13.3
Impact Factor
Theranostics 2018; 8(22):6163-6177. doi:10.7150/thno.28021 This issue Cite
Research Paper
Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction
1. Department of Cardiovascular Medicine, Xiangya Hospital, Central South University, Changsha, China 410008
2. Department of Geriatric Medicine, Xiangya Hospital, Central South University, Changsha, China 410008
3. Department of Neurobiology, Nanjing Medical University, Nanjing, China 211166
4. Department of Pathophysiology, Xiangya School of Medicine, Central South University, Changsha, China 410008
5. Institute of Medical Sciences, Xiangya Hospital, Central South University, Changsha, China 410078
6. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China, 410008
*These authors contributed equally to this article.
Abstract
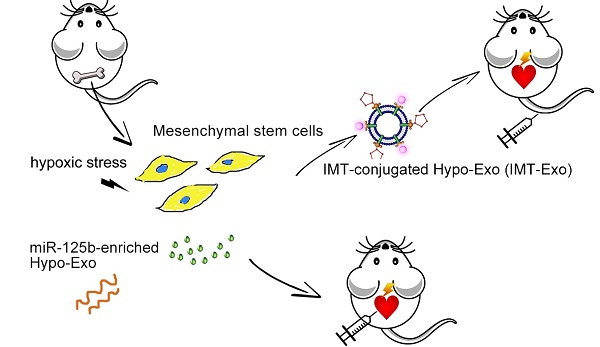
Exosomes (Exo) secreted from hypoxia-conditioned bone marrow mesenchymal stem cells (BM-MSCs) were found to be protective for ischemic disease. However, the role of exosomal miRNA in the protective effect of hypoxia-conditioned BM-MSCs-derived Exo (Hypo-Exo) remains largely uncharacterized and the poor specificity of tissue targeting of Exo limits their clinical applications. Therefore, the objective of this study was to examine the effect of miRNA in Hypo-Exo on the repair of ischemic myocardium and its underlying mechanisms. We further developed modified Hypo-Exo with high specificity to the myocardium and evaluate its therapeutic effects.
Methods: Murine BM-MSCs were subjected to hypoxia or normoxia culture and Exo were subsequently collected. Hypo-Exo or normoxia-conditioned BM-MSC-derived Exo (Nor-Exo) were administered to mice with permanent condition of myocardial infarction (MI). After 28 days, to evaluate the therapeutic effects of Hypo-Exo, infarction area and cardio output in Hypo-Exo and Nor-Exo treated MI mice were compared through Masson's trichrome staining and echocardiography respectively. We utilized the miRNA array to identify the significantly differentially expressed miRNAs between Nor-Exo and Hypo-Exo. One of the most enriched miRNA in Hypo-Exo was knockdown by applying antimiR in Hypoxia-conditioned BM-MSCs. Then we performed intramyocardial injection of candidate miRNA-knockdown-Hypo-Exo in a murine MI model, changes in the candidate miRNA's targets expression of cardiomyocytes and the cardiac function were characterized. We conjugated Hypo-Exo with an ischemic myocardium-targeted (IMT) peptide by bio-orthogonal chemistry, and tested its targeting specificity and therapeutic efficiency via systemic administration in the MI mice.
Results: The miRNA array revealed significant enrichment of miR-125b-5p in Hypo-Exo compared with Nor-Exo. Administration of miR-125b knockdown Hypo-Exo significantly increased the infarction area and suppressed cardiomyocyte survival post-MI. Mechanistically, miR-125b knockdown Hypo-Exo lost the capability to suppress the expression of the proapoptotic genes p53 and BAK1 in cardiomyocytes. Intravenous administration of IMT-conjugated Hypo-Exo (IMT-Exo) showed specific targeting to the ischemic lesions in the injured heart and exerted a marked cardioprotective function post-MI.
Conclusion: Our results illustrate a new mechanism by which Hypo-Exo-derived miR125b-5p facilitates ischemic cardiac repair by ameliorating cardiomyocyte apoptosis. Furthermore, our IMT- Exo may serve as a novel drug carrier that enhances the specificity of drug delivery for ischemic disease.
Keywords: Hypo-Exo, Nor-Exo, miR-125b-5p, myocardial infarction, IMT-Exo, apoptosis
 Global reach, higher impact
Global reach, higher impact