13.3
Impact Factor
Theranostics 2018; 8(4):1121-1130. doi:10.7150/thno.22601 This issue Cite
Research Paper
68Ga-BBN-RGD PET/CT for GRPR and Integrin αvβ3 Imaging in Patients with Breast Cancer
1. Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China
2. Department of Breast Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China
3. Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, Maryland, 20892, USA
4. Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
5. Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China
* Jingjing Zhang and Feng Mao contributed equally to this work
Received 2017-8-29; Accepted 2017-12-9; Published 2018-1-1
Abstract
Purpose: This study was to assess a gastrin-releasing peptide receptor (GRPR) and integrin αvβ3 dual targeting tracer 68Ga-BBN-RGD for positron emission tomography (PET)/computed tomography (CT) imaging of breast cancer and metastasis. Materials and Methods: Twenty-two female patients were recruited either with suspected breast cancer on screening mammography (n = 16) or underwent breast cancer radical mastectomy (n = 6). All the 22 patients underwent PET/CT at 30-45 min after intravenous injection of 68Ga-BBN-RGD. Eleven out of 22 patients also accepted 68Ga-BBN PET/CT within 2 weeks for comparison. A final diagnosis was made based on the histopathologic examination of surgical excision or biopsy. Results: Both the primary cancer and metastases showed positive 68Ga-BBN-RGD accumulation. The T/B ratios of 68Ga-BBN-RGD accumulation were 2.10 to 9.44 in primary cancer and 1.10 to 3.71 in axillary lymph node metastasis, 3.80 to 10.7 in distant lymph nodes, 2.70 to 5.35 in lung metastasis and 3.17 to 22.8 in bone metastasis, respectively. For primary lesions, the SUVmax from 68Ga-BBN-RGD PET in ER positive group was higher than that in ER negative group (P < 0.01). For both primary and metastatic lesions, SUVmean quantified from 68Ga-BBN-RGD PET correlated well with both GRPR expression and integrin αvβ3 expression. Conclusion: This study demonstrated significant uptake of a new type of dual integrin αvβ3 and GRPR targeting radiotracer in both the primary lesion and the metastases of breast cancer. 68Ga-BBN-RGD PET/CT may be of great value in discerning both primary breast cancers, axillary lymph node metastasis and distant metastases.
Keywords: BBN-RGD, PET, breast cancer, integrin αvβ3, GRPR
Introduction
Breast cancer is the most frequent malignant disease among women worldwide [1]. Early detection and accurate diagnosis and staging are very critical for patients with breast cancer to achieve effective therapy. Mammography is the recommended screening method for average-risk women by the American Cancer Society [2]. However, recent studies suggest that screening with mammography may not be as effective as originally thought and can result in significant over-diagnosis and over-treatment. Some screening results may bring patients false positive alarm which leads to follow-up examinations including biopsies [3-5]. Magnetic resonance imaging (MRI) and ultrasound are optional major modalities for diagnosis of breast cancer and recommended screening examinations for women at increased risk for breast cancer [6]. However, false-positive findings exist with all these morphological imaging modalities and a certain portion of breast cancer lesions at early stage might still be missed using MRI.
18F-FDG positron emission tomography/X-ray computed tomography (PET/CT) has become an essential imaging modality for diagnosis and staging of breast cancer [7]. It also plays an important role in evaluating regional lymph node status, monitoring treatment, and detection of recurrences [8]. However, limitations of FDG PET has been also recognized including false-positive uptake in acute and chronic infections [9] and false-negative uptake in breast cancers with less aggressive histologic subtypes [10]. Therefore, alternative PET probes targeting to tumor specific or tumor dominant antigens and receptors have been developed for detection and staging of breast cancer [11].
It has been found that both gastrin-releasing peptide receptor (GRPR) and integrin αvβ3 are overexpressed in neoplastic cells of human breast cancer [12]. GRPR is a member of the G protein-coupled receptor family of bombesin receptors, which is over-expressed in various types of cancer cells, including breast cancer [13, 14]. With high expression on both neovascular endothelial cells and tumor cells including breast cancer, integrin αvβ3 plays an important role in the regulation of tumor growth, angiogenesis, local invasiveness and metastatic potential [15-17]. Bombesin (BBN), an amphibian homolog of mammalian gastrin-releasing peptide (GRP), has been extensively used for the development of molecular probes for the imaging of GRPR after being labeled with various radionuclides [18-23]. Several bombesin related tracers have also been tested in patients with prostate cancer, breast cancer or gliomas [24-26]. The applications of integrin αvβ3 specific imaging probes, derived from RGD (arginine-glycine-aspartic acid) peptide, have also been reported in breast cancer patients [27-29].
It has been confirmed in several studies that heterodimers have advantage over the corresponding monomers due to the multi-valency effect, which lead to improved binding affinity and increased number of effective receptors [17, 30-32]. To target both GRPR and integrin αvβ3, a heterodimeric peptide BBN-RGD was synthesized from bombesin(7-14) and c(RGDyK) through a glutamate linker and then labeled with 68Ga [30]. In T47D and MDA-MB-435 breast cancer models, BBN-RGD radiotracers have shown obvious advantage and had significantly higher tumor uptake compared with monomeric RGD and monomeric BBN peptide tracer analogs at all-time points examined [30].
In this study, we evaluated the feasibility and clinical diagnosis value of 68Ga-BBN-RGD PET/CT as a novel imaging modality in patients with breast cancer, compared with 68Ga-BBN PET/CT. The correlation between imaging quantification and pathological and immunohistochemical result of GRPR and integrin αvβ3 expression within primary or metastatic breast cancer lesions was also evaluated.
Materials and Methods
This study was approved by the Ethics Committee of Peking Union Medical College Hospital and registered online at the U.S. National Institutes of Health ClinicalTrials.gov (ID: NCT02749019).
Subject Enrollment
Written informed consent was obtained from each patient before the study. The inclusion criteria were (a) female, (b) age of 18 years or older, (c) highly suspicious breast lesions on screening mammography without other therapies or underwent breast cancer radical mastectomy with confirmed pathology and suspected recurrence in another one/multiple imaging(s) follow-up results, (d) availability of histologic results from biopsy or surgery. The exclusion criteria consisted of conditions of mental illness, severe liver or kidney disease with serum creatinine > 3.0 mg/dl (270 μΜ) or any hepatic enzyme level 5 times or more than normal upper limit. Participants were also excluded if they were known to have severe allergy or hypersensitivity to IV radiographic contrast, claustrophobia to accept the PET/CT scanning, or female patients in pregnancy.
From September 2015 to April 2016, 22 patients (F, age range 29-62 y, mean age 52.5±9.3 y) were recruited to accept 68Ga-BBN-RGD PET/CT scans. Among them, 16 patients with highly suspicious breast lesions on screening mammography accepted breast ultrasound and/or magnetic resonance imaging (MRI), 6 postoperative patients accepted breast ultrasound and monitoring whole body 99mTc-MDP bone scan within 2 weeks. 11 in 22 patients also accepted 68Ga-BBN PET/CT within 2 weeks for comparison. The final diagnosis was based on the pathologic examinations. The demographics of the patients are listed in Table 1.
68Ga-BBN-RGD PET/CT
The macrocyclic chelator, 1,4,7-triaza cyclononane-N, N', N”-triacetic acid (NOTA) conjugated BBN-RGD was synthesized according to a method described in our previous publication [17]. 68Ga-BBN-RGD was prepared following the procedure reported previously [24]. The radiochemical purity was greater than 95%.
No specific subject preparation, such as fasting, was requested on the day of 68Ga-BBN-RGD PET/CT. Each patient was intravenously injected with 68Ga-BBN-RGD in a dosage of approximately 1.85 MBq (0.05 mCi) per kilogram of body weight, ranging from 75.9 to 148.0 (114.7 ± 17.1) MBq. PET/CT was performed at 25~35 min after tracer administration by using Biograph 64 Truepoint TrueV system (Siemens Medical Solutions, Knoxville, TN, USA). After a low-dose CT scan (120 kV, 35 mA, 3 mm layer, 512 × 512 matrix, 70 cm FOV), whole body PET acquisition was performed with 2 min per bed position (five to six bed positions depending on the height of the patient). The emission data were corrected for randoms, dead time, scattering, and attenuation. The conventional reconstruction algorithm was used and the images were zoomed with a factor of 1.2. The images were transferred to a MMWP workstation (Siemens) for analysis.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristics | n | % | ||
|---|---|---|---|---|
| Age (y) | ||||
| Range | 29-62 | |||
| Mean ± standard deviation | 52.5 ± 9.3 | |||
| No. of patients recruited | 22 | |||
| Newly diagnosed with suspect breast lesions | 16 | 72.7 | ||
| Malignant | ||||
| Intraductal carcinoma | 2 | 9.1 | ||
| Invasive ductal carcinoma | 3 | 13.6 | ||
| Intraductal carcinoma with infiltration | 6 | 15.7 | ||
| Invasive lobular carcinoma | 2 | 9.1 | ||
| Metaplastic Breast carcinoma | 1 | 4.5 | ||
| Benign | ||||
| Mastitis | 1 | 4.5 | ||
| Plasma cell mastitis | 1 | 4.5 | ||
| Postoperative patient | 6 | 15.7 | ||
68Ga-BBN PET/CT
8 newly diagnosed patients and 3 postoperative patients were randomly selected to accept 68Ga-BBN PET/CT within 2 weeks for comparison. No specific subject preparation including fasting, was requested on the day of 68Ga-BBN PET/CT scanning. Each patient was intravenously injected with 68Ga-BBN in a dosage of approximately 1.85 MBq (0.05 mCi) per kilogram of body weight, ranging from 77.7 to 144.3 (114.0 ± 22.3) MBq. The imaging procedure and data analysis is same as that of 68Ga-BBN-RGD PET/CT.
Image and data analysis
A Siemens MMWP workstation was used for post-processing. Visual analysis was used to determine the general biodistribution and the temporal and inter-subject stability. Regions of interest (ROIs) were drawn manually on the site of lesions using 3D ellipsoid isocontour on each image with the assistance of the corresponding CT images by two experienced nuclear medical physicians. They were different from the physicians who previously recruited the patients and interpreted the images through consensus reading and blinded to the history, other examinations, and pathologic diagnosis of the patients. Per-lesion analysis was performed for the diagnosis of breast lesions, lung, liver and bone metastases. Per-region analysis was adopted for the diagnosis of lymph node metastases because of the difficulty to correlate the lymph nodes lesion by lesion between the images and pathologic diagnosis. For semi-quantitative analysis, the results were expressed as mean and maximum standardized uptake value (SUVmean and SUVmax).
Immunohistochemical (IHC) staining
All the 16 patients with highly suspicious breast lesions on screening mammography underwent surgery breast cancer radical mastectomy within one week after 68Ga-BBN-RGD PET/CT. For each of the 6 postoperative patients, at least one part of tissue biopsy was performed. Thirty-seven specimens from primary tumor and metastases were stained with GRPR, integrin αvβ3, ER, HER-2 and Ki-67.
The representative specimens were selected by a pathologist according to the quality and quantity of the embedded tissue. All the samples were fixed with 10% neutral buffered formalin and embedded in paraffin. Five μm-thick tissue sections were blocked with endogenous peroxidase using 3% H2O2 for 20 min. Sections were then washed three times with PBS and briefly in a buffer containing 1% polymerized bovine serum albumin (BSA) and incubated with mouse anti-human monoclonal antibody against human GRPR (PA5-256791, Thermo Fisher Scientific, Rockford, IL) and integrin αvβ3 (1:200, sc-7312, Santa Cruz Biotechnology) respectively at 37 °C for 2 h. After washing with PBS, each section was incubated with corresponding horseradish peroxidase (HRP)-conjugated anti-IgG for 60 min at room temperature. Diaminobenzidine (DAB) was used as the chromogen and HE counterstaining was performed. Six fields were randomly selected from each section and observed using a light microscope (BX41, Olympus). For semi-quantification of GRPR and integrin αvβ3 expression, five entire high-power fields (×40) containing clusters of malignant cells were identified randomly per slide and scored for intensity and percentage of GRPR and integrin αvβ3 staining expression. The procedure was repeated by two independent experienced examiners. The proliferation index was calculated as (number of Ki-67-labeled cells/total number of cells) × 100% at a magnification of ×200 in five representative regions of the tumor.
The pathology was determined by two pathologists independently, and reached consensus by referring a third pathologist when there was any discrepancy. The semi-quantitative analysis was based on the following criteria. The immunostaining intensity was graded as 0, none; 1, weak; 2, moderate and 3, strong. The percentage of positive tumor cells was determined from 0 to 100. And the composite score ranging from 0 to 300 was generated by multiplying the intensity score by the percentage of cell.
Statistical analysis
The analysis was performed with the use of Prism 5.0 software (GraphPad, San Diego, CA). Continuous variables were summarized as means ± standard deviation. Categorical variables were described in numbers and percentages. The correlation between quantitative parameters was evaluated by Pearson correlation coefficient for data with normal distribution or Spearman correlation coefficient for data with skewed distribution. For the data with normal distribution, paired t test (parametric test) was used to compare the mean standard uptake value of 68Ga-BBN-RGD PET and GRPR and/or integrin αvβ3 expression from the same patients; unpaired t test was used to compare the SUVmax between the ER, HER-2 positive and negative groups. Receiver operating characteristic (ROC) curve analysis and z tests were used to compare the diagnostic performance of 68Ga-BBN-RGD PET/CT and 68Ga-BBN PET/CT. All tests were two tailed, with the significance level at 0.05.
Results
Detection of primary lesions with 68Ga-BBN-RGD PET/CT
Due to the low background in the breast area, the primary lesions can be visualized clearly with localized high signal intensities on 68Ga-BBN-RGD PET/CT with different histological subtypes (Figure 1 & 2). A total of 24 breast lesions were detected with 68Ga-BBN-RGD PET/CT in the 14 breast cancer patients including 2 intraductal carcinomas, 3 invasive ductal carcinomas, 6 intraductal carcinomas with infiltration, 2 invasive lobular carcinomas, and 1 metaplastic breast carcinoma with sizes ranging from 0.4 to 2.7 cm (mean, 1.6 ± 0.9 cm) and SUVmax from 1.7 to 8.5 (mean, 3.84 ± 2.18). The T/B ratios of 68Ga-BBN-RGD accumulation were 2.10 to 9.44 (mean, 4.74 ± 3.98). Two breast lesions from 2 patients were pathologically diagnosed as benign (mastitis and plasma cell mastitis respectively). The sizes of the lesions were 2.9 and 2.7 cm with SUVmax of 2.1 and 1.5, respectively.
In Table 2, we summarized the diagnostic value of 68Ga-BBN-RGD PET/CT. For primary breast cancer, the sensitivity, specificity, positive, negative predictive values are of 95.8% (23/24) and 60.0% (3/5), 92.0% (23/25), 75.0% (3/4), respectively.
Efficiency of 68Ga-BBN-RGD PET/CT
| Primary tumor | Lymph node metastases | |
|---|---|---|
| Sensitivity (%) | 95.8 (23/24) | 75.0 (12/16) |
| Specificity (%) | 60 (3/5) | 91.5 (54/59) |
| PPV (%) | 92.0 (23/25) | 70.5 (12/17) |
| NPV (%) | 75.0 (3/4) | 93.1 (54/58) |
| Accuracy (%) | 89.7 (26/29) | 88.0 (66/75) |
Detection of breast cancer metastases with 68Ga-BBN-RGD PET/CT
A total of 12 regions recognized as having axillary metastatic lymph nodes and sized from 0.3 to 1.7 cm (mean, 0.9 ± 0.4 cm) were detected with 68Ga-BBN-RGD PET/CT in 4 breast cancer patients. The T/B ratios of 68Ga-BBN-RGD accumulation were 1.10 to 3.71 (mean, 1.93 ± 0.85) in axillary lymph node metastases (Figure 2).
Tumor metastases to distant lymph nodes, lung and bone can also be detected with 68Ga-BBN-RGD PET/CT. For overall lymph node metastases, the sensitivity, specificity, positive, negative predictive values are of 75.0% (12/16), 91.5% (54/59), 70.5% (12/17), 93.1% (54/58), respectively. The T/B ratios of 68Ga-BBN-RGD accumulation were 3.80 to 10.7 (mean, 6.98 ± 3.04) distant lymph nodes, 2.70 to 5.35 (mean, 4.87 ± 0.34) in lung metastasis and 3.17 to 22.8 (mean, 14.9 ± 5.7) in bone metastasis, respectively (Figure 3).
68Ga-BBN-RGD PET/CT and overexpression of GRPR and integrin αvβ3
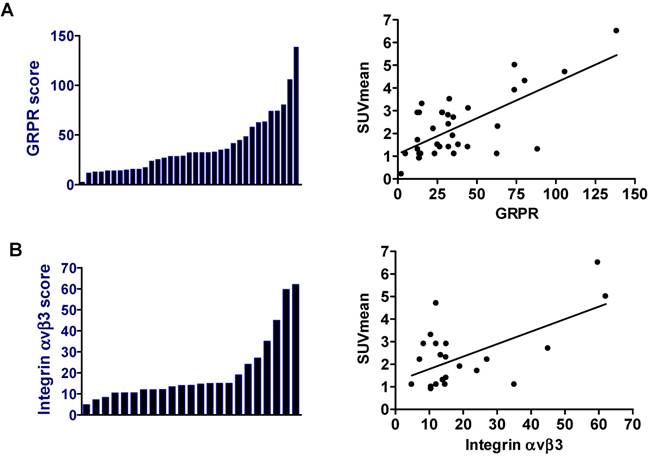
Overexpression of GRPR and integrin αvβ3 receptors on the tumor cells is expected to be the main factor for high 68Ga-BBN-RGD accumulation within tumor regions. Therefore, we performed immunostaining against GRPR and integrin αvβ3 on tumor sections. The expression of the receptor was scored by multiplying the GRPR or integrin αvβ3 immunostaining intensity and percentage of positive tumor cells. As shown in figure 4, among the 37 pathologically confirmed primary tumors and metastases, GRPR expression was absent in 2 cases (5.4%), weak in 2 cases (5.4%), moderate in 25 cases (67.6%) and strong in 8 cases (21.6%). A moderate but significant correlation between SUVmean quantified from 68Ga-BBN-RGD PET and GRPR expression was identified (r2 = 0.4791, P < 0.0001). Integrin αvβ3 expression with different extent was also found in 23 out of 37 samples. There was a significant correlation between 68Ga-BBN-RGD SUVmean and αvβ3 expression (r2 = 0.3664, P < 0.001).
68Ga-BBN-RGD PET and primary breast cancer subtypes
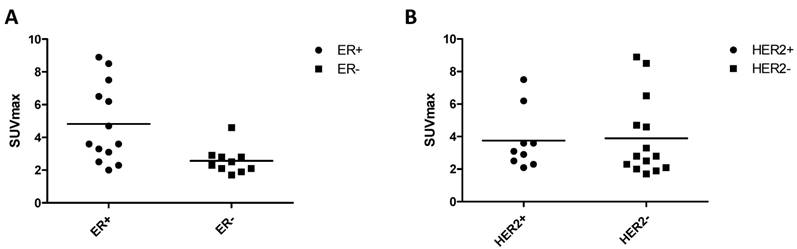
Among the 23 pathologically confirmed primary tumors, 13/23 (56.5%) lesions were identified as estrogen receptor (ER) positive and 10/23 (43.5%) negative. Meanwhile, 9/23 (39.1%) lesions were identified as human epidermal growth factor receptor-2 (HER-2) positive and 14/23 (60.9%) were negative. Ki67 proliferation index (Ki67 index) was from 5% to 90%. As shown in Figure 5, the SUVmax in 68Ga-BBN-RGD PET of ER positive group was significantly higher than ER negative group (P = 0.0083). There was no significant difference between HER-2 positive and HER-2 negative subtypes (P = 0.6589). There was also no correlation between 68Ga-BBN-RGD uptake and Ki67 index (P = 0.059).
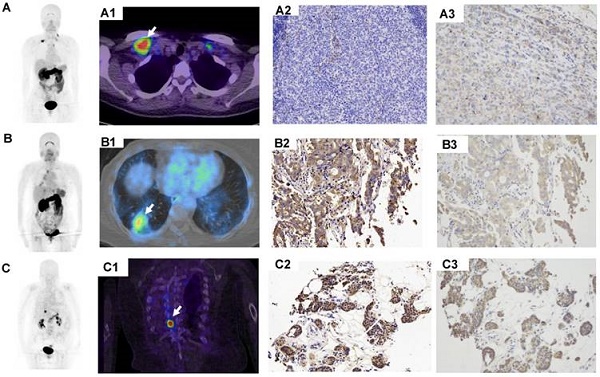
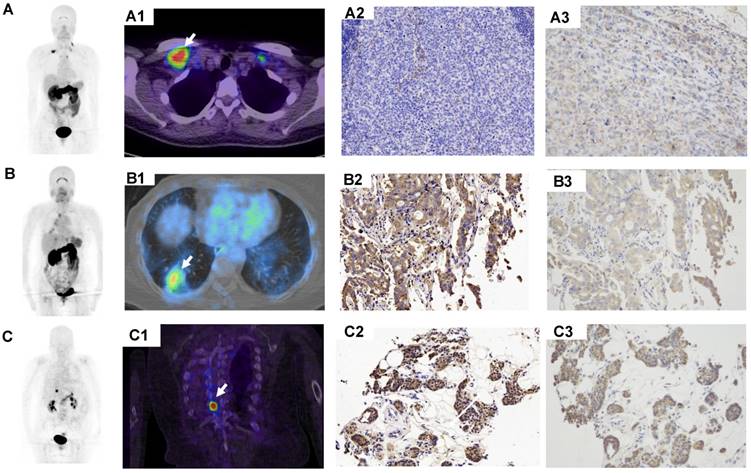
68Ga-BBN-RGD PET/CT in primary tumors with different histological subtypes of breast cancer. (A) A patient of 56y with invasive ductal carcinoma (A1-A3, arrow), estrogen receptor (ER) positive, progesterone receptor (PR) positive, human epidermal growth factor receptor- 2 (HER-2) positive. The lesion had a SUVmax of 7.5 with strong GRPR expression (A4) and weak integrin αvβ3 expression (A5). (B) A patient of 53y with invasive ductal carcinoma (B1-B3, arrow), ER negative, PR negative, HER-2 positive. The lesion had a SUVmax of 2.1 with week GRPR expression (B4) and weak integrin αvβ3 expression (B5). (C) A patient of 43y with triple negative breast cancer (TNBC) (C1-C3, arrows). The lesions had a SUVmax of 3.4 with moderate GRPR expression (C4) and weak integrin αvβ3 expression (C5).
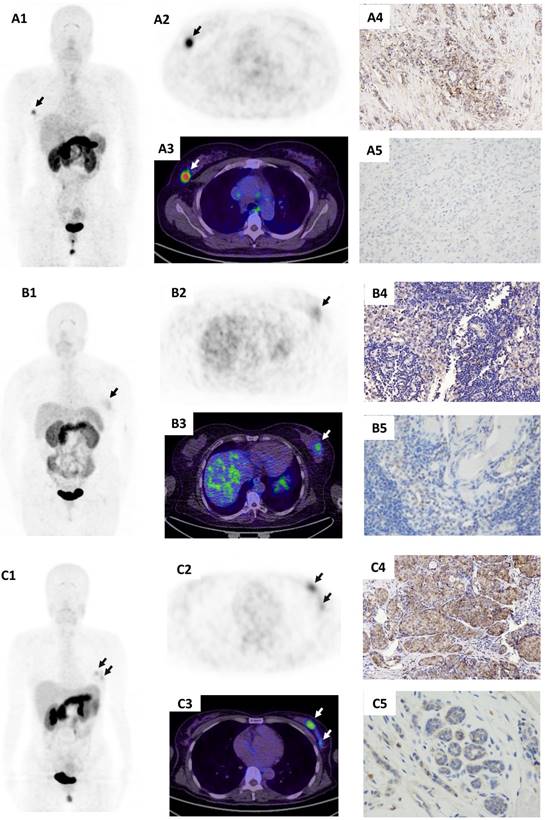
The 68Ga-BBN-RGD PET/CT for the assessment of axillary local lymph nodes in newly diagnosed breast cancer patients. (A) A patient of 47y with metaplastic breast carcinoma (spindle cell carcinoma with non-specific invasive carcinoma). The primary lesion (A2/A3) had a SUVmax of 3.3 with moderate GRPR expression (A4) and weak integrin αvβ3 expression (A5). Pathologically confirmed inflammatory lymph node was shown negative on 68Ga-BBN-RGD PET/CT (A6/A7). The lymph node showed negative GRPR expression (A8) and negative integrin αvβ3 expression (A9). (B) A patient of 62y with invasive ductal carcinoma and lymph nodes metastases. The primary tumor (B2/B3) had a SUVmax of 4.6 with weak GRPR expression (B4) and moderate integrin αvβ3 expression (B5). Lower panel revealed a pathological confirmed metastatic lymph node, which is positive on 68Ga-BBN-RGD PET/CT with a SUVmax of 1.7 (B6/B7). The lymph node shows moderate GRPR expression (B8) and negative integrin αvβ3 expression (B9).
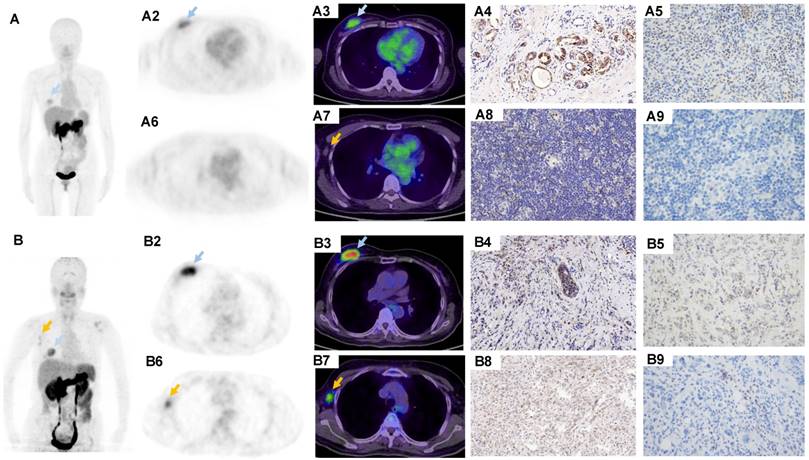
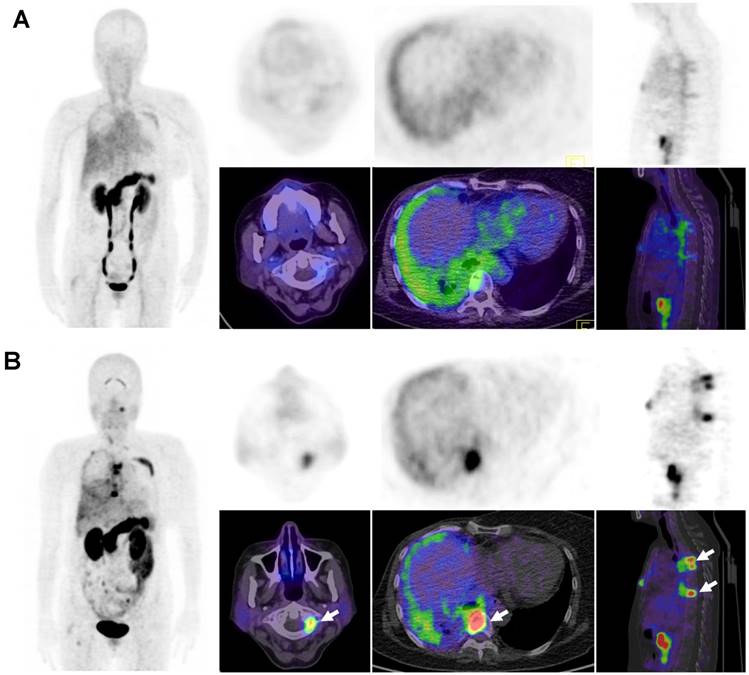
68Ga-BBN-RGD PET/CT in the metastasis of breast cancer. (A) A 43-y-old woman underwent breast cancer radical mastectomy ten months ago. 68Ga-BBN-RGD PET/CT detected multiple lymph nodes metastasis (A1) with SUVmax of 8.8. (B) A 58-year-old woman with invasive ductal carcinoma underwent breast cancer radical mastectomy 1 year ago. The pathology confirmed lung metastasis (B1) with SUVmax of 4.8 shows strong GRPR expression (B2) and strong integrin αvβ3 expression (B3). (C) A patient of 68y with invasive lobular carcinoma underwent breast cancer radical mastectomy 1 year ago. The pathology confirmed bone metastasis (C1) with SUVmax of 8.3 shows strong GRPR expression (C2) and strong integrin αvβ3 expression (C3).
(A) Expression of GRPR in primary tumor and metastasis samples from patients with breast cancer (left). Correlation of SUVmean determined by 68Ga-BBN-RGD PET and GRPR expression level determined by immunohistochemical staining (r2 = 0.4791, P < 0.0001) (right). (B) Expression of integrin αvβ3 in primary tumor and metastasis samples from patients with breast cancer (left). Correlation of SUVmean determined by 68Ga-BBN-RGD PET and integrin αvβ3 expression level determined by immunohistochemical staining (r2 = 0.3664, P < 0.001) (right).
Association of 68Ga-BBN-RGD uptake and biomarkers of breast cancer identified by pathologic diagnosis. There was significant difference between ER positive and ER negative group (A) (P=0.0485), but was no significant difference of SUVmax between HER2 positive and negative subtype (B) (P=0.4410).
Comparison of 68Ga-BBN-RGD PET/CT with 68Ga-BBN PET/CT
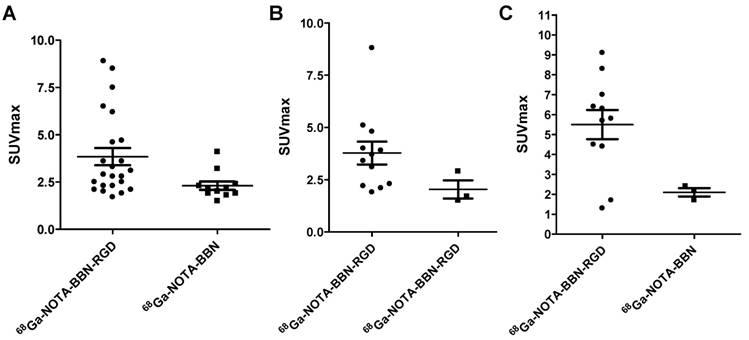
Among the 22 patients, 11 patients accepted both 68Ga-BBN-RGD PET/CT and 68Ga-BBN PET/CT within two weeks to facilitate a case-by-case comparison. As shown in Figures 6 and 7, 68Ga-BBN-RGD PET/CT showed better primary tumor detection with an overall SUVmax of 3.84 ± 2.18, which was significantly higher that of 68Ga-BBN PET/CT (2.31 ± 0.72, P < 0.05). 68Ga-BBN-RGD PET/CT also showed higher SUVmax in bone metastases than 68Ga-BBN PET/CT (5.50 ± 2.43 vs. 2.17 ± 0.57, P < 0.05). In lymph node metastases, the SUVmax of 68Ga-BBN-RGD PET/CT was higher than that of 68Ga-BBN PET/CT but without statistical significance (3.78 ± 1.90 vs. 2.03 ± 0.76, P > 0.05). With the 11 patients underwent two PET scans, 68Ga-BBN-RGD PET/CT detected 13 suspected primary tumor lesions in 9 patients, 8 metastatic lymph nodes, 9 bone metastases and 4 lung metastases. In comparison, 68Ga-BBN PET/CT detected 11 suspect primary tumor lesions in 7 patients, 3 metastatic lymph nodes, 3 bone metastases and 2 lung metastases.
Comparison of 68Ga-BBN PET/CT (A) and 68Ga-BBN-RGD PET/CT (B) in a 57-year old woman underwent breast cancer radical mastectomy and follow-up. More tumor lesions were shown with significantly higher maximum standardized uptake value (SUVmax) on 68Ga-BBN-RGD PET/CT (arrows) than that on 68Ga-BBN PET/CT.
Quantitative comparison between 68Ga-BBN-RGD PET/CT and 68Ga-BBN PET/CT in primary tumors (A), lymph node metastases (B), and bone metastases (C).
Discussion
The advantage of heterodimer over the corresponding monomers has been confirmed in numerous preclinical studies and several clinical investigations due to the multi-valency effect, resulting in improved binding affinity and increased number of effective receptors [17, 30-33]. Previously, we have demonstrated the safety and clinical diagnosis value of the heterodimeric imaging probe 68Ga-BBN-RGD PET/CT in prostate cancer patients [33]. In view of high levels of GRPR and integrin αvβ3 in breast cancers [27, 34], in this prospective pilot study we applied 68Ga-BBN-RGD PET/CT in patients with breast cancer. The results demonstrated that 68Ga-BBN-RGD is superior to the monomeric imaging probe 68Ga-BBN in visualizing both primary lesions and metastases.
To further delineate the tracer uptake in tumor region, the receptor expressions in both primary lesions and remote metastases were evaluated by immunohistochemical staining against either GRPR or integrin αvβ3. GRPR expression is positive in 35 out of 37 (94.6%) primary tumor and metastases while integrin αvβ3 is positive in 23 out of 37 samples (62.2%). Morgat et al. [34] screened invasive breast cancers by immunohistochemistry for the presence and intensity of GRPR expression and found that GRPR is overexpressed in 83% of ER-positive tumors and 94.6% of lymph node metastases. Gugger and Reubi also found GRPR to be expressed in 32/52 invasive breast carcinomas [35]. Therefore, our staining results are consistent with those reported previously. Significant correlations were identified between SUVmean determined by 68Ga-BBN-RGD PET and GRPR (r2 = 0.4791, P < 0.0001) and integrin αvβ3 (r2 = 0.3664, P < 0.001). The correlation was more significant when the sum of GRPR and integrin αvβ3 was used. However, due to the different binding affinities of BBN moiety and RGD moiety to their corresponding receptors, the simple add-up may not be the best way for the calculation.
Various GRPR targeting probes have been developed for noninvasive imaging of breast cancer. Stoykow et al. [36] reported GRPR imaging in breast cancer using the GRPR antagonist 68Ga-RM2, visualized 13/18 (72.2%) primary tumors and identified metastasis. In another pilot clinical study, 4 out of 8 (50%) breast cancer patients showed pathological uptake on PET/CT with another GRPR targeting probe 68Ga-SB3 [26]. In 11 patients with head-to-head comparison between 68Ga-BBN-RGD PET/CT and 68Ga-BBN PET/CT, primary lesions were detected in 7 out of 11 (63.6%) patients with 68Ga-BBN PET/CT while 9 out 11 (81.8%) patients were detected by 68Ga-BBN-RGD PET/CT with higher tumor SUV (P < 0.05). Moreover, the heterodimeric probe BBN-RGD is also more sensitive in tumor metastasis visualization. Increased number of effective receptors of 68Ga-BBN-RGD than 68Ga-BBN should be the main reason for the phenomenon observed, although other factors such as optimized pharmacokinetics cannot be excluded. Compared with conventional modalities, functional biomarkers based nuclear imaging provides molecular information on tumor lesions of breast cancer and may facilitate the early diagnosis, more accurate staging and individualized treatment planning for breast cancer patients [37]. The heterodimeric imaging probes may be more adapted to the molecular heterogeneity of breast cancers.
There are several limitations of this study. The first one is the relatively small number of patients which might not be sufficient to provide accurate diagnostic value of the novel PET tracer. Another limitation is false-positive cases occurred in 2/25 (8.0%) patients, along with pathologically confirmed expression of GRPR and integrin αvβ3. In addition, we were not able to compare this dual integrin αvβ3 and GRPR targeting probe with RGD PET in the same group of patients due to ethics consideration.
Overall, the accurate diagnosis and prognosis value of 68Ga-BBN-RGD warrants further larger scale clinical investigations. The relatively high SUV value also offers unique perspectives for targeted radionuclide therapy after labeling the heterodimer with therapeutic radioisotopes.
In conclusion, this study demonstrated significant uptake of a new type of dual integrin αvβ3 and GRPR targeting agent in both the primary lesion and the metastases of breast cancer. 68Ga-BBN-RGD PET/CT may be of great value in discerning both primary breast cancers and metastases, which indicates the efficiency of 68Ga-BBN-RGD PET/CT in diagnosis, staging and surgery guidance for the patients with breast cancer.
Acknowledgements
This work was supported by the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), and the National Natural Science Foundation of China projects ( 81171369, 81171370, 81271614, 81701742 and 81601551), Beijing Natural Science Foundation (7174338), PUMCH Science Fund of Key Projects for Junior faculty (pumch-2016-1.4), PUMC Youth Fund and the Foundamental Research Funds for the Central Universities (2017320003).
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29
2. Bjurstam NG, Bjorneld LM, Duffy SW. Updated results of the Gothenburg Trial of Mammographic Screening. Cancer. 2016;122:1832-5
3. Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089-96
4. Granovetter M. Inadvisable breast and prostate cancer screenings in the USA. Lancet Oncol. 2016;17:e92
5. Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013 CD001877
6. Raikhlin A, Curpen B, Warner E, Betel C, Wright B, Jong R. Breast MRI as an Adjunct to Mammography for Breast Cancer Screening in High-Risk Patients: Retrospective Review. Am J Roentgenol. 2015;204:889-97
7. Czernin J. FDG-PET in breast cancer: a different view of its clinical usefulness. Mol Imaging Biol. 2002;4:35-45
8. Lim HS, Yoon W, Chung TW, Kim JK, Park JG, Kang HK. et al. FDG PET/CT for the detection and evaluation of breast diseases: usefulness and limitations. Radiographics. 2007;27(Suppl 1):S197-213
9. Bakheet SM, Powe J, Kandil A, Ezzat A, Rostom A, Amartey J. F-18 FDG uptake in breast infection and inflammation. Clin Nucl Med. 2000;25:100-3
10. Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006;98:267-74
11. Chudgar AV, Mankoff DA. Molecular Imaging and Precision Medicine in Breast Cancer. PET Clin. 2017;12:39-51
12. Beer M, Montani M, Gerhardt J, Wild PJ, Hany TF, Hermanns T. et al. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate. 2012;72:318-25
13. Yang M, Gao H, Zhou Y, Ma Y, Quan Q, Lang L. et al. 18F-labeled GRPR agonists and antagonists: a comparative study in prostate cancer imaging. Theranostics. 2011;1:220
14. Maina T, Bergsma H, Kulkarni HR, Mueller D, Charalambidis D, Krenning EP. et al. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:964-73
15. Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91-100
16. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-87
17. Liu Z, Yan Y, Liu S, Wang F, Chen X. 18F, 64Cu, and 68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug Chem. 2009;20:1016-25
18. Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32:733-40
19. Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC. et al. [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci U S A. 2007;104:12462-7
20. Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR. et al. microPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med. 2004;45:1390-7
21. Mansi R, Wang X, Forrer F, Waser B, Cescato R, Graham K. et al. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur J Nucl Med Mol Imaging. 2011;38:97-107
22. Carlucci G, Kuipers A, Ananias H, de Paula Faria D, Dierckx R, Helfrich W. et al. GRPR-selective PET imaging of prostate cancer using [18F]-lanthionine-bombesin analogs. Peptides. 2015;67:45-54
23. Pourghiasian M, Liu Z, Pan J, Zhang Z, Colpo N, Lin K-S. et al. 18F-AmBF3-MJ9: A novel radiofluorinated bombesin derivative for prostate cancer imaging. Bioorg Med Chem. 2015;23:1500-6
24. Zhang J, Li D, Lang L, Zhu Z, Wang L, Wu P. et al. 68Ga-NOTA-Aca-BBN(7-14) PET/CT in healthy volunteers and glioma patients. J Nucl Med. 2016;57:9-14
25. Sah BR, Burger IA, Schibli R, Friebe M, Dinkelborg L, Graham K. et al. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J Nucl Med. 2015;56:372-8
26. Maina T, Bergsma H, Kulkarni HR, Mueller D, Charalambidis D, Krenning EP. et al. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:964-73
27. Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P. et al. Patterns of αvβ3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med. 2008;49:255-9
28. Mittra ES, Goris ML, Iagaru AH, Kardan A, Burton L, Berganos R. et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182-91
29. Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30-47
30. Li Z-B, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. Journal of Nuclear Medicine. 2008;49:453-61
31. Liu Z, Yan Y, Liu S, Wang F, Chen X. 18F, 64Cu, and 68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjugate Chem. 2009;20:1016-25
32. Eder M, Schafer M, Bauder-Wust U, Haberkorn U, Eisenhut M, Kopka K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate. 2014;74:659-68
33. Zhang J, Niu G, Lang L, Li F, Fan X, Yan X. et al. Clinical Translation of a Dual Integrin αvβ3- and gastrin-releasing peptide receptor-targeting PET radiotracer, 68Ga-BBN-RGD. J Nucl Med. 2017;58:228-34
34. Morgat C, MacGrogan G, Brouste V, Velasco V, Sevenet N, Bonnefoi H. et al. Expression of gastrin-releasing peptide receptor (GRPR) in breast cancer and its association with pathological, biological and clinical parameters: a study of 1432 primary tumors. J Nucl Med. 2017;58:1401-7
35. Gugger M, Reubi JC. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am J Pathol. 1999;155:2067-76
36. Stoykow C, Erbes T, Maecke HR, Bulla S, Bartholoma M, Mayer S. et al. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics. 2016;6:1641-50
37. Dalm SU, Verzijlbergen JF, De Jong M. Review: receptor targeted nuclear imaging of breast cancer. Int J Mol Sci. 2017;18:260
Author contact
![]() Corresponding author: Zhaohui Zhu (zhuzhhcn); Xiaoyuan Chen (shawn.chengov)
Corresponding author: Zhaohui Zhu (zhuzhhcn); Xiaoyuan Chen (shawn.chengov)
 Global reach, higher impact
Global reach, higher impact