13.3
Impact Factor
Theranostics 2018; 8(12):3348-3365. doi:10.7150/thno.23453 This issue Cite
Review
Extracellular Vesicles as Markers and Mediators in Sepsis
1. Ludwig Boltzmann Institute for Experimental and Clinical Traumatology, Trauma Research Center of AUVA, Vienna, Austria.
2. Department of Anaesthesia, General Intensive Care and Pain Management, Medical University of Vienna, Vienna, Austria.
Received 2017-10-23; Accepted 2018-3-14; Published 2018-5-23
Abstract
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. It remains a highly lethal condition in which current tools for early diagnosis and therapeutic decision-making are far from ideal. Extracellular vesicles (EVs), 30 nm to several micrometers in size, are released from cells upon activation and apoptosis and express membrane epitopes specific for their parental cells. Since their discovery two decades ago, their role as biomarkers and mediators in various diseases has been intensively studied. However, their potential importance in the sepsis syndrome has gained attention only recently. Sepsis and EVs are both complex fields in which standardization has long been overdue. In this review, several topics are discussed. First, we review current studies on EVs in septic patients with emphasis on their variable quality and clinical utility. Second, we discuss the diagnostic and therapeutic potential of EVs as well as their role as facilitators of cell communication via micro RNA and the relevance of micro-organism-derived EVs. Third, we give an overview over the potential beneficial but also detrimental roles of EVs in sepsis. Finally, we focus on the role of EVs in selected intensive care scenarios such as coagulopathy, mechanical ventilation and blood transfusion. Overall, the prospect for EV use in septic patients is bright, ranging from rapid and precise (point-of-care) diagnostics, prevention of harmful iatrogenic interventions, to using EVs as guides of individualized therapy. Before the above is achieved, however, the EV research field requires reliable standardization of the current methods and development of new analytical procedures that can close the existing technological gaps.
Keywords: sepsis, inflammation, point-of-care, microparticles, exosomes
Introduction
Sepsis was recently redefined as life-threatening organ dysfunction caused by a dysregulated host response to infection. For decades, it has remained a highly lethal condition in which dependable diagnostics and therapeutic decision-making are far from optimal. Currently, sepsis incidence is estimated at 270 cases per 100,000 persons/year followed by an approximate 26% mortality rate [1,2]. Programs such as the Surviving Sepsis Campaign [3] and World Sepsis Day have increased awareness to the global burden of sepsis as well as the related diagnostic/ therapeutic difficulties. Both the Surviving Sepsis Campaign and the most recent Sepsis-3 definitions stress a dire need for reliable biomarkers. The current Surviving Sepsis Guidelines are limited to a weak recommendation regarding the use of procalcitonin for shortening/discontinuation of ongoing antimicrobial treatment only and encourage evaluation of further markers, e.g., for renal dysfunction and coagulopathy [2,3]. An ideal sepsis biomarker should possess a diagnostic and predictive value (high sensitivity, high specificity), display low donor variability, be measured in a rapid and cost-effective bedside assay and withstand validation in multicenter trials [4]. Establishing a biomarker of sepsis should not be merely limited to correlating its concentration in the blood (and/or other fluids) with a particular disease phase and/or clinical outcome. It should also govern further management, e.g., the initiation of further diagnostics, escalation or de-escalation of a specific antibiotic regimen and initiation or discontinuation of invasive and extracorporeal treatments.
Many compounds with biological impact to the host defense are detectable in body fluids at elevated levels during sepsis and other states accompanied by an acute inflammatory response. Several inflammatory cytokines (circulating peptides) like tumor necrosis factor (TNF)-alpha, interleukin (IL)-1-beta and IL-6 have been identified as markers of sepsis severity and outcome predictors, and were targeted in several clinical trials [5-8]. The failure of the trials targeting the “cytokine storm” was surprising and disappointing; it is now known that their design was based on a false assumption that deaths from sepsis were primarily attributable to the overwhelming synthesis of cytokines (i.e., hyperinflammation) [9,10]. The newest evidence shows that sepsis responses are multifactorial and fluctuate rapidly [9]. Thus, it is not the abundance of one particular cytokine but rather a specific complex inflammatory “signature” that accurately depicts a patient's immune status as well as the probable prognosis and response to treatment [9,11].
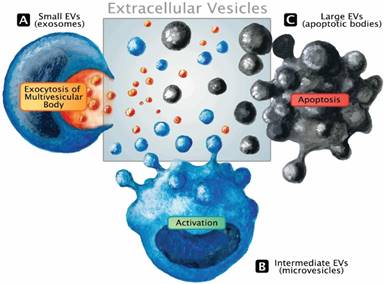
Only recently, the role of exosomes in critical illness has been summarized [12]. This small population (size range 30-100 nm) of EVs derived from the endosomal system represents only a subset of the extracellular messenger pool. EVs are 30 nm to several micrometers in size, formed either by outward budding of the plasma membrane upon activation, during programmed cell death (i.e., apoptosis), or by exocytosis of multivesicular bodies [13]. They express epitopes on their membranes that are specific for their parental cells. A review on their function in health and disease has been published recently [14]. Systematic assessment of EVs in sepsis is an area of diagnostics that may support the developing concept of patient-tailored therapy by filling up the wide biomarker gap. Release of EVs represents an immediate cell communication mechanism that fulfills three major functions: a) transfer of an expression pattern to other cells, b) dissemination of the membrane-bound mediators within the liquid phase and c) rapid rearrangement of the cell surface. Like all messengers, EVs can immediately modify a phenotype of neighboring cells in a paracrine fashion but also bring forward an altered micro-milieu to other tissues by transferring the membrane composition and/or expression pattern to other regions of the circulation (endocrine release). Figure 1 illustrates the different types of EVs and basic modes of cellular release. The existing literature suggests that EV monitoring can effectively aid in the identification and characterization of specific pathophysiological blueprints of a given septic patient as well as in assessing the efficacy of various therapeutic regimens available to that patient. Over the last two decades, a large number of original studies investigating EVs in sepsis have been published. However, more is not always better, and a large number of experiments does not automatically ensure clinical progress.
Release mechanisms and the extracellular vesicle pool. EVs constitute a dynamic pool with a considerable range in size. The classic term microparticles (100-1000 nm, intermediate size range) contains the EV fraction that is sometimes referred to as microvesicles or ectosomes and is released from the cell surface in response to activation (B). Although larger, apoptotic bodies that emerge during the disintegration of dying cells well range into the size spectrum of apoptotic microparticles, making it impossible to distinguish the underlying biogenesis solely based on dimensions (C). Exosomes emerge from the endosomal network and are considered the smallest fraction of the EV pool. Undergoing active packaging processes, exosomes are eventually liberated by the fusion of the multivesicular body with the surface membrane (A).
This review has several objectives. First, we provide an analysis of the 40 existing studies on EVs in septic patients (summarized in Table 1) with a focus on their study design. This is important given that EVs have been suggested to serve as diagnostic biomarkers [15], while the methods for their detection and data interpretation in complex diseases such as sepsis are still under development. This translates into large variability in the quality, usefulness and/or comparability among the existing sepsis-related studies. Second, we focus on the role of EVs as biomarkers and mediators in sepsis as well as emphasize their potential utility to guide treatments and their role as potential targets for treatment. Third, we debate whether EVs are a meaningful part of the adequate evolutionary host response or also act as involuntary players of the inflammatory response [15]. Finally, we characterize the influence of EVs in the exemplary, most severe clinical manifestation of sepsis (i.e., disseminated intravascular coagulation, DIC) and common treatment routines (i.e., blood transfusion, mechanical ventilation) used in the intensive care unit (ICU). While a systematic analysis of all existing in vitro and preclinical studies was not the main scope of this review, several preclinical papers relevant to the discussed topics are also included.
Heterogeneity versus standardization: critical assessment of EVs studies
In order to be useful, data from in vitro/ex vivo, preclinical (no patients involved) studies and clinical (patients-based) studies must meet several stringent criteria including: 1) best possible match between the research model and a given clinical disease scenario, 2) an adequate study design, sample sources and sampling time points, 3) adequate statistical power, 4) standardized detection assays and 5) reproducibility. In a complex disorder like sepsis, mediators like EVs dynamically fluctuate and those changes may play a different (if not opposite) role in various body compartments as well as in the local and systemic circulation.
The intensive care unit (ICU) patient populations in general, but especially those fulfilling sepsis criteria [2], are extremely heterogeneous. They present with variations in genetic background, age, gender, accompanying diseases, chronically prescribed medications, source of infection (medical or surgical) and pathogens involved. Heterogeneity has been one of the major hindrances in sepsis research, particularly affecting the development of therapies. On the one hand, studies performed in heterogeneous cohorts better reflect the entire population and are easier to power, making any novel findings more robust and applicable to a wider target population. Conversely, large patient cohorts can also mask potential benefits (and harms) of any novel treatment in defined, smaller patient sub-groups. Although retrospective analyses may enable post-hoc detection of such benefits, the investigative value of such findings is weaker. Below, we present several key elements that modify the perception and understanding of EV-based research in the clinical context. The “sepsis” diagnosis encompasses patients with a multitude of scenarios by which the microbial invasion develop. To our knowledge, only one recent study compared the role of EVs among subsets of septic patients based on the site of infection [16].
For example, at least 19 of the analyzed studies (Table 1) were performed in cohorts with mixed sites of infection (i.e., pneumonia, intra-abdominal infection, urinary tract infection, necrotizing fasciitis, wound infection, meningitis, mediastinitis) and underlying pathologies and injuries (i.e., trauma, cancer, immunosuppression). The relatively small number of existing clinical EVs studies exacerbates the difficulty of any reliable cross-comparisons. It is currently unclear whether the origin of the infection focus in any way modulates the EVs characteristics and their signaling routes and/or diagnostic potential. Although different anticoagulants for blood samples were used, citrate predominated. This is appropriate as citrate is recommended for achieving stable EVs counts throughout the initial processing period [17]. In the context of potential EV utilization, the heterogeneity of the sepsis population remains one of the most significant hurdles, necessitating careful planning (e.g., only including patients with site-specific sepsis focus) and reliance on clear definitions and reporting.
Most recently, sepsis has been redefined as a dysregulated host response to infection and its gradation largely rests upon the state of organ dysfunction present in the patient [2]. The latter element partly overlaps with the earlier definition of severe sepsis [18] and can occur in the presence of underlying circulatory and cellular/metabolic abnormalities which are profound enough to additionally increase mortality (defined as “shock”). Given that sepsis and septic shock often develop from an initially non-life-threatening infection, the influence of EVs during this phase may be misinterpreted if patients enrolled for clinical studies are not meticulously selected based on the exact diagnosis and/or disease phenotype. In general, most EV studies were performed in patients suffering from severe sepsis (i.e., including organ dysfunction) according to the former definition, which is referred to as 'sepsis' according to the recent Sepsis-3 definitions [2] (see Table 1). In at least 14 studies, the septic patients were in the state of shock. It is unknown whether re-analysis of sepsis studies using the new Sepsis-3 definitions for enrollment (and thus redistributing groups) would change their conclusions; new studies selecting patients based on the new Sepsis-3 have been only recently emerging [16]. Interestingly, redistribution of patients according to the Sepsis-3 definition did not affect conclusions drawn in the recent study by Matsumoto et al. [19].
In patients, the exact time of the onset of a septic episode is typically unknown. Repetitive blood sampling from intravascular catheters at least once daily is a routine practice in the ICU. In the studies analyzed in Table 1, EVs were characterized based on only one sampling time-point in 23 studies, and on multiple time-points (i.e., time course) in 17 studies. Additionally, marked variation existed regarding the time that elapsed between sepsis diagnosis and the draw of the first blood sample enrolled into the study. These factors related to timing of samples may hinder precise and/or protracted characterization of EV-related events in a rapidly fluctuating disease. Another critical factor is the choice of optimal and defined inclusion/exclusion criteria. EVs are ubiquitously detectable and have pleiotropic effects. Therefore, multiple preexisting disorders may confound the EV concentration in septic patients. The most frequent exclusion criteria were: a) diabetes mellitus [20], b) statin treatment [21], c) drugs altering platelet function [22,23], d) recent blood product transfusion [22,23], e) any hematologic malignancy, and f) other types of cancer [24]. As a result, a substantial part of average ICU septic patients was excluded, potentially rendering the population represented by the existing studies too specific for unbiased comparison with the general ICU population. Future studies could address this problem by maximally reducing exclusion criteria while ensuring a meticulous documentation of patient characteristics. This could be supported by an EV-oriented checklist that helps authors to include the most important patient information and minimal experimental requirements for definition of EVs and their functions [25,26]. Table 1 shows that those studies that described mortality rates in sepsis and/or septic shock reported them within a relatively narrow range. This is appropriate given that it enables cross-comparison among studies. Of note, sepsis mortality was above 50% in four studies [27-30].
In summary, the analyzed studies (Table 1) enrolled highly heterogeneous septic patient populations with a) diverse exclusion criteria, b) different sites of infection, c) varying disease severity and phenotype, and d) mostly single or double sampling time-points only. Therefore, any future sepsis studies targeting EV sequelae should account for the above discussed elements in their study design. Any improvement of study inter-comparability by amenable standardization procedures will directly enhance their clinical translatability.
Characteristics of clinical patient studies investigating the role of extracellular vesicles (EV) in sepsis.
| Study | Diagnosis | Mortality | Time to first sample | Sampling time points | Anticoagulant | Cohort size | EV sources | Summary of findings |
|---|---|---|---|---|---|---|---|---|
| 1. Larsson (1996) [151] | Gram-negative sepsis on ICU | Unknown | At day of ICU admission | Day 1, 2, 4 and 6 | Citrate | Sepsis (n=4) | Platelets | 3 out of 4 septic patients showed high PEV count (no statistics). |
| 2. Lundahl (1996) [152] | Mixed ICU population | Unknown | Unknown | Once | Citrate | ICU patients (n=19), healthy (n=20) | Platelets | Increased PEV (no statistics) in 2 out of 8 patients who died shortly after sampling. |
| 3. Nieuwland (2000) [53] | Meningococcal septic shock | 5 survivor, 2 non-survivors | <24 h | At 0, 4, 10, 16, 24, 36 h | ethylene diamine tetraacetic acid | Septic shock (n=7), healthy (n=5) | Platelets, ECs, granulocytes, monocytes, erythrocytes | Only PEV and GEV increased in septic vs. healthy on admission. Only GEV decreased within 10h of admission. |
| 4. Joop (2001) [28] | Mixed severe sepsis | 4 survivors, 5 non-survivors at day 28 | Unknown | Once | Citrate | Severe sepsis (n=9), healthy (n=14) | Platelets, erythrocytes, ECs, granulocytes, TF+ cells | AnnV+/CD61+ higher, AnnV-/CD61+ lower, AnnV-/glycophorinA+ higher, AnnV-/ CD62E+ lower, AnnV+/ CD66b+ and AnnV-/ CD66b+ higher, AnnV+/CD142+ lower in sepsis vs. healthy. |
| 5. Ogura (2001) [92] | Mixed sepsis, trauma + SIRS, all with C-reactive protein >10 mg/dl | Unknown | 2-7 d post trauma | Once | Citrate | Sepsis (n=14), SIRS (n=12) healthy (n=12) | Platelets | PEV/platelet count higher in sepsis vs. healthy, no difference trauma vs. sepsis. |
| 6. Fujimi (2002) [153] | Mixed sepsis with CRP>10 mg/dL | Unknown | <24 h | Once | Heparin | Sepsis (n=21), healthy (n=21) | Granulocytes | GEV counts higher in septic vs. healthy. Enhanced expression of CD11b in sepsis vs. healthy on GEV < 1 µm but not GEV > 1 µm. |
| 7. Janiszewski (2004) [154] | Septic shock | Unknown | <24 h post diagnosis | Once | Heparin | Septic shock (n=16), healthy (n=6) | Platelets, leukocytes, monocytes, granulocytes, ECs | PEVs exposed p22phox and gp91phox, exhibited intrinsic ROS production and enhanced apoptosis (vs. healthy volunteers) after incubation with ECs or vascular smooth muscle cells. Effects reversible by addition of ROS antagonists. |
| 8. Soriano (2005) [30] | Mixed severe sepsis | 51.4% at day 28 | 24-48 h after organ failure | At admission, day 1 and day 2 | Citrate | Severe sepsis (n=35), healthy (n=45) | ECs, platelets | EEV, not PEV and EEV-monocyte conjugate count was higher in septic patients on day 1 vs. healthy controls. EEV-monocyte conjugates at all time points were higher in non-survivors vs. survivors. |
| 9. Gambim (2007) [155] | Mixed septic shock | Unknown | <24 h after diagnosis | Once | Citrate | Septic shock (n=12), healthy (n=10)) | Platelets | EV from human platelets were similar to septic patients-derived PEV after in vitro exposure to NONOate and LPS, but not to TNF-alpha or thrombin, generated superoxide and nitric oxide. ECs incubated with EVs underwent caspase-3 activation/apoptosis, inhibited by ROS antagonists. |
| 10. Azevedo (2007) [156] | Septic shock | Unknown | <48 h after diagnosis | Once | Heparin | Septic shock (n=55), healthy (n=12) | Platelets | Exosomes from septic patients decreased maximal DT and positive dT/dt in rat papillary muscle preparations and positive dT/dtmax in isolated rabbit hearts pre-exposed to LPS. Exosomes from septic patients showed intrinsic NO production and induced myocardial NO content. No effects of exosomes from septic patients on isolated rabbit heart not pre-exposed to LPS and rat aortic ring contractility. |
| 11. Huisse (2008) [157] | Severe sepsis | 14% in-hospital mortality | Unknown | Once | Citrate | Severe sepsis (n=14), heat-stroke (n=18), healthy (n=18) | Total EVs, platelets, monocytes, granulocytes, ECs | Severely septic patients showed lower PEVs, increased GEVs and MEVs and similar EEVs vs. healthy volunteers. |
| 12. Mostefai (2008) [105] | Mixed septic shock | 28% at day 28 | 10 h post ICU admission | Once | Citrate | Septic shock (n=36), ICU non-septic (n=18) | Total EVs, platelets, ECs, leukocytes, granulocytes, monocytes, erythrocytes | Total EVs, PEVs, EEVs, L-Selectin+EVs and P-Selectin+EVs were increased, LEVs were decreased, and GEVs, MEVs, EryEVs and AnnV+ EVs were not different in septic vs. non-septic ICU patients. |
| 13. Pérez-Casal (2009) [158] | Severe sepsis, rhAPC treatment | Unknown | At start of rhAPC infusion | Days 0, 1 and 4 of rhAPC infusion | Citrate | Severe sepsis with rhAPC (n=4), severe sepsis without rhAPC (n=4) | EPCR+ EVs | Co-localization of EPCR and APC on EVs from septic patients during, but not before, rhAPC infusion. APC on EPCR+ EVs was higher during vs. before (individual controls) rhAPC treatment and vs. non-rhAPC-treated controls. |
| 14. Forest (2010) [159] | SIRS, mixed sepsis or septic shock | 0% in all SIRS patients, 0% in <50 y sepsis, 26% in ≥75 y sepsis | Within 1 h of hospital admission | Once | Citrate | <50y SIRS (n=26), <50y sepsis (n=27), ≥75y SIRS (n=31), ≥75y sepsis (n=27) | ECs, erythrocytes, platelets | Lower EEVs, but the same EV procoagulant activity in older vs. younger patients. Lower EEVs in sepsis vs. SIRS in young patients. Lower EV procoagulant activity in sepsis vs. SIRS in both old and young patients. Elderly sepsis non-survivors had higher EEVs vs. elderly survivors. |
| 15. Rank (2011) [160] | Sepsis | Unknown | Before conditioning | Twice a week for 30 days, thereafter once a week until discharge | Citrate | hematopoietic stem-cell transplantation (n=19), incl. infection/sepsis (n=15) | Erythrocytes | EryEVs level was affected only by development of graft-versus-host-disease but not conditioning therapy, total body irradiation, high-dose chemotherapy, in vivo T-cell depletion, aplasia or engraftment in uncomplicated patients, infectious complications and/or sepsis. |
| 16. Pérez-Casal (2011) [70] | Severe sepsis (Pneumonia or intraabdominal infection), rhAPC indication | 36% at day 28 | Start of RhAPC | Before RhAPC infusion and at day 2, 3, 4, 5 and 6 | Citrate | Severe sepsis with rhAPC (n=25) or without rhAPC (n=25), healthy (n=6) | EPCR+EV, APC+EV, myeloid cell line | Increased circulating EV carrying EPCR and APC expression after rhAPC. Decrease of endothelial permeability by APC+EVs via PAR-1. |
| 17. Prakash (2012) [101] | Septic peritonitis, mixed ICU with suspected ventilator-associated pneumonia or lung donors | Unknown | Unknown | Once | N/A (abdominal lavage fluid or BAL fluid within 2h of collection) | Septic peritonitis (n=3), control abdominal lavage fluid from non-septic laparotomy (n=4), ICU suspected pneumonia BAL (n=33), control BAL from lung donor (n=2) | Granulocytes, platelets, ECs, erythrocytes | GEV only present in inflamed foci. THP-1 cells were activated by phagocytosis of GEV. |
| 18. Woth (2012) [55] | Mixed severe sepsis | 21% | At admission to the ICU | At admission, day 3 and day 5 | Citrate | Severe sepsis (n=33), healthy (n=20) | Platelets | Elevated AnnV+ EV and PEV in septic vs. healthy on admission. Higher AnnV+ EV and PEV in fungal vs. non-fungal septic patients on day 1. CD42+ EV increased in fungal vs. non-fungal sepsis at all times and PAC1+ EVs at day 1 and day 5. |
| 19. Timár (2013) [103] | S. aureus bacteremia and fever | Unknown | Within 24 h after fever | Once | Unknown | Bacteremia (n=12), healthy (n=6) | Granulocytes | GEV 5-6 fold higher in serum and bacterial-EV aggregates larger with serum of bacteremic patients vs. healthy. |
| 20. Tőkés-Füzesi (2013) [22] | Severe sepsis | 15% at day 28 | Within 24 h of diagnosis of severe sepsis | At admission, day 3 and day 5 | Citrate | Severe sepsis (n=37) or non-septic ophthalmic patients (n=20) | Platelets, monocytes, myeloid cell line | Increased total, CD41+, CD42a+, and PAC1+ EVs in septic vs. healthy. Increased total, CD41+, and CD13+ EVs on admission in septic patients with renal dysfunction. Negative correlation of CD42a+ PEVs with blood urea nitrogen and creatinine concentrations in sepsis. |
| 21. Mostefai (2013) [161] | Septic shock | 12.5% at day 28 | 10±4 h after ICU admission | Once | Unknown | Septic shock (n=16) | Unknown | Septic EVs augmented histamine-induced contraction in human tissue-engineered vascular media. Septic EV treatment increased cyclo-oxygenase-1 and IL-10 expression of IL-10 (but not IL-1α, IL-1β, IL-6). |
| 22. Van Ierssel (2013) [162] | Mixed severe sepsis or septic shock | 14% at day 28 | At admission (<72 h of sepsis diagnosis) | Once | Citrate | Severe Sepsis (n=30), healthy (n=15) | ECs | EEV not different between septic and healthy. |
| 23. Delabranche (2013) [122] | Mixed septic shock with DIC | 29.3% at day 28 | At diagnosis of septic shock | At admission (day 1), day 2, day 3 and day 7 | Unknown | Septic shock (n=92) incl. (n=40) with DIC | Procoagulant EVs (prothrombinase assay), ECs, leukocytes | CD11a+ and CD105+ EVs were increased in DIC at admission. Increased CD105+ EVs had higher and CD31+ EVs lower odds ratios for DIC. |
| 24. Dalli (2014) [106] | Sepsis caused by CAP | Unknown | <24h from admission | Once | Unknown | Sepsis survivor (n=25), sepsis non-survivor (n=25), healthy (n=15) | Granulocytes | Alpha-2-macroglobulin+ EVs were higher in sepsis survivors vs. non-survivors and healthy volunteers. High alpha-2-macroglobulin+ EVs correlated with better outcome. |
| 25. Hellum (2014) [132] | Meningitis with or without shock | 61% | <4 h from admission and symptoms <72 h | Once | Citrate | Meningitis and septic shock (n=13) and meningitis (n=10), healthy (n=6) | PhtdSer+ EVs | Faster and more efficient thrombin generation by EVs in shock vs. no shock and strong correlation with LPS level in shock. |
| 26. Exline (2014) [110] | Sepsis | 38% in-hospital | <24 h admission | At admission and after 48h | Unknown | Sepsis (n=34), non-infected critically-ill controls (n=16) | Total EV | Higher EV-derived caspase-1 activity on day 1 and day 3 in septic vs. non-septic patients. EVs from septic patients on day 1 induced lymphocyte apoptosis. |
| 27. Woei-A-Jin (2014) [56] | Community-acquired febrile E.coli urinary tract infection | 0% | <24 h of symptoms | At admission and on the 3 days thereafter | ethylene diamine tetraacetic acid | Febrile urinary tract infection (n=215), healthy volunteers (n=19) | Monocytes | Higher TF+ EV activity on admission in patients with higher APACHE II score categories, but weak correlation with soluble E-selectin, soluble vascular cell adhesion molecule, thrombin-antithrombin-complexes and procalcitonin. TF+ EV activity was higher in patients vs. healthy. |
| 28. Matsumoto (2015) [125] | Mixed severe Sepsis + DIC | 12.5% overall ICU mortality | <24 h of diagnosis | Once | Citrate | Severe sepsis (n=24), trauma SIRS (n=12), cerebral hemorrhage SIRS (n=6), healthy (n=23) | ECs | TF+ EEV, TM+ EEV and EPCR+ EEV were increased in both septic shock and trauma vs. healthy; lack of correlation with APACHE II and sequential organ function score; moderate-to-strong correlation with the International Society of Thrombosis and Haemostasis DIC score. |
| 29. Herrmann (2015) [27] | Sepsis (Gram- and Gram+, possibly polymicrobial infections, 62.5% pulmonary, 37.5% abdominal) | Sepsis 40%, ICU control 15% | Within 72 h of ICU admission | Once | Heparin | Sepsis (n=14), SIRS (n=8) | Granulocytes | CD11β+/CD18+ and CD11β+/CD177+ EVs were higher in sepsis vs. control. Similar IL-6 plasma release by septic vs. control. Procoagulant activity increased, clotting time decreased, and bacteria aggregation activity higher in sepsis vs. control. |
| 30. Zhang (2016) [23] | Mixed sepsis | Unknown | <24 h of disease | Once | Citrate | Septic (n=15), healthy (n=10) | Platelets, ECs, erythrocytes, leukocytes, granulocytes, T-lymphocytes, monocytes | All PhtdSer+ EVs were increased in sepsis vs. healthy. EVs induced coagulation at PhtdSer-exposing sites in vitro; partly reversed by PhtdSer antagonism. |
| 31. Trepesch (2016) [163] | Mixed sepsis, severe sepsis, septic shock, survivor vs. non-survivors | 36.7% ICU mortality | At sepsis diagnosis | Daily up to 7 days, then every second day up to day 13 | Unknown | Sepsis (n=6), severe sepsis (n=4), septic shock (n=20) | PhtdSer+ EVs | The amount of PhtdSer+ EVs was not associated with mortality and organ dysfunction. |
| 32. Delabranche (2016) [133] | Mixed septic shock | total 34.4%, without DIC 28.3%, with DIC 45.2% | <6 h of septic shock diagnosis | At admission (day 1), day 3, and day 7 | Unknown | Septic shock (n=259) incl. with DIC at admission (n=61), with DIC within first 24h (n=32) | procoagulant (PhtdSer+) EV, leukocytes, apoptotic ECs, platelets, monocytes/macrophages | Increased CD105+ EVs and decreased CD31+ EVs were associated with DIC. Increased CD11a+ EVs/leukocytes supported leukocyte activation. |
| 33. Lehner (2016) [29] | Septic shock | ICU-mortality 66.7%, hospital-mortality 70% | Median (25-75% interquartile range) 12.6 h (3.1-21) from diagnosis | Once | Citrate | Septic shock (n=30), healthy (n=18) | ECs, leukocytes, platelets | Low but increased counts of EEVs (CD144+, CD62E+, CD106+) and increased CD31+/CD41- EVs vs. healthy. Correlation between CD31+/CD41- EVs and leukocytes. Increased levels of CD41+ EVs and CD31+/CD41-/AnnV- EVs in 48h non-survivors. CD144+, CD62E+ and CD106+ EVs not different between DIC and non-DIC. |
| 34. Stiel (2016) [164] | Septic shock with and without DIC | 7-day mortality: septic shock with DIC 20%; septic shock without DIC 5,5% | At admission | Once | Citrate | Septic shock: with DIC (n=35), without DIC (n=65) | Granulocytes | CD66+ GEV/neutrophilic granulocyte count higher in septic shock with DIC vs. without DIC. |
| 35. O'Dea (2016) [48] | Major burn injury vs. severe sepsis | Severe sepsis: ICU-mortality (27%), hospital-mortality (33%) | Within 24 h of burn injury, within 48 h of sepsis diagnosis | At admission and on day 2 (burn injury), once (sepsis) | Heparin | Major burn injury (n=15), severe sepsis (n=15), healthy (n=12) | Leukocytes, monocytes, granulocytes, ECs | EVs increase on admission in burns vs. healthy. Only CD45+/Cd14+ MEVs and CD66b+/CD11b+ GEVs increased in sepsis vs. healthy. CD45+/Cd14+ MEVs and CD105+ EEVs lower in sepsis vs. burns. CD45+ LEVs and CD66b+/CD11b+ GEVs increased in dying vs. surviving burn patients. No difference between dying and surviving sepsis patient. |
| 36. Matsumoto (2017) [19] | Mixed SIRS vs. severe sepsis | 16% | Within 24 h of injury/diagnosis | Once | Citrate | Trauma patients (24), severe sepsis (n=25), healthy (n=23) | Monocytes | Increased AnnV+/CD13+/CD142+ EVs in both trauma and severe sepsis vs. healthy. Moderate association between AnnV+/CD13+/CD142+ EVs and APACHE II score, IL-6, Injury Severity Score (in trauma), and International Society of Thrombosis and Haemostasis-DIC score (in sepsis). |
| 37. Panich (2017) [165] | Sepsis, septic shock | Sepsis with AKI 11.4%, Sepsis without AKI 6.7% | At admission | Daily day 1 to day 7 | N/A (Urine) | Sepsis with AKI (n=79), sepsis without AKI (n=60), healthy (n=8) | Exosomes | Activating transcriptional factor-3+ exosomes increased on day 1 of admission in sepsis with AKI vs. sepsis without AKI. Area under receiver operating curve for AKI 0.84. |
| 38. Reithmair (2017) [66] | Sepsis, septic shock | unknown | Day 0 | Day 0 and day 4 | Serum | Sepsis (n=22), healthy (n=23) | miRNA in serum, blood cells and EVs (exosomes, Tumor susceptibility gene 101+) by next generation sequencing | Compartment-specific (serum, cellular, extracellular) differences over time. |
| 39. Lehner (2017) [73] | Severe sepsis or sepsis shock, medical ICU | 33% ICU survival, 25% hospital survival | On average 7 h post start hemofiltration | Once | Citrate | Severe sepsis or septic shock (n=12) | Microparticles expressing platelet endothelial cell adhesion molecule (from platelets, leukocytes and/or ECs) and platelets. | Increase of CD31+/CD41- EVs post-filter vs. pre-filter. Decreased total PhtdSer-exposing EVs and decreased TF+ EVs post-filter vs. pre-filter. No PhtdSer-exposing EVs and no TF activity measurable in ultrafiltrate. |
| 40. Lashin (2017) [16] | Sepsis due to CAP or fecal peritonitis | 50% in sepsis due to CAP, 50% in sepsis due to fecal peritonitis (selected based on survival) | Day 1 post ICU admission | Day 1, 3, and 5 post ICU admission | ethylene diamine tetraacetic acid | CAP (n=60), fecal peritonitis (n=40), healthy (n=10) | Granulocytes, monocytes, T-lymphocytes, platelets, erythrocytes, and ECs | Circulating GEVs, MEVs, T-lymphocyte-derived EVs, and alpha-2-macroglobulin+ EVs (from all studied cell types) at day 1 were higher in CAP vs. fecal peritonitis and healthy volunteers, EEVs only vs. healthy, PEVs and EryEVs were not different. Alpha-2-macroglobulin+ EVs were higher in survivors of CAP, but not in fecal peritonitis. |
AKI: acute kidney injury; AnnV +/-: Annexin V positive/negative; APC: activated protein C; BAL: broncho-alveolar lavage; CAP: community acquired pneumonia; CD: cluster of differentiation; DIC: disseminated intravascular coagulation; EC: endothelial cell; EEVs: endothelial cell derived EVs; EPCR: endothelial protein C receptor; EryEVs: erythrocyte-derived EVs; EVs: extracellular vesicles; GEVs: granulocyte-derived EVs; ICU: intensive care unit; IL: interleukin; LEVs: leukocyte-derived EVs; LPS: lipopolysaccharide; MEVs: monocyte-derived EVs; PEVs: platelet-derived EVs; PhtdSer: phosphatidylserine; rhAPC: recombinant human APC; ROS: reactive oxygen species; SIRS: non-infectious systemic inflammatory response syndrome; TF: tissue factor; TNF: tumor necrosis factor.
The following search terms were used (PubMed; accessed on 15 Feb 2018): “extracellular vesicles OR microvesicles OR microparticles OR exosomes AND sepsis AND patients”. This search yielded 85 studies with following exclusions: 14 reviews, 1 editorial, 1 acute respiratory distress syndrome oriented study: 7 in vitro studies, 8 animals studies, 6 studies without EVs assessment, 7 studies without/unclear number of septic patients, 1 article in Chinese language. The final selection included 40 studies in English language that analyzed the abundance, composition or effect of EVs in samples from septic patients.
Diagnostic and/or therapeutic potential of EVs in sepsis
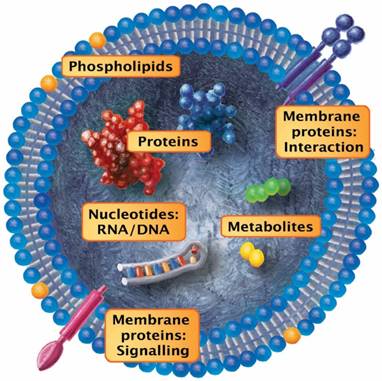
Once prompted by activation and/or apoptosis, EVs that shed from the cell surface typically retain the membrane composition (or the main features thereof) of the cells they originated from. For example, the release of these vesicles from the endothelium and monocytes can be effectively simulated by incubation with endotoxins such as bacterial lipopolysaccharides (LPS), pro-inflammatory cytokines or TNFα [31]. Although extracellular vesicles differ in size, composition and biogenesis, some basic characteristics can be summarized. Irrespective of the dimensions, EVs represent spherical, subcellular compartments that are composed of a phospholipid bilayer and various membrane-bound or plasmatic cargo molecules (Figure 2). Depending on the EV release process, their membrane retains the pattern of the cell surface they originated from. Given that EVs are released from all known tissue types, the retained transmembrane molecules and phospholipids interact with countless cellular processes. The smaller EV fraction (which arises from the early endosome) and the multivesicular bodies display membrane features that resemble organelles of those cells but undergo additional fine-tuned wrapping mechanisms.
Prototypic vesicle. Although extracellular vesicles differ in size, composition and biogenesis, some basic characteristics can be summarized: Irrespective of the dimensions, EVs represent spherical, subcellular compartments that are composed of a phospholipid bilayer and various membrane-bound or plasmatic cargo molecules. Depending on the process during which they are released, the EV membrane retains the pattern of the cell surface they originated from. Given that EVs are released from all known tissue types, the maintained transmembrane molecules and phospholipids interact with countless cellular processes. The smaller EV fraction (which arises from the early endosome) and the multivesicular bodies display membrane features that resemble these cell organelles but are subject to fine-tuned wrapping mechanisms. In general, the EV content includes proteins/peptides, smaller metabolites, as well as nucleotide sequences.
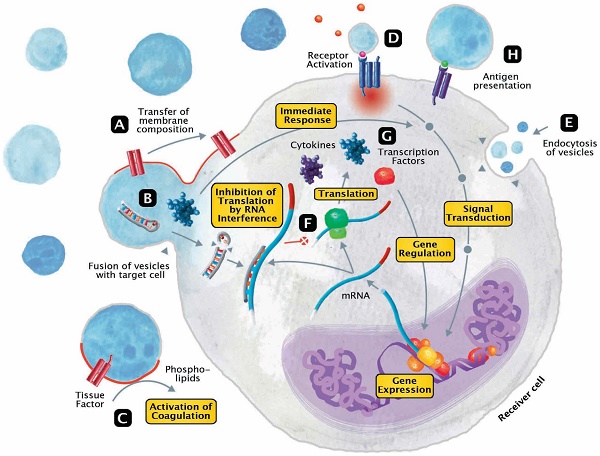
The released EVs become potential mini-messengers which may function as biomarkers but may also rapidly disseminate, magnify and/or perpetuate the processes triggered by the parent cell. EVs represent cellular shuttles that are capable of transferring a variety of compounds between cells. In general, the EV content includes proteins/peptides, smaller metabolites as well as nucleotide sequences. Upon fusion, vesicles rapidly modify the membrane composition of the receiver cell by transferring phospholipids and transmembrane molecules (Figure 3A). Meanwhile, a variety of cargo molecules are directly ejected into the plasma of the cell (Figure 3B). Compounds like growth factors, cytokines or other mediators are able to provoke an immediate metabolic response and to directly interfere with the receiver cell's signal transduction. During states of systemic inflammation and cell activation, tissue factor (TF) and phosphatidylserine (PhtdSer)-bearing vesicles represent microcarriers for the dissemination of a procoagulant phenotype (Figure 3C). Any component of the vesicle membrane can function as a ligand for receptors at the surface of the receiver cell, thereby triggering multiple responses (Figure 3D). During endocytosis, vesicles retain their membrane integrity and are engulfed by invagination (Figure 3E). Aside from proteins and metabolites that directly provoke the transduction of signals, other content like miRNAs are capable of silencing the expression of genes (Figure 3F). By altering the posttranscriptional processing on an mRNA level, this interference not only affects the synthesis of signaling molecules but secondarily also the expression of transcription factors (Figure 3G). Finally, EVs also play a role in fundamental mechanisms of immunity, including cytokine synthesis and antigen presentation (Figure 3H).
Any compound to serve as a potential biomarker must be stable enough to enable a reproducible detection in the hospital setting. Availability of sensitive assays to quantify cellular and/or circulating compounds and metabolites (including their genomic or transcriptional fingerprints) has enabled us to identify “signatures” of potentially diagnostically relevant biomarkers in virtually any biological sample, such as the blood [32], urine [33,34], cerebrospinal [35] and nasal [36] fluid.
EVs represent cellular mediators that are released from all known cell types via a number of evolutionarily conserved mechanisms [37]. Sepsis triggers a multifaceted cellular activation including massive shedding of membrane vesicles from various cell subtypes [38]. Although other methods have been recently attempted [39], flow cytometry is currently the main technology for the high-throughput characterization of EVs. When EVs are used for further investigation, fluorescence-activated cell sorting, a specific form of flow cytometry by which cells are sorted based on their light scattering and fluorescence characteristics can be used. While flow cytometry by itself is reliable and sensitive, the standardization procedures for the detection of EVs are not. Currently, the utility/fidelity of EVs as biomarkers cannot be judged without defining clear standard operation procedures. Specifically, a reproducible detection and in-depth characterization of circulating EVs depends on two key variables: a) sample preparation (e.g., anticoagulation, storage time and temperature, agitation during manipulation or transport) [17,26] and b) precisely defined flow cytometry settings [40]. The international societies have been striving to reach an acceptable consensus on standardized sample preparation, documentation and measurement procedures [26,41-44]. Recently, the applicability of size-calibrated beads for the standardization of the EVs count analysis using flow cytometry was tested on platelet-free plasma from healthy volunteers by 44 laboratories using 14 different types of cytometers (with central collection and analysis of raw data). Although the test demonstrated an acceptable variability among most commercial cytometers [40], these results need to be verified using clinical samples. Another important limitation of flow cytometry is that a large fraction of EVs remains under the lower limit of detection of the majority of available commercial cytometers (currently around 0.3 µm). This caveat should not be ignored given that the value of EVs as biomarkers may lie in recognizing changes in the ratios of specific EVs subpopulations and/or their presence/absence in a biological material. Such an EV-based approach has shown its value as a diagnostic support in the trauma setting [45].
From the technological standpoint, for EVs smaller than 0.3 µm, the method of choice is nanoparticle tracking analysis, which enables detection of specific antigens with fluorescence filter sets [46]. Although technologically advanced, this method has not yet been verified for EV-based diagnostics in septic patients. Regarding timing, a number of new point-of-care technologies that may deliver results within 2 hours are currently being studied. For example, Herrmann et al. used the aggregation of granulocyte-derived EVs (GEVs) and bacteria for the differentiation of infectious versus non-infectious inflammatory states using a microfluidic chip [47,48]. Another promising method is the lateral flow immunoassay: a one-step chromatographic immunoassay that has undergone continuous performance improvement by the use of modern labels such as gold nanoparticles, magnetic particles, carbon nanoparticles, colored latex beads, quantum dots, organic fluorophores, enzymes and liposomes. Oliviera-Rodriguez et al. have recently tested the value of this assay for the detection of (CD63 and CD9-positive) platelet-derived EVs (PEVs) in human plasma and identified gold nanoparticles as the optimal label [49,50]. Future studies testing the applicability of the lateral flow immunoassay for the early detection of sepsis and its complications using EVs derived from other sources are warranted. Table 2 identifies the main developmental directions needed for closing the current technological gap in EV research.
The most relevant technological challenges in the field of EV.
| detection of EVs below 0.3 µm (i.e., nanoparticles) precise receptor/cargo-based EV characterization differentiation between EV-associated and non-EV-associated miRNA in plasma development of quick point-of-care tests based on EVs development of EV-based vaccination techniques elimination of detrimental EVs from the bloodstream |
The main portion of the EV pool under steady-state conditions is platelet-associated (i.e., resting/activated platelets, megakaryocytes) [51,52]. In pathological conditions, however, EVs are mostly derived from activated platelets, endothelial cells (ECs) and leukocytes. These EVs accumulate rapidly in the circulation and are readily detectable by flow cytometry [53,54]. The presence/absence, dynamic changes and/or composition of circulating EVs have a potential to be harnessed as fine-tuned diagnostic descriptors of ongoing pathophysiological inflammatory processes in sepsis (and beyond). In addition to the considerable role of EVs as markers of a systemic inflammatory response, an elevation of TF-bearing vesicles could be considered as an early indicator of a generalized (in some cases even focal) infection. TF-positive EVs could be used to guide further or more aggressive treatment with antimicrobials [55,56]. The mediator/effector role of PhtdSer/ TF-expressing EVs in septic coagulopathy (blood clotting disorder) is detailed in the last subchapter in this review.
Besides analysis of EVs on the basis of vesicle surface antigens (the most important marker molecules displayed in Table S1), identification of cargo like micro-ribonucleic acid (miRNA) also appears to be an interesting research tool [57]. MiRNAs are short (19-25 nucleotides) noncoding sequences that are both actively and passively incorporated into EVs and that are capable of regulating gene expression by interfering with the translation of messenger RNA. In a preclinical sepsis study, miR-223 containing EVs released by mesenchymal stem cells attenuated cecal ligation and puncture-induced cardiac dysfunction in mice by reducing the Sema3a and Stat3 transcription factors [58]. Moreover, miR-223 is involved in the modulation of hematopoiesis and is seen as a promising indicator of immune response dysregulation [59,60]. Combined with the expression pattern of other miRNAs, miR-223 could potentially help to distinguish between non-septic and septic patients [61]. Studies in cancer patients have shown that miRNA phenotypes are consistent and reproducible among individuals and serve as a cluster of markers for a number of diseases [62,63]. Other miRNAs, such as miR-15a, miR-30d-5p, miR-30a-5p, miR-192-5p, miR-26a-5p, miR-23a-5p, and miR-191-5p appear to be capable of differentiating septic patients and those with non-infectious inflammation (also called systemic inflammatory response syndrome, SIRS) [64,65]. Some miRNAs are detectable in exosomes and serum only, whereas others are also present in multiple cell types [66]. Moreover, miR-21 has been shown to be an essential part of the protective effect of remote ischemic preconditioning in sepsis [67]. Another recent report demonstrated EVs as messengers that can suppress miRNA expression and activate downstream gene expression in the central nervous system during inflammatory states [68]. However, research on the utility of EV-derived nucleotide sequences is in its infancy. Amplification and detection of miRNAs is usually performed via reverse transcriptase polymerase chain reaction and next-generation sequencing. However, these methods do not adequately discriminate between circulating, Argonaute protein-bound miRNA and miRNA incorporated to EVs. Studies investigating miRNA in sepsis (not focused on EV detection) may in fact have measured EV-associated miRNA (and vice versa). Innovative detection technologies to bypass this limitation are urgently needed (Table 2). Given that there are no clear guidelines regarding EV-RNA analytics, any findings should be interpreted with caution [69].
In addition to their role as biomarkers, circulating EVs constitute a dynamic pool of messengers that allow an organism to rapidly respond to altered physiological conditions. Thus, in their mediator-like character, they are capable of conveying a multitude of information to even distant tissues within the body. In a short-range communication format, EVs released by various cells could affect other immuno-competent cells/tissues in their immediate surroundings modulating, for example, a focal inflammatory process. Additionally, EVs may also support cell communication over longer distances. For example, analogous to the soluble endothelial protein C receptor (EPCR), EVs expressing EPCR are able to bind activated protein C (APC) upon external administration of recombinant human protein C in septic patients in order to exert its effects at distant vascular sites [70]. This mode of mediation appears to be especially relevant in diseases involving endothelial activation and microvascular dysfunction (e.g., sepsis) given that they are accompanied by a robust release of EVs. Sepsis-induced acute kidney injury (AKI) is associated with an impairment of endothelial barrier function and the sequestration of (endothelial cell-derived) EVs is thought to be a substantial causative factor in AKI pathophysiology [71]. In the future, excessive EVs could be eliminated using continuous veno-venous hemofiltration [72] or be used for optimizing filtration fractions during hemofiltration [72,73].
Modes of interaction of EVs with receiver cells. EVs represent cellular shuttles that are capable of transferring a variety of compounds between cells. (A) Upon fusion, vesicles rapidly modify the membrane composition of the receiver cell by transferring phospholipids and transmembrane molecules. (B) Meanwhile, a variety of cargo molecules are directly ejected into the plasma of the cell. Compounds like growth factors, cytokines or other mediators are able to provoke an immediate metabolic response and to directly interfere with the receiver cell's signal transduction. (C) During states of systemic inflammation and cell activation, tissue factor and phosphatidylserine-bearing vesicles represent microcarriers for the dissemination of a procoagulant phenotype. (D) Any component of the vesicle membrane can function as a ligand for receptors at the surface of the receiver cell, thereby triggering a multitude of responses. (E) During endocytosis, vesicles retain their membrane integrity and are engulfed by invagination. (F) Aside from proteins and metabolites that directly provoke the transduction of signals, other content like micro ribonucleic acids (RNA) are capable of silencing the expression of genes. (G) By altering the posttranscriptional processing on a messenger RNA level, this interference not only affects the synthesis of signaling molecules but secondarily also the expression of transcription factors. (H) Finally, EVs also play a role in fundamental mechanisms of immunity, including cytokine synthesis and antigen presentation.
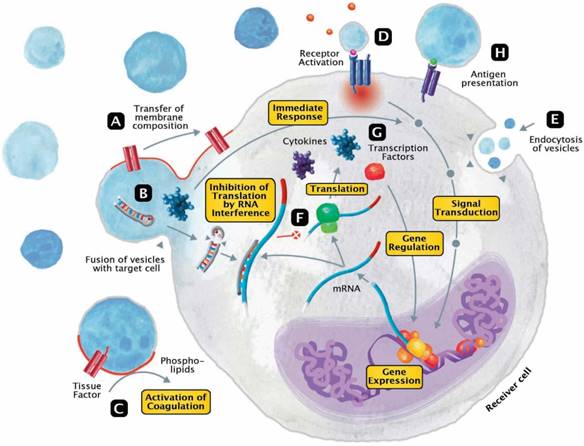
In summary, EVs appear to simultaneously serve as both markers and mediators in sepsis syndromes. This creates a multidirectional opening for their potential utilization: either diagnostic, therapeutic or both (depending on a specific sepsis scenario/ phenotype and objective).
EVs in sepsis: a friend or foe?
EVs are involved in severe pathophysiological events such as infections and/or inflammatory processes. The existing literature [74] implies that EVs play an important role in sepsis and septic shock. Given that both the pathogen and the host appear to rely on EVs as their tool of either attack or defense, it needs to be precisely deciphered which EVs populations mediate and promote progression of sepsis and which support its resolution and propel healing. The most intuitive separation can be done based on the source of EV release.
Bacteria, viruses and fungi responsible for septic infections are all well-capable of producing and secreting varieties of membrane vesicles, yet without the specific surface lipid composition seen in eukaryotic microbes [75-81]. Gram-positive and Gram-negative bacteria, as the most frequent cause of sepsis [82], can release EVs (in Gram-negative termed outer membrane vesicles) that may carry toxins, adhesins, proteolysins and other virulence factors relevant for the development of the systemic inflammatory response [83-86] and/or subsequent dysfunction of organs and tissues. Incubation of C. albicans-derived EVs with bone marrow-derived macrophages resulted in synthesis of IL-10, IL-12, transforming growth factor-beta and nitric oxide, as well as increased expression of major histocompatibility class II and cluster of differentiation (CD)-86 on macrophages [87] - all suggesting a strong immunomodulating potential for fungal EVs during infections [80]. Similarly, development of sepsis, a leading cause of death in HIV type 1 patients [88], is promoted by exosomes from infected cells containing the accessory extracellular viral protein Nef, which was shown to induce apoptosis of CD4-T-cells [89]. Viruses are also capable of controlling the release and content of EVs produced by infected cells [90] to facilitate the viral spread. In this context, the foes could potentially become friends by protecting vulnerable cohorts of patients against specific hospital pathogens (e.g., S. aureus or E. coli) to prevent secondary infections. For example, bacterial protoplast-derived nanovesicles have been used to vaccinate mice against these germs in models of pneumonia and peritonitis [91]. In the context of the growing antibiotic resistance crisis, this could be a life-saving preventive option (Table 2).
The second major pool of EVs stems from the host organism and can be equally bio-active as the EVs released by microorganisms. Key sources of host EVs during sepsis are circulating cells such as platelets and leukocytes [92,93], the latter being predominantly innate immune cells such as neutrophilic granulocytes, macrophages, dendritic and natural killer (NK) cells [94]. In sepsis, especially neutrophilic granulocytes show an increased activation but also higher apoptosis rates [95,96]—the two main conditions that trigger a robust EV release [97-100]. Unlike the microbial EVs with predominantly detrimental effects (as perceived based on the current literature), the action of the host-released EVs appears to be more heterogeneous. A number of protective EV-related mechanisms were recently implied. In a clinical study, GEVs were found in the infectious foci rather than in the circulation [101]. Due to the functional relationship to their parent cells, GEVs can exhibit antibacterial effects, support the production of inflammatory mediators [102-104] and/or protect from vascular dysfunction [103,105]. Selected danger-associated EV subsets (containing alpha-2-macroglobulin excreted by neutrophilic granulocytes) were shown to improve resolution of infection by enhancing bacterial clearance in a mouse sepsis model [105,106] and by interfering with leukocyte-trafficking in vitro [107]. Similarly, when THP-1 monocytes phagocytized GEVs which were previously isolated from peritoneal and broncho-alveolar lavages of patients with surgical sepsis, their general activity and phagocytic capacity significantly increased in vitro [101,105]. In septic rats, EVs produced by immature dendritic cells reduced mortality by diminishing TNFα and high mobility group box-1 release and supported engulfment of apoptotic cells [108].
In contrast, the emerging evidence demonstrates that some EVs released by leukocytes in sepsis can be harmful. For example, in a porcine model of endotoxemia, danger-associated populations of PEVs robustly increased in dying but not in surviving animals [109]. Furthermore, lymphocyte apoptosis, one of the major abnormalities observed in both pre-and clinical sepsis [110,111], was induced by caspase-1 released from circulating EVs in septic patients [110]. While the precise EV source was not determined in that study, peripheral blood monocytes are potential candidates given that they were shown to release EVs containing caspase-1 which induced apoptosis of smooth vascular muscle cells in vitro [112]. Blocking the release of EVs from bacteria-infected macrophages by pre-treatment with a sphingomyelinase inhibitor in septic mice had cardioprotective effects and prolonged survival [113]. It has also been suggested that EVs contribute to the development of sepsis-induced acute lung injury [94]. Intratracheal application of EVs released from LPS-primed alveolar macrophages to C57BL/6 mice provoked their alveolar macrophages to release EVs, which caused an increased expression of adhesion molecules, protein and neutrophilia [94]. In another example, dendritic cells activated by LPS released EVs that stimulated epithelial cells to secrete chemokines (e.g., IL-8 and Chemokine (C-C motif) ligand 5) which are important soluble components of a physiological innate immune response but can simultaneously fuel sepsis by excessive cytokine release [114]. Circulating EVs (without classification) containing reactive oxygen species (ROS) from LPS-stimulated mice induced vascular leakage and cardiac dysfunction in C57BL/6 mice [115]. The roles of exosomes in septic cardiac dysfunction have recently been reviewed [116].
Many other leukocyte subpopulations are capable of EV release but their role in sepsis has not yet been adequately investigated. NK cells are a suitable example. In contrast to other immune cells, NK cells produce EVs constitutively and independently of their parent cell activation status. NK cells contain so-called killer proteins such as Fas ligand and perforin that influence the tumor growth and immune system homeostasis [117]. Interestingly, NK cell-derived vesicles only act against the activated immune cells. This indicates that they could actually modulate septic processes by controlling the expansion and proliferation of stimulated immune cells. Another example is the potential function of EVs in adaptive immunity. Stimulated CD4+ and CD8+ T-cells can induce cytokine production (TNF-alpha, IL-1-beta, soluble IL-1 receptor antagonist) by human monocytes by either direct contact or by releasing EVs as messengers [118].
In summary, the existing evidence strongly suggests that EVs have both protective and detrimental roles, either locally or at distant sites. Whether EVs assume the shape of a friend or foe appears to partly depend on their origin (e.g., microorganism vs. host) and/or specific pathophysiological trait examined.
Role of EVs in specific subsets of septic patients
Role of EVs in disturbed coagulation
Although initiation of thrombotic pathways may inhibit dissemination of bacteria through the body, it may also lead to excessive activation and consumption of coagulation components. With their procoagulant potential, EVs are likely involved in the pathophysiology of DIC in septic patients [119]. The development of DIC is a severe sepsis complication; its manifestation is associated with doubled mortality rates and is characterized by the massive consumption of coagulation factors and platelets and the excessive infiltration of thrombi in the microcirculation. It is estimated that approximately 35% of sepsis patients develop DIC [120]. Currently, the management of sepsis complicated by DIC comprises combating the underlying infection by surgical source control, broad spectrum antibiotics and general goal directed therapy [121] with organ function support and/or replacement therapy. So far, other treatments have not shown to be beneficial in large prospective, randomized trials. In patients who do not present with DIC in the initial phase of sepsis, early assessment of the risk for developing DIC would be beneficial. Thus, determination of EVs may aid in assessing such a risk and in identifying possible targets for treatment. EVs may exhibit direct procoagulant properties via exposure of PhtdSer on their surface, a cell membrane phospholipid that supports the assembly of coagulation enzymes and TF, which is the main initiator of the coagulation cascade [38,122]. In sepsis, procoagulant (PhtdSer-exposing) EVs (typically identified by Annexin V staining and flow cytometry) are mainly released by platelets (PEVs), but also by endothelial cells (EEVs) and monocytes (MEVs) [28]. TF is the prime initiator of the coagulation cascade whereas PhtdSer serves as a catalyst for the activation of coagulation factors. Both functions make this vesicle subset a highly potent vector for the dissemination of a pro-coagulant phenotype throughout the circulation [23,123]. As discussed above, the main source of TF in plasma is PEVs [124]. Together with the up-regulation of TF on endothelial cells and monocytes, as well as the general activation of prothrombotic and fibrinolytic pathways, blood-borne TF-positive EVs are therefore also co-responsible for the prothrombotic milieu that underlies DIC [122,125]. This highly lethal complication of sepsis (associated with doubled mortality) is characterized by the massive consumption of coagulation factors and platelets and the excessive infiltration of thrombi in the microcirculation.
Several observations also support the more indirect role of EVs as 'vehicle carriers' that link inflammation to coagulation. Our lab recently showed that the release of EVs is a danger signal that eventually promotes the transition to a pro-thrombotic phenotype [126] and that IL-33 is involved in increased TF activity in EEVs [149]. The release of vesicles can occur downstream of multiple signal transduction pathways, depending on the initial receptor-activation by specific ligands. Sculpting and budding at the cell membrane or within the multivesicular bodies is mediated by highly conserved vesicular trafficking entities like the endosomal sorting complexes required for transport [127]. During inflammatory activation of cells, the increase in intracellular calcium results in the activation of calpains (calcium-activated neutral cysteine proteases) and scramblase and the loss of phospholipid bilayer asymmetry [128,129].
Furthermore, in vitro and in vivo endothelial and systemic activation by thrombin/CD40L and LPS enhanced EVs carrying matrix metallopeptidase 10 and CD40L. Elevation in circulating matrix metallopeptidase 10 and CD40L was associated with an increased mortality in septic patients who had enhanced thrombin formation [130]. Boisramé-Helms et al. showed that inoculation with EVs derived from septic rats into a cohort of healthy rats lowered their arterial pressure (not observed when 'donor' animals were treated with APC) [131]. Also blockage of calpains appears to exert therapeutic effects. Zafrani et al. studied the role of calpains in the development of DIC during sepsis in a clinically relevant animal sepsis model; they observed a survival benefit by attenuation of DIC after blocking calpains by overexpressing its endogenous inhibitor calpastatin [148].
EVs appear as promising markers of patients with DIC. For example, in meningococcal sepsis patients with multiple organ dysfunction, circulating EVs were mainly platelet-derived and their number was almost 15-fold higher compared to healthy controls [53]. Furthermore, EVs exposing TF were decreased in patients with sepsis and multiple organ dysfunction [28]. However, another study in patients with meningococcal septic shock demonstrated higher procoagulant activity of EVs compared to non-shock patients [132]. Overall, septic patients would greatly benefit from early markers that can reliably predict DIC, enabling in turn early and adequate treatment stratification. CD105+ EVs and CD31+ EVs are promising marker candidates that warrant further validation [122,133].
Role of EVs in blood transfusion
Septic patients regularly develop anemia, often necessitating transfusions of erythrocyte concentrates in case of insufficient oxygen delivery. Transfusion of blood components has been associated with adverse effects such as increased risk of infection [134]. However, the exact mechanisms of this phenomenon remain elusive. It has been recently suggested that EVs present in blood products trigger various inflammatory pathways [135-140]. Vlaar et al. showed a release of TNF-alpha, IL-6 and IL-8 after whole blood was incubated with the supernatant of red blood cell (RBC) concentrates, which was associated with the storage time of the RBC concentrate and the amount of EVs present in the supernatant [141]. Interestingly, no pro-inflammatory response was observed when the supernatant was depleted from EVs before incubation. The same authors showed that endothelial cells incubated with monocytes expressed more intercellular adhesion molecule-1 and E-Selectin only in the presence of EV-containing RBC supernatants. EVs were phagocytized by monocytes in a complement receptor 3-dependent fashion and elicited expression of Von Willebrand factor but not TF [142]. In murine transfusion models, EVs from RBCs affected the pulmonary endothelium via adhesion molecules [143] and thrombin-dependent complement activation [144]. The existence of such an activation effect is supported by pre-clinical evidence: hemorrhaged mice resuscitated with RBC and plasma rapidly accumulated neutrophilic granulocytes in the lungs when the resuscitation fluids contained RBC-derived EVs [145]. Those EVs also induced increased neutrophilic CD11b expression after i.v. administration in mice and in vitro incubation with human neutrophilic granulocytes [145]. The occurrence of acute lung injury by blood product-derived EVs may be explained by the two-hit phenomenon: the clinical condition of the critically ill patient leads to polymorphonuclear granulocyte recruitment to the activated pulmonary microvascular endothelium (first hit) which is further aggravated by the activation of adherent PMNs by blood product transfusion (second hit). This can eventually lead to destruction of pulmonary ECs, capillary leakage and acute respiratory distress syndrome.
Role of EVs in mechanical ventilation
Mechanical ventilation may result in ventilator-induced lung injury. It appears that EVs can influence the pathophysiology of this and other lung-related conditions in the ICU patients. For example, increased EVs have been recently associated with a reduced risk of the acute respiratory distress syndrome [146]. More specifically, Mutschler et al. investigated the effects of mechanical ventilation upon EVs present in the broncho-alveolar lavage fluid of ventilated pigs and human patients at post-operative extubation [147]. They found that EVs are activated and adhere to neutrophil granulocytes in the pulmonary air-blood interface and suggested the use of EVs to guide the mechanical ventilation strategy. For example, a defined EV concentration could aid in deciding whether a patient with acute respiratory distress syndrome requires temporal extracorporeal membrane oxygenation in order to prevent any further lung injury from mechanical ventilation.
Few pre-clinical and in vitro studies further underline an active role of EVs in ventilator-induced lung injury. A pathological pulmonary EC stretching model (mimicking excessive tidal volume ventilation) induced EV generation which was counteracted by the caspase inhibitor Z-VAD [148] and was independent of co-treatment with thrombin, LPS, the Rho kinase inhibitor Y-27632 and calpeptin (a calpain inhibitor). In another study, high (in contrast to low) tidal volume ventilation in healthy mice increased systemic levels of pulmonary endothelium (CD31+)-derived EVs and decreased CD31 in the lung tissue. The same mechanism was investigated using a stretch model of human pulmonary EC which shed (Annexin V and CD31 positive) EEVs [149]. Those EEVs induced lung inflammation when instilled intratracheally into healthy mice. Subsequent proteomic analysis found multiple common molecules shared by the different EEV populations, e.g., CD31 [149]. The above examples strongly imply that disintegration of the pulmonary endothelial barrier (due to high volume ventilation) provokes shedding of EVs that express several adhesion molecules which likely exert strong systemic downstream effects upon inflammatory processes [150]. In a recent study, elevation of EVs in plasma was inversely associated with the risk for the development of acute respiratory distress syndrome in a group of critically ill patients with varying underlying pathologies. Interestingly, the closer post-hoc analysis revealed that this association was true in the septic patient cohort (representing less than half of the total studied population) but not in non-septic patients [146].
Conclusions and outlook
Within the last two decades, the multifaceted roles of EVs in inflammation have entered the focus of sepsis research. Given that both sepsis and EVs are two very complex fields and the existing evidence regarding the intricacies of EVs in sepsis is limited, no definitive connecting lines can be currently drawn between those two entities. However, a number of promising speculations pertaining to the role of EVs in sepsis are justified.
First, it is convincing that EVs have a strong role as mediators in sepsis. It has also become apparent that EVs can act both as friends and foes in systemic inflammatory reactions; their friendly or hostile character largely depends on the EV origins and cargo they carry. The latter element makes specific EV subsets potential candidates for drug delivery systems. In the future, the above EV properties could facilitate more precise and individualized treatment approaches.
The identification of specific EVs subsets remains fundamental for the isolation and application in diagnostic and/or therapeutic procedures. At the moment, however, it is clear that the restrictions of characterization (hence our biological understanding of EVs) are limited by the methodological isolation/detection boundaries. The gold standard for high-throughput detection of EVs based on surface antigens is flow cytometry with a current detection limit of well above 0.3 µm. To our knowledge, nanoparticle tracking analysis has not yet been tested in septic patients. Although size transition of EVs is fluent, discrimination of EV subsets is largely based on their dimension, a measure that ignores the biogenesis of the vesicles. Development of new methodologies to rapidly assess circulating miRNA and miRNA incorporated to EVs should have a high priority. Furthermore, quick point-of-care tests such as microfluidic chip tests and lateral flow immunoassays have a great potential to be used with plasma of septic patients, but need additional testing. Given their strong immunomodulatory capabilities and vast diversity of cargo molecules, EVs present a considerable therapeutic potential for the treatment of sepsis. It is not impossible to imagine that EVs could serve in the future ICU as new types of vaccines against secondary infections (Table 2).
Overall, the potential utility points for EVs in sepsis (and other ICU conditions) are multifaceted, reaching from preventive to diagnostic and therapeutic. Yet, before any of these points migrate into the clinic, basic methodological challenges in EVs detection have to be solved and any viable application of EVs must be first a) standardized for detection/description, b) evaluated for clinical applicability, c) validated in multi-center randomized clinical trials and d) thoughtfully adopted for clinical use.
Abbreviations
AKI: acute kidney injury; AnnV +/-: Annexin V positive/negative; APC: activated protein C; BAL: broncho-alveolar lavage; CAP: community acquired pneumonia; CD: cluster of differentiation; DIC: disseminated intravascular coagulation; EC: endothelial cell; EEVs: endothelial cell derived EVs; EPCR: endothelial protein C receptor; EryEVs: erythrocyte-derived EVs; EVs: extracellular vesicles; GEVs: granulocyte-derived EVs; ICU: intensive care unit; IL: interleukin; LEVs: leukocyte-derived EVs; LPS: lipopolysaccharide; MEVs: monocyte-derived EVs; miRNA: micro ribonucleic acid; NK: natural killer; PEVs: platelet-derived EVs; PhtdSer: phosphatidylserine; RBC: red blood cell; ROS: reactive oxygen species; SIRS: non-infectious systemic inflammatory response syndrome; TF: tissue factor; TNF: tumor necrosis factor.
Supplementary Material
Supplementary Table S1.
Acknowledgements
The authors sincerely thank Dr Marcin F. Osuchowski for taking the back seat in this project and helping to pilot it to the happy end. The authors are indebted to Prof Edit I. Buzás and Prof Mervyn Singer for providing useful advices and constructive criticism. We thank Dr James Ferguson for the linguistic editing. Partly supported by FWF Science Fund, grant number T707-B13.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fleischmann C, Scherag A. et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Crit Care Med. 2016;193:259-272
2. Singer M, Deutschman CS, Seymour CW. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801-810
3. Rhodes A, Evans LE, Alhazzani W. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304-377
4. Walley KR. Biomarkers in sepsis. Curr Infect Dis Rep. 2013;15:413-420
5. Fisher CJ Jr, Slotman GJ, Opal SM. et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12-21
6. Abraham E, Wunderink R, Silverman H. et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934-941
7. Cronin L, Cook DJ, Carlet J. et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430-1439
8. Calandra T, Baumgartner JD, Grau GE. et al. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990;161:982-987
9. Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ. et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247-1288
10. Van der Poll T, Van de Veerdonk FL, Scicluna BP. et al. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407-420
11. Bozza FA, Salluh JI, Japiassu AM. et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49
12. Terrasini N, Lionetti V. Exosomes in critical illness. Crit Care Med. 2017;45:1054-1060
13. Van der Pol E, Boing AN, Gool EL. et al. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14:48-56
14. Yanez-Mo M, Siljander PR, Andreu Z. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066
15. Reid VL, Webster NR. Role of microparticles in sepsis. Br J Anaesth. 2012;109:503-513
16. Lashin HMS, Nadkarni S, Oggero S. et al. Microvesicle subsets in sepsis due to community acquired pneumonia compared to faecal peritonitis. Shock. 2018;49:393-401
17. Wisgrill L, Lamm C, Hartmann J. et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytometry A. 2016;89:663-672
18. Levy MM, Fink MP, Marshall JC. et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29:530-538
19. Matsumoto H, Yamakawa K, Ogura H. et al. Clinical significance of tissue factor and CD13 double-positive microparticles in Sirs patients with trauma and severe sepsis. Shock. 2017;47:409-415
20. Sabatier F, Darmon P, Hugel B. et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840-2845
21. Tramontano AF, O'Leary J, Black AD. et al. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34-38
22. Tokes-Fuzesi M, Woth G, Ernyey B. et al. Microparticles and acute renal dysfunction in septic patients. J Crit Care. 2013;28:141-147
23. Zhang Y, Meng H, Ma R. et al. Circulating microparticles, blood cells, and endothelium induce procoagulant activty in sepsis through phosphatidylserine exposure. Shock. 2016;45:299-307
24. Furie B, Furie BC. Cancer-associated thrombosis. Blood Cells Mol Dis. 2006;36:177-181
25. Lotvall J, Hill AF, Hochberg F. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913
26. Witwer KW, Buzas EI, Bemis LT. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360
27. Herrmann IK, Bertazzo S, O'Callaghan DJ. et al. Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale. 2015;7:13511-13520
28. Joop K, Berckmans RJ, Nieuwland R. et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810-820
29. Lehner GF, Harler U, Haller VM. et al. Characterization of microvesicles in septic shock using high-sensitivity flow cytometry. Shock. 2016;46:373-381
30. Soriano AO, Jy W, Chirinos JA. et al. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Crit Care Med. 2005;33:2540-2546
31. Zipperle J, Schlimp CJ, Holnthoner W. et al. A novel coagulation assay incorporating adherent endothelial cells in thromboelastometry. Thromb Haemost. 2013;109:869-877
32. Arraud N, Linares R, Tan S. et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614-627
33. Knepper MA, Pisitkun T. Exosomes in urine: who would have thought..? Kidney Int. 2007;72:1043-1045
34. Hiemstra TF, Charles PD, Gracia T. et al. Human urinary exosomes as innate immune effectors. J Am Soc Nephrol. 2014;25:2017-2027
35. Basso M, Bonetto V. Extracellular vesicles and a novel form of communication in the brain. Front Neurosci. 2016;10:127
36. Lasser C, O'Neil SE, Ekerljung L. et al. RNA-containing exosomes in human nasal secretions. Am J Rhinol Allergy. 2011;25:89-93
37. Buzas EI, Gyorgy B, Nagy G. et al. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356-364
38. Zafrani L, Ince C, Yuen PS. Microparticles during sepsis: target, canary or cure? Intensive Care Med. 2013;39:1854-1856
39. Van der Pol E, Coumans FA, Grootemaat AE. et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12:1182-1192
40. Cointe S, Judicone C, Robert S. et al. Standardization of microparticle enumeration across different flow cytometry platforms: results of a multicenter collaborative workshop. J Thromb Haemost. 2017;15:187-193
41. Kalra H, Simpson RJ, Ji H. et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450
42. Van Deurn J, Mestdagh P, Agostinis P. et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228-232
43. Pathan M, Keerthikumar S, Chisanga D. et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J Extracell Vesicles. 2017;6:1321455
44. Kim DK, Lee J, Kim SR. et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015;31:933-939
45. Matijevic N, Wang YW, Holcomb JB. et al. Microvesicle phenotypes are associated with transfusion requirements and mortality in subjects with severe injuries. J Extracell Vesicles. 2015;4:29338
46. Dragovic RA, Gardiner C, Brooks AS. et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7:780-788
47. Lasser C, O'Neil SE, Shelke GV. et al. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J Transl Med. 2016;14:181
48. O'Dea KP, Porter JR, Tirlapur N. et al. Circulating microvesicles are elevated acutely following major burns injury and associated with clinical severity. PLoS One. 2016;11:e0167801
49. Oliveira-Rodriguez M, Lopez-Cobo S, Reyburn HT. et al. Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids. J Extracell Vesicles. 2016;5:31803
50. Oliveira-Rodriguez M, Serrano-Pertierra E, Garcia AC. et al. Point-of-care detection of extracellular vesicles: sensitivity optimization and multiple-target detection. Biosens Bioelectron. 2017;87:38-45
51. Italiano JE Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578-584
52. Rank A, Nieuwland R, Delker R. et al. Cellular origin of platelet-derived microparticles in vivo. Thromb Res. 2010;126:e255-e259
53. Nieuwland R, Berckmans RJ, McGregor S. et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930-935
54. Tripisciano C, Weiss R, Eichhorn T. et al. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7:6522
55. Woth G, Tokes-Fuzesi M, Magyarlaki T. et al. Activated platelet-derived microparticle numbers are elevated in patients with severe fungal (Candida albicans) sepsis. Ann Clin Biochem. 2012;49:554-560
56. Woei AJF, Van der Starre WE, Tesselaar ME. et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014;133:799-803
57. Crescitelli R, Lasser C, Szabo TG. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677
58. Wang X, Gu H, Qin D. et al. Exosomal miR-223 Contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep. 2015;5:13721
59. Johnnidis JB, Harris MH, Wheeler RT. et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125-1129
60. Wang JF, Yu ML, Yu G. et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394:184-188
61. Wang HJ, Zhang PJ, Chen WJ. et al. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg. 2012;73:850-854
62. Chen X, Ba Y, Ma L. et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006
63. Mitchell PS, Parkin RK, Kroh EM. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513-10518
64. Caserta S, Kern F, Cohen J. et al. Circulating plasma microRNAs can differentiate human sepsis and systemic inflammatory response syndrome (SIRS). Sci Rep. 2016;6:28006
65. Wang H, Zhang P, Chen W. et al. Evidence for serum miR-15a and miR-16 levels as biomarkers that distinguish sepsis from systemic inflammatory response syndrome in human subjects. Clin Chem Lab Med. 2012;50:1423-1428
66. Reithmair M, Buschmann D, Marte M. et al. Cellular and extracellular miRNAs are blood-compartment-specific diagnostic targets in sepsis. J Cell Mol Med. 2017;21:2403-2411
67. Jia P, Wu X, Dai Y. et al. MicroRNA-21 Is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit Care Med. 2017;45:e703-e710
68. Balusu S, Van Wonterghem E, De Rycke R. et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med. 2016;8:1162-1183
69. Mateescu B, Kowal EJ, Van Balkom BW. et al. Obstacles and opportunities in the functional analysis of extracellular vesicle. J Extracell Vesicles. 2017;6:1286095
70. Perez-Casal M, Thompson V, Downey C. et al. The clinical and functional relevance of microparticles induced by activated protein C treatment in sepsis. Crit Care. 2011;15:R195
71. Souza AC, Yuen PS, Star RA. Microparticles: markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and AKI. Kidney Int. 2015;87:1100-1108
72. Abdelhafeez AH, Jeziorczak PM, Schaid TR. et al. Clinical CVVH model removes endothelium-derived microparticles from circulation. J Extracell Vesicles. 2014;3:23498
73. Lehner GF, Harler U, Feistritzer C. et al. Hemofiltration induces generation of leukocyte-derived CD31+/ CD41- microvesicles in sepsis. Ann Intensive Care. 2017;7:89
74. Lehner GF, Brandtner A.K, Joannidis M. Microvesicles in sepsis: implications for the activated coagulation system. Vincent JL, ed. Annual update in intensive care and emergency medicine 2017, Springer International Publishing AG. 2017:29-39
75. Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948-1957
76. Lee EY, Choi DY, Kim DK. et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425-5436
77. Kim JH, Lee J, Park J. et al. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97-104
78. Thay B, Wai SN, Oscarsson J. Staphylococcus aureus alpha-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS One. 2013;8:e54661
79. Olaya-Abril A, Prados-Rosales R, McConnell MJ. et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014;106:46-60
80. Joffe LS, Nimrichter L, Rodrigues ML. et al. Potential roles of fungal extracellular vesicles during infection. mSphere. 2016;1:e00099-16
81. Anderson MR, Kashanchi F, Jacobson S. Exosomes in viral disease. Neurotherapeutics. 2016;13:535-546
82. Vincent JL, Sakr Y, Sprung CL. et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344-353
83. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163-184
84. Lindmark B, Rompikuntal PK, Vaitkevicius K. et al. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009;9:220
85. Park KS, Choi KH, Kim YS. et al. Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PLoS One. 2010;5:e11334
86. Shah B, Sullivan CJ, Lonergan NE. et al. Circulating bacterial membrane vesicles cause sepsis in rats. Shock. 2012;37:621-628
87. Vargas G, Rocha JD, Oliveira DL. et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015;17:389-407
88. Japiassu AM, Amancio RT, Mesquita EC. et al. Sepsis is a major determinant of outcome in critically ill HIV/AIDS patients. Crit Care. 2010;14:R152
89. Lenassi M, Cagney G, Liao M. et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110-122
90. Wurdinger T, Gatson NN, Balaj L. et al. Extracellular vesicles and their convergence with viral pathways. Adv Virol. 2012;2012:767694
91. Kim OY, Choi SJ, Jang SC. et al. Bacterial protoplast-derived nanovesicles as vaccine delivery system against bacterial infection. Nano Lett. 2015;15:266-274
92. Ogura H, Kawasaki T, Tanaka H. et al. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001;50:801-809
93. Pugholm LH, Baek R, Sondergaard EK. et al. Phenotyping of leukocytes and leukocyte-derived extracellular vesicles. J Immunol Res. 2016;2016:6391264
94. Soni S, Wilson MR, O'Dea KP. et al. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016;71:1020-1029
95. Ebong SJ, Call DR, Bolgos G. et al. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12:118-126
96. Fialkow L, Fochesatto FL, Bozzetti MC. et al. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care. 2006;10:R155
97. Gasser O, Hess C, Miot S. et al. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243-257
98. Cerri C, Chimenti D, Conti I. et al. Monocyte/macrophage-derived microparticles up-regulate inflammatory mediator synthesis by human airway epithelial cells. J Immunol. 2006;177:1975-1980
99. Midura EF, Prakash PS, Johnson BL III. et al. Impact of caspase-8 and PKA in regulating neutrophil-derived microparticle generation. Biochem Biophys Res Commun. 2016;469:917-922
100. Nusbaum P, Laine C, Bouaouina M. et al. Distinct signaling pathways are involved in leukosialin (CD43) down-regulation, membrane blebbing, and phospholipid scrambling during neutrophil apoptosis. J Biol Chem. 2005;280:5843-5853
101. Prakash PS, Caldwell CC, Lentsch AB. et al. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg. 2012;73:401-406
102. Dalli J, Montero-Melendez T, Norling LV. et al. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics. 2013;12:2205-2219
103. Timar CI, Lorincz AM, Csepanyi-Komi R. et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121:510-518
104. Lorincz AM, Schutte M, Timar CI. et al. Functionally and morphologically distinct populations of extracellular vesicles produced by human neutrophilic granulocytes. J Leukoc Biol. 2015;98:583-589
105. Mostefai HA, Meziani F, Mastronardi ML. et al. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med. 2008;178:1148-1155
106. Dalli J, Norling LV, Montero-Melendez T. et al. Microparticle alpha-2-macroglobulin enhances pro-resolving responses and promotes survival in sepsis. EMBO Mol Med. 2014;6:27-42
107. Dalli J, Norling LV, Renshaw D. et al. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512-2519
108. Miksa M, Wu R, Dong W. et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected]. J Immunol. 2009;183:5983-5990
109. Eriksson M, Nelson D, Nordgren A. et al. Increased platelet microvesicle formation is associated with mortality in a porcine model of endotoxemia. Acta Anaesthesiol Scand. 1998;42:551-557
110. Exline MC, Justiniano S, Hollyfield JL. et al. Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis. PLoS One. 2014;9:e90968
111. Hotchkiss RS, Swanson PE, Cobb JP. et al. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298-1307
112. Sarkar A, Mitra S, Mehta S. et al. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4:e7140
113. Essandoh K, Yang L, Wang X. et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015;1852:2362-2371
114. Klima J, Grafnetterova J, Kubelka Z. et al. Therapeutic monitoring of metipamide during antihypertensive therapy. J Chromatogr. 1989;488:295-300
115. Mu X, Wang X, Huang W. et al. Circulating exosomes isolated from septic mice induce cardiovascular hyperpermeability through promoting podosome cluster formation. Shock. 2018;49:429-441
116. Monteiro VVS, Reis JF, de Souza GR. et al. Dual behavior of exosomes in septic cardiomyopathy. Adv Exp Med Biol. 2017;998:101-112
117. Lugini L, Cecchetti S, Huber V. et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189:2833-2842
118. Scanu A, Molnarfi N, Brandt KJ. et al. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83:921-927
119. Oehmcke S, Westman J, Malmstrom J. et al. A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes. PLoS Pathog. 2013;9:e1003529
120. Levi M, Van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38-44
121. Rivers E, Nguyen B, Havstad S. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377
122. Delabranche X, Boisrame-Helms J, Asfar P. et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 2013;39:1695-1703
123. Wang JG, Manly D, Kirchhofer D. et al. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092-1098
124. Martinez de LS, Roncal C, Calvayrac O. et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2012;32:1477-1487
125. Matsumoto H, Yamakawa K, Ogura H. et al. Enhanced expression of cell-specific surface antigens on endothelial microparticles in sepsis-induced disseminated intravascular coagulation. Shock. 2015;43:443-449
126. Holnthoner W, Bonstingl C, Hromada C. et al. Endothelial cell-derived extracellular vesicles size-dependently exert procoagulant activity detected by thromboelastometry. Sci Rep. 2017;7:3707
127. Beer KB, Wehman AM. Mechanisms and functions of extracellular vesicle release in vivo-What we can learn from flies and worms. Cell Adh Migr. 2017;11:135-150
128. Lee SK, Yang SH, Kwon I. et al. Role of tumour necrosis factor receptor-1 and nuclear factor-kappaB in production of TNF-alpha-induced pro-inflammatory microparticles in endothelial cells. Thromb Haemost. 2014;112:580-588
129. Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27:31-39
130. Muller I, Klocke A, Alex M. et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476-478
131. Boisrame-Helms J, Delabranche X, Degirmenci SE. et al. Pharmacological modulation of procoagulant microparticles improves haemodynamic dysfunction during septic shock in rats. Thromb Haemost. 2014;111:154-164
132. Hellum M, Ovstebo R, Brusletto BS. et al. Microparticle-associated tissue factor activity correlates with plasma levels of bacterial lipopolysaccharides in meningococcal septic shock. Thromb Res. 2014;133:507-514
133. Delabranche X, Quenot JP, Lavigne T. et al. Early detection of disseminated intravascular coagulation during septic shock: a multicenter prospective study. Crit Care Med. 2016;44:e930-e939
134. Rohde JM, Dimcheff DE, Blumberg N. et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317-1326
135. Xiong Z, Cavaretta J, Qu L. et al. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610-621
136. Xie RF, Hu P, Wang ZC. et al. Platelet-derived microparticles induce polymorphonuclear leukocyte-mediated damage of human pulmonary microvascular endothelial cells. Transfusion. 2015;55:1051-1057
137. Rubin O, Delobel J, Prudent M. et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744-1754
138. Kent MW, Kelher MR, West FB. et al. The pro-inflammatory potential of microparticles in red blood cell units. Transfus Med. 2014;24:176-181
139. Gao Y, Lv L, Liu S. et al. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2013;105:11-17
140. Almizraq RJ, Seghatchian J, Acker JP. Extracellular vesicles in transfusion-related immunomodulation and the role of blood component manufacturing. Transfus Apher Sci. 2016;55:281-291
141. Straat M, Boing AN, Tuip-De BA. et al. Extracellular vesicles from red blood cell products induce a strong pro-inflammatory host response, dependent on both numbers and storage duration. Transfus Med Hemother. 2016;43:302-305
142. Straat M, Van Hezel ME, Boing A. et al. Monocyte-mediated activation of endothelial cells occurs only after binding to extracellular vesicles from red blood cell products, a process mediated by beta-integrin. Transfusion. 2016;56:3012-3020
143. Chang AL, Kim Y, Seitz AP. et al. Erythrocyte-derived microparticles activate pulmonary endothelial cells in a murine model of transfusion. Shock. 2017;47:632-637
144. Cumpelik A, Ankli B, Zecher D. et al. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann Rheum Dis. 2016;75:1236-1245
145. Belizaire RM, Prakash PS, Richter JR. et al. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg. 2012;214:648-655
146. Shaver CM, Woods J, Clune JK. et al. Circulating microparticle levels are reduced in patients with ARDS. Crit Care. 2017;21:120
147. Mutschler DK, Larsson AO, Basu S. et al. Effects of mechanical ventilation on platelet microparticles in bronchoalveolar lavage fluid. Thromb Res. 2002;108:215-220
148. Vion AC, Birukova AA, Boulanger CM. et al. Mechanical forces stimulate endothelial microparticle generation via caspase-dependent apoptosis-independent mechanism. Pulm Circ. 2013;3:95-99
149. Letsiou E, Sammani S, Zhang W. et al. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am J Respir Cell Mol Biol. 2015;52:193-204
150. Cabrera-Benitez NE, Valladares F, Garcia-Hernandez S. et al. Altered profile of circulating endothelial-derived microparticles in ventilator-induced lung injury. Crit Care Med. 2015;43:e551-e559
151. Larsson A, Lundahl T, Eriksson M. et al. Endotoxin induced platelet microvesicle formation measured by flow cytometry. Platelets. 1996;7:153-158
152. Lundahl TH, Lindahl TL, Fagerberg IH. et al. Activated platelets and impaired platelet function in intensive care patients analyzed by flow cytometry. Blood Coagul Fibrinolysis. 1996;7:218-220
153. Fujimi S, Ogura H, Tanaka H. et al. Activated polymorphonuclear leukocytes enhance production of leukocyte microparticles with increased adhesion molecules in patients with sepsis. J Trauma. 2002;52:443-448
154. Janiszewski M, Do Carmo AO, Pedro MA. et al. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit Care Med. 2004;32:818-825
155. Gambim MH, do Carmo AO, Marti L. et al. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107
156. Azevedo LC, Janiszewski M, Pontieri V. et al. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care. 2007;11:R120
157. Yin R, Dai T, Avci P. et al. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol. 2013;13:731-762
158. Perez-Casal M, Downey C, Cutillas-Moreno B. et al. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and anti-inflammatory effects. Haematologica. 2009;94:387-394
159. Forest A, Pautas E, Ray P. et al. Circulating microparticles and procoagulant activity in elderly patients. J Gerontol A Biol Sci Med Sci. 2010;65:414-420
160. Rank A, Nieuwland R, Toth B. et al. Microparticles for diagnosis of graft-versus-host disease after allogeneic stem transplantation. Transplantation. 2011;92:244-250
161. Mostefai HA, Bourget JM, Meziani F. et al. Interleukin-10 controls the protective effects of circulating microparticles from patients with septic shock on tissue-engineered vascular media. Clin Sci (Lond). 2013;125:77-85
162. Van Ierssel SH, Van Craenenbroeck EM, Hoymans VY. et al. Endothelium dependent vasomotion and in vitro markers of endothelial repair in patients with severe sepsis: an observational study. PLoS One. 2013;8:e69499
163. Trepesch C, Nitzsche R, Glass A. et al. High intravascular tissue factor-but not extracellular microvesicles-in septic patients is associated with a high SAPS II score. J Intensive Care. 2016;4:34
164. Stiel L, Delabranche X, Galoisy AC. et al. Neutrophil fluorescence: a new indicator of cell activation during septic shock-induced disseminated intravascular coagulation. Crit Care Med. 2016;44:e1132-e1136
165. Panich T, Chancharoenthana W, Somparn P. et al. Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrol. 2017;18:10
Author Biographies
Pierre Raeven completed medical school at Maastricht University, The Netherlands. He then joined the Ludwig Boltzmann Institute for Experimental and Clinical Traumatology as a research fellow in Marcin F. Osuchowski's group. Pierre obtained his PhD degree (with distinction) from the Medical University of Vienna for his thesis on the role of plasminogen activator inhibitor type 1 during experimental trauma and sepsis. Pierre currently works as a resident trainee and clinical scientist in anesthesiology and intensive care medicine at the Medical University of Vienna, Austria.
Johannes Zipperle obtained a bachelor's degree in general biology and a master's degree in molecular cell biology from the University of Innsbruck, Austria, where he focused on cardiovascular physiology. During his studies, he worked as an emergency medical technician and moved his research interests towards critical care medicine. After a research fellowship at Harvard Medical School in Boston, Johannes is currently doing his PhD at the Ludwig Boltzmann Institute for Experimental and Clinical Traumatology where he works on cellular mechanisms in trauma-induced coagulopathy.
Susanne Drechsler obtained her diploma in veterinary medicine at the University of Veterinary Medicine in Vienna, Austria. She then enrolled for a doctoral program to study the effects of age and gender in a mouse model of posttraumatic sepsis in Marcin F. Osuchowski's group at the Ludwig Boltzmann Institute for Experimental and Clinical Traumatology and received her DVM degree from the University of Veterinary Medicine in Vienna, Austria. Susanne currently leads a FWF Hertha Firnberg project investigating the complement system in posttraumatic sepsis.
![]() Corresponding author: Pierre Raeven MD, PhD, Department of Anaesthesia, General Intensive Care and Pain Management, Medical University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria. Tel.: +43 (0)1 40400 - 41020; Fax: +43 (0)1 40400 - 41040; Email: pierre.raevenac.at
Corresponding author: Pierre Raeven MD, PhD, Department of Anaesthesia, General Intensive Care and Pain Management, Medical University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria. Tel.: +43 (0)1 40400 - 41020; Fax: +43 (0)1 40400 - 41040; Email: pierre.raevenac.at
 Global reach, higher impact
Global reach, higher impact