# These authors contributed equally.
Abstract
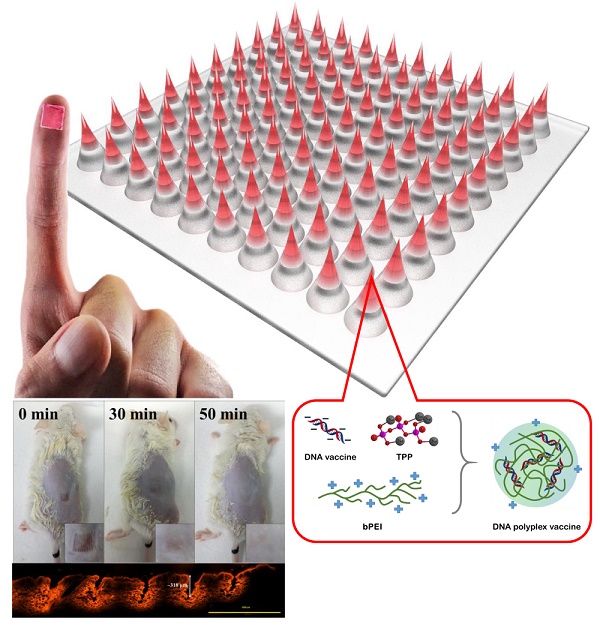
The strong immunogenicity induction is the powerful weapon to prevent the virus infections. This study demonstrated that one-step synthesis of DNA polyplex vaccine in microneedle (MN) patches can induce high immunogenicity through intradermal vaccination and increase the vaccine stability for storage outside the cold chain. More negative charged DNA vaccine was entrapped into the needle region of MNs followed by DNA polyplex formation with branched polyethylenimine (bPEI) pre-coated in the cavities of polydimethylsiloxane (PDMS) molds that can deliver more DNA vaccine to immune-cell rich epidermis with high transfection efficiency. Our data in this study support the safety and immunogenicity of the MN-based vaccine; the MN patch delivery system induced an immune response 3.5-fold as strong as seen with conventional intramuscular administration; the DNA polyplex formulation provided excellent vaccine stability at high temperature (could be stored at 45ºC for at least 4 months); the DNA vaccine is expected to be manufactured at low cost and not generate sharps waste. We think this study is significant to public health because there is a pressing need for an effective vaccination in developing countries.
Keywords: dissolvable microneedles, self-assembly, thermostability, DNA vaccine, intradermal delivery.
 Global reach, higher impact
Global reach, higher impact