13.3
Impact Factor
Theranostics 2017; 7(10):2649-2651. doi:10.7150/thno.19866 This issue Cite
Editorial
S100-SPECT uncovers cellular and molecular events of pre-metastatic niche formation and following organ-specific cancer metastasis
Department of Cell Biology, Okayama University Graduate School of Medicine and Dentistry, Okayama-shi 700-8558, Japan
Published 2017-6-25
Abstract
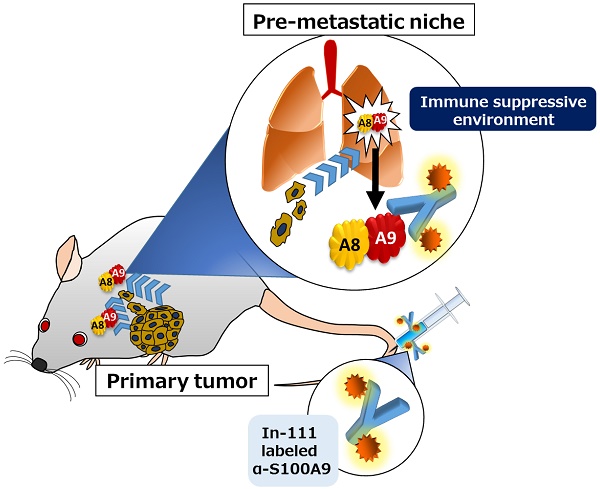
Great progress has been made in in vivo imaging for cancer metastasis. Eisenblaetter et al. developed an innovative S100A8/A9-specific single photon emission computed tomography (SPECT) probe for imaging and succeeded in detecting the metastatic organ favored by the cancer before the cancer arrival. By utilizing this advanced method, researchers have also found that cancer-derived monocytes are the main source of the abundant production of S100A8/A9 in the pre-metastatic area. The CCL2-CCR2 axis is associated with S100A8/A9 production. Clinical establishment of this technique is expected to enable accurate prediction and monitoring of cancer metastasis during therapy.
Keywords: In vivo imaging, S100A8/A9, organ-specific metastasis, pre-metastatic niche, monocytes, inflammation, CCR2, CCL2
Due to its life-threatening characteristics, metastatic dissemination of cancer cells to remote areas within the body is the most problematic aspect of cancer diseases, and the establishment of advanced diagnostic and therapeutic approaches to regulate metastasis is therefore required to fight against cancer. To do so, we need to delve more into the principles of cancer on a biological level as well as on a molecular level. Cancer cells frequently exhibit organ-specific metastasis, for which the “seed and soil” theory (“seed” referring to cancer cells and “soil“ referring to the favored distant target organ in metastasis) has long been proposed 1. However, the molecular mechanisms of this concept have yet to be systematically mapped out.
An impressive amount of evidence regarding the S100 protein family functioning as an important player in the aforementioned theory is continuously being compiled. S100 proteins are a family of low-molecular-weight proteins characterized by an EF-hand calcium-binding motif. There are at least 20 different S100 proteins in humans. Among these, a heterodimer complex composed of the S100A8 and S100A9 proteins referred to as S100A8/A9 is remarked. It was first reported that S100A8/A9, which was coincidentally found to be abundant in lung cells, exhibits a “soil signal” that attracts cancer cells having Toll-like receptor 4 (TLR4), which itself is considered to be a “soil sensor” on cancer cells, by functioning as its ligand 2, 3. This causes distant melanoma cells to approach the lung area as a preferred metastasis or “seeding” site. Consecutively, the receptor for advanced glycation end products (RAGE) was also included as a soil sensor, a ligand for S100A8/A9 in cancer metastasis 4. Our own investigations also showed increasing evidence of yet other unknown important soil sensors for S100A8/A9, namely, EMMPRIN, NPTN and MCAM 5, 6, 7. Collectively we have been gradually unveiling soil sensors that allow cancer cells to travel to favored soil signal organs. However, the mechanism by which cancer cells influence the creation of cancer-favorable microenvironments within the metastatic organ is still a mystery due to the elaborate network of various normal cells such as stromal and immune cells and the molecules needed for their interaction.
Eisenblaetter et al. 8 first tried to understand this complex mechanism by using an innovative S100A8/A9-specific single photon emission computed tomography (SPECT) probe for imaging in a transplantable syngeneic mouse model of breast cancer that exhibits a tendency to metastasize into the lung area. Using their newly developed imaging agent, they succeeded in clearly presenting the in vivo visualization of S100A8/A9 functioning as a very strong marker for cancer attraction, and they clarified the mechanism of marker production through certain immunomodulation that is linked to systemic metastatic niche formation. The presence of the transplanted cancer cells spontaneously induced proinflammatory monocytes belonging to myeloid derived suppressor cells (MDSC), which were specifically defined by the authors as CCR2highCXCR1low cells, in the remote pre-metastatic lung area. Interestingly, the induced monocytes played a significant role in the establishment of an immunosuppressive environment enriched with T-reg cells and with few NK cells. The SPECT showed abundant production and release of S100A8/A9 in the cancer-derived CCR2highCXCR1low cells within the pre-metastatic region. The authors also found that the abundance of cancer-attractive S100A8/A9 was triggered by stimulation of the cells with the CCR2 ligand, CCL2, which could be supplied by either the cancer itself or cancer-associated fibroblasts. This CCL2-CCR2 axis in the cancer-derived CCR2highCXCR1low cells might also be involved in the enhanced vascular permeability in the metastatic process of cancer cells, since Hiratsuka et al. recently showed that abundant secretion of serum amyloid A3, a strong permeability mediator, was induced by the axis activation and worked in the process 9. Thus, the authors presented invaluable results showing that (1) S100A8/A9 is an accurate new imaging biomarker to predict metastatic niche formation with higher reliability in lungs before breast cancer metastasis and (2) a large amount of S100A8/A9 is released from cancer-derived CCR2highCXCR1low monocyte cells and is in charge of the pre-metastatic niche formation with immunosuppression.
Here, an idea regarding the involvement of other S100 proteins in cancer-derived metastatic niche formation was born. There have been many reports indicating that not only S100A8/A9 but also other S100 family proteins act cooperatively at specific stages, such as epithelial-mesenchymal transition (EMT), floating, adhesion, invasion, growth and in elaborate cancer metastatic processes. In support of the studies described above, Hoshino et al. reported important findings for the “seed and soil” theory. They found that cancer-secreted exosomes function as key players to determine the destination of cancer cells to certain organs such as the lungs or liver. The exosomes in the blood stream are incorporated into specific organ cells such as fibroblasts in the lungs or Kupffer cells in the liver. These cells would then in turn highly express and secrete various S100 family proteins. In the end, the expressed S100 proteins would function as critical target signals making a pre-metastatic niche and trigger cancer metastasis to their target organs 10. Therefore, other S100 proteins also have a great potential to become valuable target molecules for diagnostic imaging markers. Thus, the author's results are indeed helpful for this scope of work and for understanding organ-specific cancer metastasis.
On the other hand, accumulating evidence has indicated that the S100A8/A9 heterodimer appears at a high level in lesional sites and in blood flow in the presence of not only cancers but also a broad range of severe inflammatory diseases such as psoriasis, atopic dermatitis, atherosclerosis and diabetes. If a cancer patient has such an inflammatory disease, the severe inflammation may cause an increased background in the imaging of S100A8/A9 that hampers accurate assessment. Further comprehensive studies should show whether S100A8/A9 is a significantly valuable agent with flexibility in several clinical settings.
Cancer metastasis from the primary site to a distant organ is the most serious problem for cancer processes because it is a life-threatening situation. The establishment of advanced diagnostic and therapeutic approaches for regulating metastasis is therefore strongly desired. Hence, the author's principle in cancer metastasis obtained from the novel imaging work will lead to the establishment of highly innovative means to predict, overcome and overpower cancer metastasis in the near future.
References
1. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101
2. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369-1375
3. Hiratsuka S, Watanabe A, Sakurai Y. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349-1355
4. Saha A, Lee YC, Zhang Z, Chandra G, Su SB, Mukherjee AB. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem. 2010;285:10822-10831
5. Hibino T, Sakaguchi M, Miyamoto S. et al. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 2013;73:172-183
6. Ruma IM, Putranto EW, Kondo E. et al. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin Exp Metastasis. 2016;33:609-627
7. Sakaguchi M, Yamamoto M, Miyai M. et al. Identification of an S100A8 receptor neuroplastin-β and its heterodimer formation with EMMPRIN. J Invest Dermatol. 2016;136:2240-2250
8. Eisenblaetter M, Flores-Borja F, Lee JJ. et al. Visualization of Tumor-Immune Interaction - Target-Specific Imaging of S100A8/A9 Reveals Pre-Metastatic Niche Establishment. Theranostics. 2017;7(9):2392-2401 doi:10.7150/thno.17138
9. Hiratsuka S, Ishibashi S, Tomita T. et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun. 2013;4:1853
10. Hoshino A, Costa-Silva B, Shen TL. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-335
Author contact
![]() Corresponding author: Masakiyo Sakaguchi, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama-shi, Okayama 700-8558, Japan; E-mail: masa-sokayama-u.ac.jp, Phone number: +81-86-235-7395; Fax: +81-86-235-7400
Corresponding author: Masakiyo Sakaguchi, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama-shi, Okayama 700-8558, Japan; E-mail: masa-sokayama-u.ac.jp, Phone number: +81-86-235-7395; Fax: +81-86-235-7400
 Global reach, higher impact
Global reach, higher impact