13.3
Impact Factor
Theranostics 2019; 9(11):3075-3093. doi:10.7150/thno.31858 This issue Cite
Review
Supramolecular nanotheranostics based on pillarenes
1. State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China
2. Department of Endoscopics, China-Japan Union Hospital of Jilin University, Jilin University, 126 Xiantai Street, Changchun 130033, P. R. China
3. School of Chemistry and Chemical Engineering, Tianjin Key Laboratory of Organic Solar Cells and Photochemical Conversion, Tianjin University of Technology, Tianjin 300384, P. R. China
4. The State Key Laboratory of Refractories and Metallurgy, School of Chemistry & Chemical Engineering, Wuhan University of Science and Technology, Wuhan 430081, P. R. China
Received 2018-11-28; Accepted 2019-3-1; Published 2019-5-18
Abstract
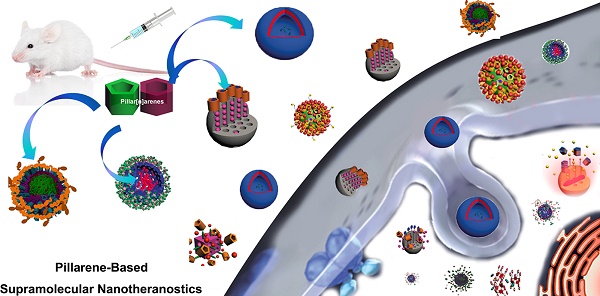
With the rapid development of supramolecular chemistry and nanomaterials, supramolecular nanotheranostics has attracted remarkable attention owing to the advantages compared with conventional medicine. Supramolecular architectures relying on non-covalent interactions possess reversible and stimuli-responsive features; endowing supramolecular nanotheranostics based on supramolecular assemblies great potentials for the fabrication of integrated novel nanomedicines and controlled drug delivery systems. In particular, pillarenes, as a relatively new class of synthetic macrocycles, are important candidates in the construction of supramolecular therapeutic systems due to their excellent features such as rigid and symmetric structures, facile substitution, and unique host-guest properties. This review summarizes the development of pillarene-based supramolecular nanotheranostics for applications in biological mimicking, virus inhibition, cancer therapy, and diagnosis, which contains the following two major parts: (a) pillarene-based hybrid supramolecular nanotheranostics upon hybridizing with porous materials such as mesoporous silica nanoparticles, metal-organic frameworks, metal nanoparticles, and other inorganic materials; (b) pillarene-based organic supramolecular therapeutic systems that include supramolecular amphiphilic systems, artificial channels, and prodrugs based on host-guest complexes. Finally, perspectives on how pillarene-based supramolecular nanotheranostics will advance the field of pharmaceuticals and therapeutics are given.
Keywords: Drug delivery, hybrid materials, macrocyclic arenes, nanotheranostics, supramolecular chemistry
1. Introduction
In the course of human history, none has attracted much intense attention and fascinated the enthusiasm of people than the pursuit of improving the quality of life. However, human beings always suffer from a large number of diseases during the lives, which cause harms to bodies or even death. Cancer, for example, is the leading cause of death worldwide and the burden of cancer deaths is still increasing. Around 18 millions of new cancer cases have been reported in 2018, and 9.6 million deaths were caused by cancers, according to the reports by WHO. Besides, non-communicable diseases such as cardiovascular disease (CVD) and diabetes, communicable diseases such as acquired immune deficiency syndrome (AIDS), flu and other viral/bacterial infections, are threatening our health and lives. Scientific community has devoted to develop new therapeutic approaches and medicines to improve drug effectiveness and decrease the grim side effects [1, 2]. Hart-fought progress has been made in the fields of biological materials, diagnosis and treatments [3, 4]. The aims are to acquire more in-depth knowledge of life course and consequently improve the quality of lives [5].
Supramolecular chemistry is based on non-covalent interactions that can associate the building blocks together to endow them with reversible and stimuli-responsive properties [6]. Non-covalent interactions including electrostatic interactions, π-π stacking interactions, cation-π interactions, hydrophobic effects, and hydrogen bonding interactions are the basic forces in living bodies to maintain the growth and reproduction. Therefore, supramolecular architectures are the ideal model to mimicking biological actions. Thus, researchers have made efforts to integrate supramolecular architectures into medicines or to prepare new drugs for theranostics [7]. The multiple functions of supramolecular architectures and nanomaterials integrated in uniform systems bestow them the opportunities to construct novel nanomedicines, achieving the tumor-targeting drug delivery and specific antibacterials or virus inhibitors [8, 9].
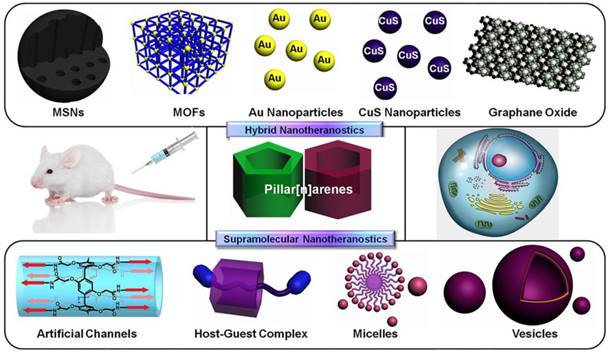
Supramolecular macrocycles, including crown ethers, cyclodextrins, cucurbiturils, calixarenes, and pillararenes (or pillarenes), have typical cavity structures, which can host small molecules based on host-guest interactions [10]. In particular, pillarenes and their derivatives, as a relatively new class of macrocycles first reported by Ogoshi et al. in 2008, have been extensively investigated during the past decade. Many pillarene derivatives have been synthesized and their host-guest properties were studied [11-16]. Their applications have developed rapidly in the areas of materials science [17-20], biological science [21], and industrial engineering [22-23], because of their versatile substitution [24, 25], rigid structures, and typical host-guest properties [26-29]. Pillarenes exhibit remarkable potentials in biomedical science via constructing supramolecular systems to achieve drug design and effective therapeutic [30-32]. Prodrugs can be prepared through the design and synthesis of pillarene derivatives. Meanwhile, a variety of supramolecular architectures such as vesicles and nanoparticles can be further fabricated via self-assembly of supramolecular amphiphiles [33,34] that can load cargos inside [35]. On the other hand, pillarenes can be hybridized with many inorganic materials, such as mesoporous silica nanoparticles (MSNs), metal-organic frameworks (MOFs), carbon materials, and metal nanoparticles. Taking advantages of the reversible and stimuli-responsive features of pillarene-based host-guest systems, precise drug delivery and controllable drug release could be realized (Figure 1) [36, 37].
Schematic illustration of the classification of pillarene-based supramolecular nanotheranostics. Pillarenes have been hybridized with mesoporous silica nanoparticles (MSNs), metal-organic frameworks (MOFs), metal nanoparticles, graphene oxide, etc. The pillarene-based supramolecular therapeutic systems are mainly constructed from their synthetic modifications, host-guest complexes, and the supramolecular assembly architectures.
In this review, we summarize recent advances in supramolecular nanotheranostics based on pillarenes, which include pillarene-based biological systems, supramolecular amphiphilic systems, and pillarene-based hybrid materials, and their applications in drug delivery, cell imaging, antibacterials, virus inhibitor, and so forth.
2. Supramolecular hybrid therapeutic systems based on pillarenes
2.1 Supramolecular nanotheranostic platforms based on MSNs equipped with pillarene nanovalves
MSNs are one kind of well-defined mesoporous silica nanomaterials with superior characteristics such as high specific surface area, good biocompatibility, tunable pore size, uniform morphologies, mature synthetic methods, facile surface modification, and stable structures. MSNs can be used as inorganic supports to load cargo molecules and protect them from enzymatic degradation and microbial attack, facilitating the targeted drug delivery and controlled release of drugs to the intended cells and tumors. Considering the desirable features of MSNs, diverse gatekeepers to keep cargo within the porous carriers have been designed and prepared since the discovery of the controlled cargo delivery system based on coumarin-modified MCM-41 nanoparticles reported in 2003 [38]. Researchers have been working hard to design a variety of gatekeepers that can be responsive to different external stimuli including light, chemical signals, pH, temperature, oxidation-reduction, enzyme, and biological inputs.
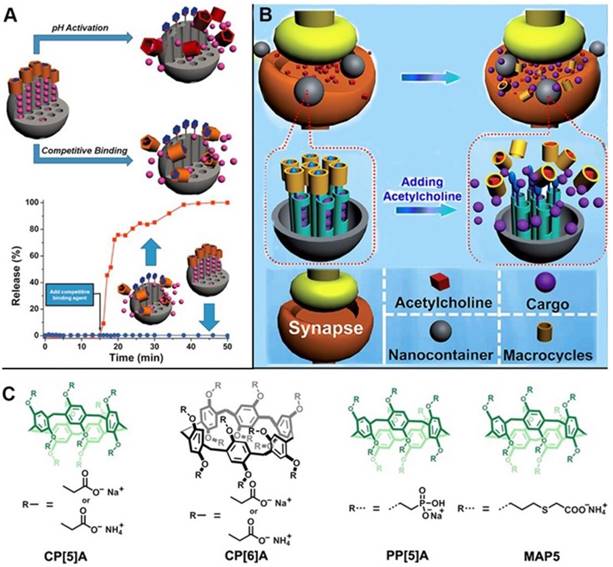
Pillarenes with attractive structural and host-guest properties are promising candidates to fabricate supramolecular nanovalves. In 2013, the first pillarene-based gatekeeper anchored on the pore orifices of MSNs for on-command cargo release was designed and constructed by us [39]. The terminal pyridinium stalks modified on the surface of MSNs were encircled by carboxylatopillar[5]arenes (CP[5]A), and could be activated by pH changes and/or by adding competitive binding agents so that the trap and release of dye or drug molecules could be achieved (Figure 2A) [34]. In the same year, our group reported the first enzyme-responsive supramolecular nanovalves consisting of MSNs surface-modified with the choline moieties encircled by the sulfonatocalix[4]arene (SC[4]A), an analogue of pillarenes [40]. Since then, several pillarene-based supramolecular nanovalve systems were designed and prepared. For example, based on the previous study, we subsequently reported the acetylcholine (ACh)-triggered drug delivery systems based on MSNs functionalized by supramolecular nanovalves. CP[5]A capped on the stalks would be dethreaded from the stalks and the loaded cargo molecules would be released upon increasing the concentration of competitive agents, i.e., ACh, suggesting its great potential for the treatment and recovery of Parkinson's disease (Figure 2B) [41].
(A) Illustration of pillar[5]arene-based supramolecular nanovalve on MSNs, regulating the release of cargos in response to pH changes and competitive agents; (B) Illustration of cargo release from MSN-based nanocarriers in response to acetylcholine. (C) Chemical structures of pillarene derivatives. Reproduced with permission from [39] and [41], copyright 2013, 2014, respectively, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Schematic illustrations of (A) pillar[6]arene-based triple-responsive drug delivery system constructed from MSNs; Reproduced with permission from [42], copyright 2014 American Chemical Society, (B) CP[5]A-based bistable [2]pseudorotaxane-modified MSNs for triple-responsive controlled release; Reproduced with permission from [43], copyright 2016 Royal Society of Chemistry, (C) near-infrared (NIR) light responsive hybrid nanocontainers embedded with Au nanorods for drug controlled release; Reproduced with permission from [45], copyright 2017 American Chemical Society, (D) pH-responsive drug delivery systems based on hollow MSNs and pillar[5]arene. Reproduced with permission from [47], copyright 2019 Royal Society of Chemistry.
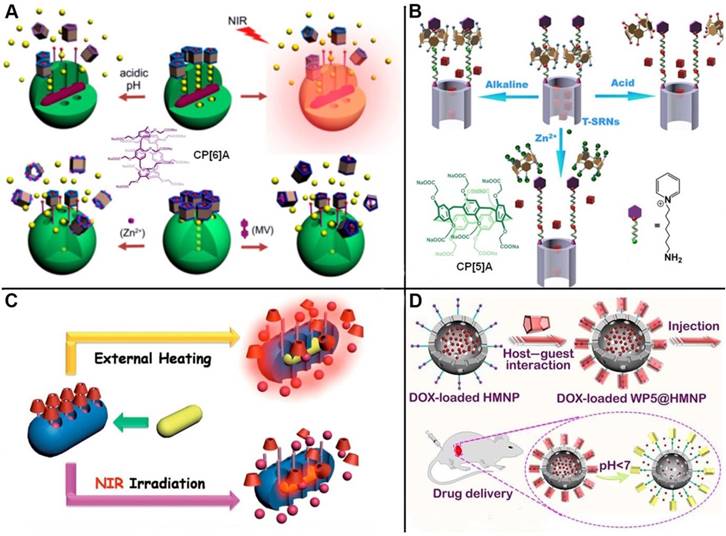
Taking advantage of the typical reversible and stimuli-responsive properties of pillarene-based host-guest systems as non-covalent binding, some multi-stimuli responsive supramolecular nanovalves on MSNs have also been constructed and showcased in literature. Du and coworkers developed dimethylbenzimidazolium and bipyridinium stalks-modified MSNs to encapsulate cargos, where carboxylatopillar[6]arene (CP[6]A) acted as supramolecular gating entities encircled on the stalks. Coordination chemistry was introduced to activate the nanovalves by metal chelating with the carboxylate groups of CP[6]A. Thus, the cargo release can be achieved upon lowing pH or adding competitive binding agents for dethreading of CP[6]A from the stalks, or adding metal ions to competitively chelate with CP[6]A to rob them from the stalks, which can meet requirements of multi-responsiveness and targeted drug delivery (Figure 3A) [42]. Fu and coworkers fabricated a triple-stimuli-responsive nanovalve consisting of CP[5]A-based bistable [2]pseudorotaxanes-modified MSNs. The doxorubicin (DOX) drugs were encapsulated within the pores and blocked by the host-guest complexes of CP[5]A and 1,6-hexanediammonium. Controlled cargo release was realized upon exposure to acid, alkali pH or Zn2+. In addition, MTT assays and in vitro release experiments were carried out to demonstrate that the nanocontainers can be applied for targeted drug delivery (Figure 3B) [43].
The sealing of the pores of MSN solid support after loading anticancer drugs is very important to avoid the leakage of drugs before they arrive at the lesion sites. Polymers have been integrated with the nanocarriers to lower the premature release of cargo. We designed and reported the layer-by-layer (LbL) coated MSNs (LbL-MSNs), with good biocompatibility, negligible premature drug leakage, and dual-responsive drug release. The ambient LbL consisted of bis-aminated poly(glycerol methacrylate)s (BA-PGOHMAs) and cucurbit[7]uril (CB[7]), in which CB[7] acted as the bridge to hold the two bis-aminated polymeric layers via host-guest interactions. In the acidic conditions or after adding competitive binding agents, such as adamantaneamine hydrochloride (AH) or hydronium ions, controlled release was realized and DOX molecules could escape from the pores to achieve the anticancer goal. Significantly, operating pH to acid condition matches the acidifying endosomal environments of HeLa cancer cells, indicating their great potentials to wipe out the cancer cells. The in vitro and in vivo release experiments of DOX-loaded LbL-MSNs materials were performed, indicating the materials are capable of delivering anticancer drug in specific cells and inhibit the tumor growth [44].
To expand the applications of pillarene switches-gated MSNs, the nanocontainers hybridized with other metal nanoparticles with unique characteristics, for example, Au nanoparticles (AuNPs) or Au nanorods (AuNRs). In 2014, we constructed a near-infrared (NIR) light responsive hybrid nanocontainer (AuNR@MSN), in which AuNRs was coated by MSNs and the surface was further installed with SC[4]A-based supramolecular switches. "Ladder-type" pulsatile release was successfully achieved upon irradiation of NIR light, relying on the plasmonic photothermal conversion of AuNRs (Figure 3C) [45]. In 2017, Du and coauthors designed a kind of novel supramolecular nanovalves were constructed by MSNs functionalized with choline or pyridinium stalks encircled by phosphonated pillar[5]arenes (PPA[5]). PPA[5]-based supramolecular switches as the gatekeepers minimized the premature drug release as compared with CP[5]A-based version. The controlled drug release could be activated by lowing pH value, Zn2+ coordination, and adding competitive agents. AuNRs were embedded into the PPA[5] supramolecular switches modified MSNs. The resulted hybrid nanocontainers realized the controlled drug release upon being irradiated by NIR light, and enhanced the release efficiency and antitumor efficacy due to the synergistic effects, which indicated their promising potentials in tumor photothermo-chemotherapy [46].
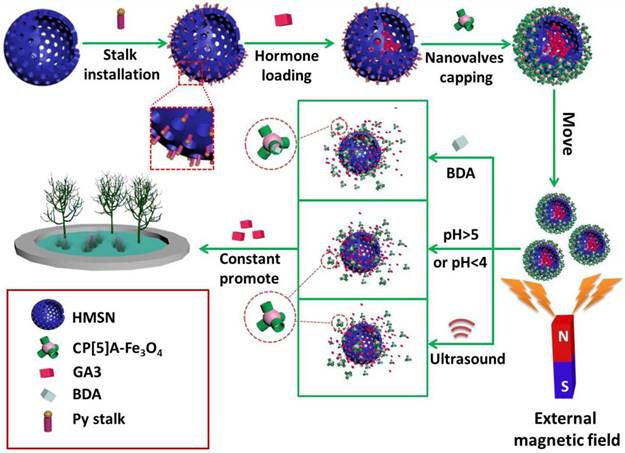
To satisfy the demand of encapsulating drug molecules of different sizes and properties, a variety of inorganic supports have also been designed and prepared. Huang and co-workers constructed the supramolecular nanovalves consisting of hollow MSNs (HMSNs) modified by CP[5]A-based host-guest complexation, releasing DOX readily and controllably under low pH (Figure 3D) [47]. Interestingly, we fabricated a hollow MSN-based hormone delivery system with multi-stimuli responsive property (Figure 4). HMSNs were capped by CP[5]A-functionalized Fe3O4 magnetic nanoparticles which served as nanovalves, and gibberellin acid (GA3) was encapsulated within the mesopores. Controlled release of GA3 was regulated by lowing or raising pH values, adding 1,4-butanediamine (BDA), and ultrasound. The magnetic hybrid material was successfully used in the control of plant growth like Arabidopsis thaliana (A. thaliana) and cabbages, suggesting their promising application in the promotion of agricultural industry development [48].
Schematic illustration of the construction of pillarene-magnetic nanoparticles-gated hollow MSNs (HMSNs) for magnetic operated, multi-stimuli responsive hormone release to promote the growth of plants. Reproduced with permission from [48], copyright 2019 Royal Society of Chemistry.
2.2 Supramolecular nanotheranostic platforms based on MOFs equipped with pillarene nanovalves
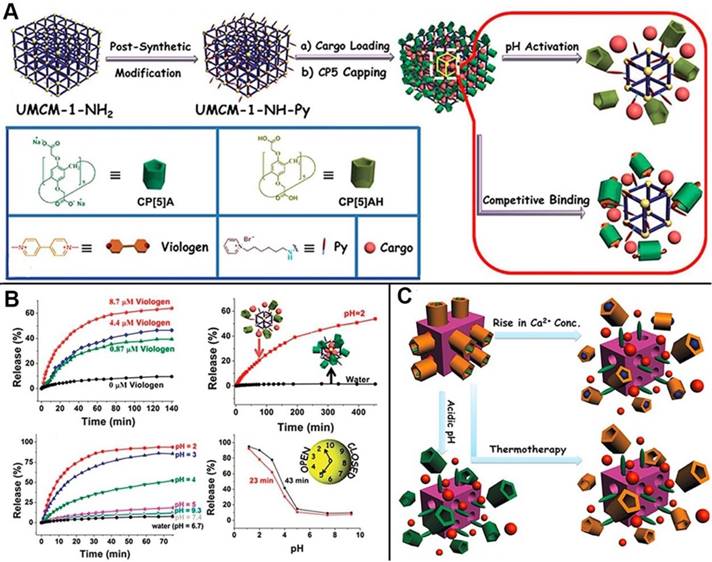
MOFs, as advanced inorganic-organic hybrid porous materials, also serve as one of the most promising candidates to construct drug delivery systems for next-generation cancer theranostics because of their desirable characteristics such as robust structures, large pore sizes for drug loading, versatile functionality, high surface areas, and good biocompatibility. Several MOF-based drug delivery systems have also been reported [49]. In 2015, the first mechanized nanoMOFs equipped with CP[5]A-based supramolecular switches as the gates to encapsulate drugs within the pores was reported by us, representing a new class of theranostic platform (Figure 5A). The delivery of therapeutic agents was controlled and regulated by adjusting pH or adding competitive binding agents (Figure 5B) [50]. Subsequently, another theranostic nanoplatform constructed from Zirconium MOFs (Zr-MOFs) by our research group. Positive charged stalks encircled with negatively charged CP[5]A were functionalized on MOF surfaces to block the loaded drugs inside the pores, and the cargo release was triggered by addition of Zn2+, indicating the hybrid materials are promising for brain disease therapy [51]. Meanwhile, Ca2+, pH and thermo triple-responsive theranostic Zr-MOFs installed with CP[5]A-based supramolecular switches were obtained for on-command drug delivery potentially in bone diseases. The variations of pH and Ca2+ concentrations inside the bone tumor cells triggered the drug release to achieve efficient treatment (Figure 5C). Simultaneously, adjusting temperature regulated the drug release in the above mechanized MOFs, which is also one of the popular cancer therapy techniques named as hyperthermia. The supramolecular nanocarriers fabricated from mechanized Zr-MOFs exhibited negligible premature release and cytotoxicity, providing a good opportunity for developing ideal therapeutic drug delivery systems for bone regeneration, brain recovery, and cancer therapy [52].
(A) Schematic illustration of stimuli-responsive nanocarriers based on mechanized nanoMOFs with surface-installed, positively-charged stalks encircled by pillarenes. The mechanized UMCM-1-NH2 nanocarriers can be operated either by pH changes or by competitive binding to regulate the release of cargos such as DOX; (B) Release curves represent the controlled drug delivery performance of MOF-based nanocarriers; Reproduced with permission from [50], copyright 2015 Royal Society of Chemistry. (C) Illustration of stimuli-responsive mechanized Zr-MOFs, operated by pH changes, Ca2+ concentration changes, and heating to regulate the release of 5-Fu. Reproduced with permission from [52], copyright 2016 Royal Society of Chemistry.
Schematic representation of (A) the fabrication process and operation of CP[6]A-based Fe3O4@UiO-66 theranostic nanoplatform and the structures of the representative building blocks; Reproduced with permission from [53], copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, and (B) UiO-66 MOFs serving as nanoplatform for dual targeted chemophotothermal therapy of cervical cancer. Reproduced with permission from [54], copyright 2018 American Chemical Society.
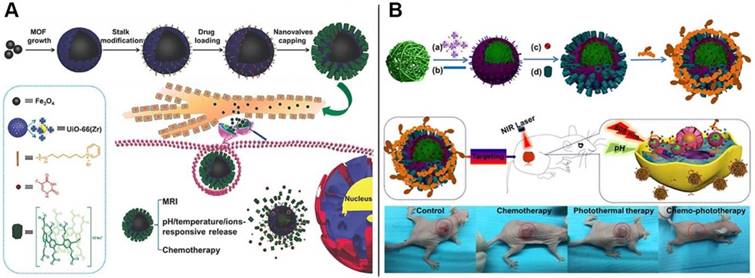
Inspired by the hybrid nanocontainers constructed from MSNs, we envisioned that the mechanized inorganic platforms embedded with metal nanoparticles could be endowed with unique features such as light-responsiveness. Thus, we reported the construction of the intelligent theranostic core-shell hybrids, comprising of Fe3O4 magnetic nanoparticles as the core, UiO-66 MOF with large loading capacity as the shell, and CP[6]A-based pseudorotaxanes anchored on the surface as the gatekeepers, which integrated the multi-functions including tumor microenvironment-activated drug release (triple responsiveness towards pH, temperature and competitive binding agents), effective chemotherapy, sustained release ascribed to the tight host-guest interactions, and superior abilities of magnetic resonance imaging (MRI). The new theranostic nanoplatform with tight nanovalves and multi-functions paves a new way to design controllable sustained drug delivery systems and improve the therapeutic effects (Figure 6A) [53]. We also designed and prepared another multifunctional supramolecular nanotheranostic material via LbL strategy, in order to improve the efficiency of cancer therapy. First, polypyrrole nanoparticles (PPy NPs) with photothermal conversion capability were coated with UiO-66 MOFs with high loading capacity as the nanocontainers (Figure 6B). Then CP[6]A were introduced to encircle the stalks onto the surface to result in supramolecular nanovalves to regulate drug release. Finally, folic acid conjugated polyethyleneimine (PEI-Fa) capable of targeting certain cancer cells was covered onto the nanoplatform surface through electrostatic interactions. Integrating the whole functions of each components, the multifunctional supramolecular nanoplatforms with the functions of NIR or pH responsive drug release and targeted delivery exhibited efficient synergistic chemophotothermal therapy for cervical cancer, as proved by in vitro and in vivo data, indicating the good efficacy in tumor inhibition [54].
2.3 Supramolecular nanotheranostic platforms based on other inorganic materials equipped with pillarene nanovalves
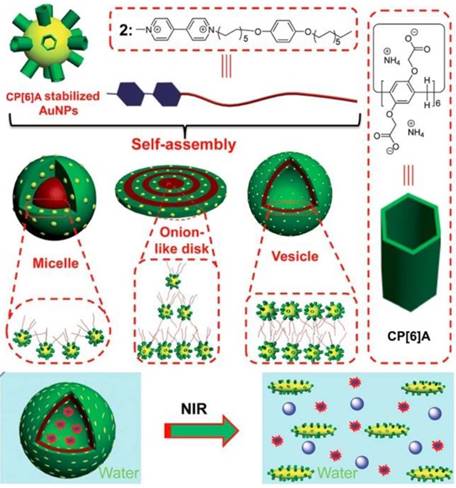
Metal nanoparticles have been used to hybridize with MSNs or MOFs to construct multi-functional materials, taking advantage of the optical or chemical properties of metal nanoparticles. Pillarenes with typical substitutions can also stabilize metal nanoparticles directly and show unique biological applications. Huang and co-workers prepared the CP[6]A-stabilized AuNPs and AuNRs (Figure 7). Different morphologies including micelles, onion-like disks, and vesicles were fabricated through the self-assembly of CP[6]A-stabilized AuNPs and paraquat derivative G1 with hydrophobic chains. Furthermore, supramolecular hybrid vesicle was constructed from the assembly of CP[6]A-stabilized AuNRs and G1, encapsulating cargo within the hollow hearts and achieving the controlled drug release upon decreasing pH or irradiating by NIR light [55]. Cell membrane proteins play the key roles in cell life, e.g., signaling cascades, transportation, and intercellular communication. Therefore, the separation of cell membrane proteins is significant to figure out the structures and their operation for cell life regulation. However, the low concentration and purity of membrane proteins limit their isolating efficiency. So, Huang, Shan, Mao and their coworkers reported a new method for separation and enrichment of cell membrane proteins based on host-guest recognition of per-phosphate pillar[5]arene (PP[5]A) and magnetic solid-phase extraction. PP[5]A rings were modified on the surface of magnetic Fe3O4 nanoparticles, which were used for separation and enrichment of guest-tagged cell membrane proteins upon exposure to magnetic field [56].
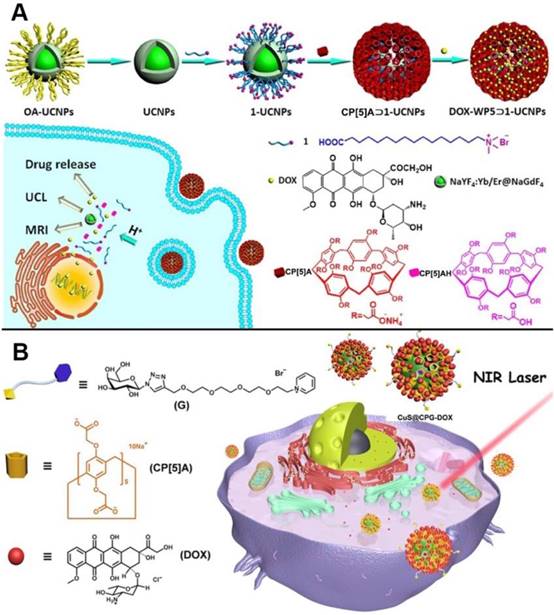
Drug delivery systems comprising of pillarenes hybridized metal nanoparticles were also constructed. In 2018, upconversion nanoparticles (UCNPs) were integrated with CP[5]A to prepare a novel supramolecular upconversion nanosystems via the host-guest recognitions (Figure 8A). DOX as one kind of chemotherapeutic drug was loaded in the nanosystems. The DOX-loaded upconversion nanomaterials could enter into HeLa cells by endocytosis and the acidic conditions in cytoplasm of cancer cells could make CP[5]A protonated, resulting in drug release. Intriguingly, the hybrid nanosystems could be used as MRI contrast agent for cancer diagnosis and treatment due to their MRI and upconversion luminescent imaging along with the drug release, indicating their bioimaging-guided therapeutic applications [57]. Yu, us, and coworkers also designed and constructed the CP[5]A-modified CuS nanoparticles, which were further coated with the galactose derivative as the liver cancer target sites (Figure 8B). The resulting hybrid materials possessed multiple functions of targeting, chemotherapy, and photothermal therapy. In vitro and in vivo experiments demonstrated the NIR-responsive hybrid nanosystem integrated the multiple advantages including good biocompatibility, enhanced therapeutic effect, and excellent tumor inhibition [58].
Graphene, especially its other forms like graphene oxide (GO) or reduced graphene oxide (RGO), have attracted great attention due to their unique electronic and optical properties. Many hybrid materials based on graphene have been prepared and their applications including electronic devices, energy storage, and optical devices have been well demonstrated. In 2014, Zhao and coauthors reported the pillarene-modified GO for in vitro Raman imaging, suggesting the potentials of the hybrid materials in dual-mode diagnostics [59]. In 2016, Li and co-workers reported the hybrid materials fabricated from hydrazino-pillar[5]arene functionalized GO, providing the fluorescent probe for paraquat through the fluorescence competition displacement in living cells and mice [60]. Recently, ultrasound and photoacoustic signal nanoamplifier, comprising of pillar[6]arene-based host-guest ensemble and GO, was constructed through π-π stacking for the first time. CO2 bubbles were generated on the surface of the material, resulting from photothermal effect and bicarbonate counterions mediated by NIR light, achieving the strong enhancement of ultrasound and photoacoustic that holds great potential in diagnostics [61].
Schematic illustration of the self-assembly of WP6-stabilized AuNPs and a guest into various hybrid nanostructures in water and the NIR-triggered vesicle-to-micelle transition, resulting in the release of encapsulated calcein. Reproduced with permission from [55], copyright 2014 Royal Society of Chemistry.
Schematic representation of (A) the construction of upconversion nanoparticle-based nanosystem and its application in drug release and cell imaging; Reproduced with permission from [57], copyright 2018 American Chemical Society, and (B) the assembly of galactose derivative (G) with CP[5]A on CuS@CP NPs for DOX loading and release. Reproduced with permission from [58], copyright 2018 American Chemical Society.
3. Supramolecular therapeutic systems with pillarenes as core components
3.1 Artificial channels constructed from pillarenes
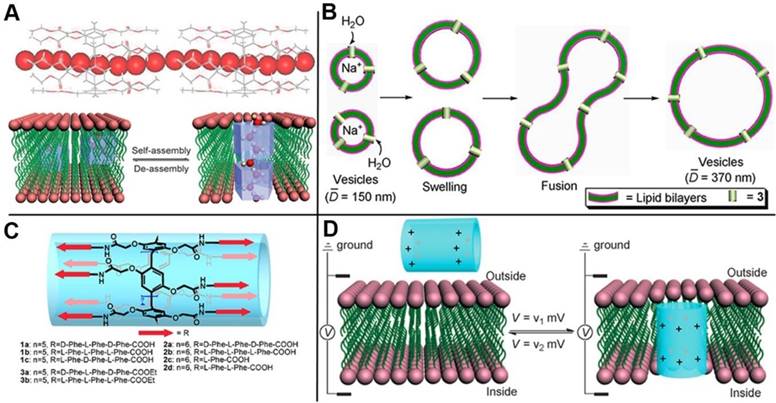
Cytomembrane that consists of lipid bilayer and transmembrane channel proteins on lipid bilayer play a crucial role in vital process to maintain the transmittance of substance in and out of the cells. The channels possess very important functions to prevent cells from microbial invasion. Channelopathy can be induced if the transmembrane channels are aberrant, for instance, flu for H+ channel, epilepsia for K+ channel, and cystic fibrosis for Cl- channel. Therefore, insuring the effectiveness of the transmembrane channels and fabricating highly controlled transmembrane channels are necessary. Inspired by the pillar shapes of pillarenes and their versatile functionalities, Hou and coworkers designed and synthesized a series of artificial transmembrane channels based on pillarenes and realized the controlled transmission of protons (Figure 9A) [62], water (Figure 9B) [63], and amino acids (Figure 9C) [64] across the cytomembrane, respectively. Furthermore, they designed a strategy to drive and control the artificial channels' insertion into or leaving from a lipid bilayer, reaching the goal of regulating the transmembrane transport by voltage (Figure 9D) [65]. Phosphatidylcholine is the major component of mammalian cells, while phosphatidylglycerol is the major component of prokaryotic cells. Based on this difference, membrane-active therapeutic agents can be designed by synthetic channels with selective target toward different cells. Hou and coworkers synthesized a new unimolecular pillar[5]arene channel that selectively and efficiently inserted into the lipid bilayer of mammalian cells by electrostatic interactions, resulting in its strong ability to destroy cancer cells. This study also provided us a new method to distinguish normal and cancer cells by constructing stimuli-responsive channels [66].
(A) Partial X-ray crystal structure of bridged pillar[5]arene, indicating that water molecules (CPK model) were induced to form linear water wires in organic nanotubes (stick model). Reproduced with permission from [62], copyright 2011, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Schematic representation of the increase in vesicle size caused by outside-to-inside water transport. Reproduced with permission from [63], copyright 2012 American Chemical Society. (C) The structure of artificial channels for amino acids transmission. Reproduced with permission from [64], copyright 2013 American Chemical Society. (D) Schematic presentation for the voltage-driven channel inserting into and leaving of lipid bilayer. Reproduced with permission from [65], copyright 2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
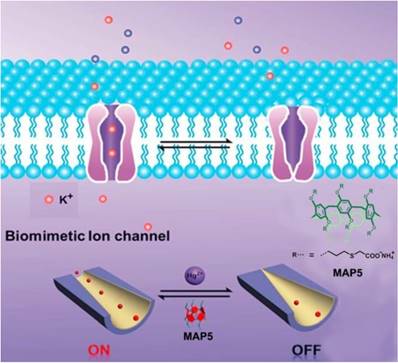
In 2016, Li and coworkers synthesized an artificial nanochannel assembled from mercaptaoacetic acid pillar[5]arene (MAP5), modulating the tunable mercury ion-gate. Mercury ion is toxic and harmful for organisms, which can damage the brain and kidneys. The toxicity of mercury ion was resulted from blocking potassium ion channels. In order to investigate the pathology and toxicology, it is necessary to mimic the complicated biological process. The ''on-off" switch in the presence and absence of mercury ion was actualized by potassium ion transport (Figure 10). Meanwhile, the nanochannel functionalized with MAP5 can selectively detect mercury ions and serve as the nanoelectronic logic devices [67]. Li, Sun and coworkers also reported the temperature-sensitive artificial ion channels by introducing pillar[5]arene-based host-guest complex. The surface change of the ion channel was changed by controlling the pillar[5]arene-based host-guest interactions, leading to the switch of ion transport from cations to anions [68]. Nome, Garcia-Rio and coworkers synthesized a supramolecular artificial enzyme for phosphate diester hydrolysis based on imidazole-functionalized pillar[5]arenes [69]. These studies opened a new window in understanding the sophisticated biological process and suggested potential applications of pillarenes in the construction of different therapeutic agents.
Schematic diagram of the design of a biomimetic mercury ion-gated nanochannel. Reproduced with permission from [67], copyright 2014 Royal Society of Chemistry.
3.2 Supramolecular theranostics based on host-guest recognition of pillarenes
Pillarenes possess unique host-guest interactions towards cationic guests or electron-deficient neutral guests due to their electron-rich cavities. Significantly, pillarenes and their derivatives also exhibit specific recognitions to molecules important for biological processes. Li, Jia and co-workers already reported the selective complexation between CP[5]A and amino acids, which have been playing the key role to sustain physiological processes because they are the basic building blocks for construction of proteins and metabolites [70]. A novel fluorescent sensor for ʟ-tryptophan based on functionalized pillar[5]arene was designed and synthesized, taking advantage of the efficient binding affinity of the pillar[5]arene derivative towards ʟ-tryptophan [71].
Except fluorescent sensors for amino acids, pillarene-based host-guest interactions have also been applied to light up cells and other organelle. For instance, Huang and coworkers synthesized two tetraphenylethene (TPE)-based guest molecules with aggregation-induced emission (AIE) property, containing electron-deficient paraquat groups (TPE-PQ) and electron-rich naphthalene (TPE-NP), respectively. Thus, difunctional negatively charged pillar[6]arene can include the paraquat groups within the cavity and form stable inclusion complex with TPE-PQ. Meanwhile, TPE-NP and TPE-PQ can form complex based on the charge-transfer (CT) interactions between naphthalene and paraquat groups. The host-guest complex was disassociated and AIE-enhanced CT complex between TPE-PQ and TPE-NP formed upon lowing pH, enabling the application in cancer cell imaging due to the more acidic endosomes [72]. They also developed a pillar[5]arene-based [2]rotaxane with AIE-active stoppers, which can be used for the specific imaging of mitochondria. Furthermore, DOX was introduced into the [2]rotaxane by covalent imine bonds, serving as anticancer drugs trigged upon hydrolysis of imine bonds [73]. Adjustable magnetization transfer MRI was realized based on the complex of water-soluble pillar[5]arene and 129Xe, indicating that pillarene derivatives are the promising candidates to design biosensors to expand the diagnosis [74]. Evtugyn and coworkers reported the electrochemical sensor prepared by modifying carbon black and pillar[5]arene on glassy carbon electrode, which could be applied for the detection of organic acid and DNA [75].
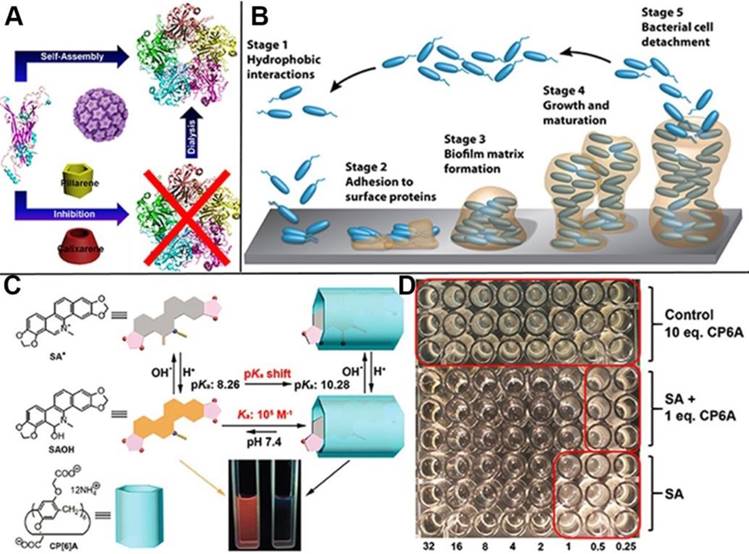
Virus and bacterial, especially those with infectivity and pathogenicity, threaten human health severely. Great effort has been devoted to the exploration and development of medicines and strategies to prevent them colonization and proliferation. For instance, many type of vaccines have been developed to protect people against human papillomavirus (HPV), as the causative agent of anogenital cancer, especially cervical cancer. Pillarenes and calixarenes as the macrocycles exhibit selective binding toward Arg and Lys at the monomer interface of HPV16 L1 pentamer, indicating their potentials to inhibit the formation of HPV16 L1 virus (Figure 11A). The experimental results showed that pillarenes was more efficient than calixarenes in inhibiting the pentamer formation, suggesting that they can be used as virus inhibitor for therapeutic demand [76]. Taking advantage of the facile functionalization of pillarenes, Imberty, Vidal and coauthors synthesized pentavalent pillar[5]arene derivatives with five galactose groups and five fucose groups, respectively, since glycoclusters have the potential to disrupt bacterial biofilm and serve as the therapeutic alternatives to conventional antibiotics. Their binding affinity to three types of bacterial lectins was investigated, demonstrating these pillarene-based glycoclusters exhibited multivalent and selective binding to pathogenic bacterial lectins such as BambL from Burkholderia ambifaria and LecA and Lec B from Pseudomonas aeuruginosa, indicating their potentials as therapeutic alternatives in treating injection [77]. Li, Cui and coworkers investigated the complexation of CP[6]A and sanguinarine alkaloid (SA), as a physiologically active substance, discovering that CP[6]A has not only increased the solubility of SA in water, but also enhanced the antibacterial activity by encapsulating SA in cavity (Figure 11C, D) [78]. In 2018, Pisagatti, Notti and coauthors developed a new type of pillar[5]arene-based multilayer carried antibiotic drugs, i.e., levofloxacin and amikacin, via host-guest interactions. The multilayer was endowed with antiadhesive and sustained antibiotic release properties, which was effective for drastically reducing the adhesion and the proliferation of Gram-positive and Gram-negative bacteria [79]. Considering most of bacterial injections are along with biofilm formation and the bacterial are against antibiotics with the protection of biofilms, Cohen, Fridman and coworkers reported the cationic pillarene derivatives to inhibit the biofilm formation effectively (Figure 11B). The positive charges, pillarene structures and host-guest interactions of pillarenes contributed to the inhibition of biofilm formation. Neither membrane damage nor cytotoxicity was caused by the cationic pillarenes, indicating their therapeutic applications related to the effects of biofilm [80]. They also designed and synthesized the phosphonium-substituted pillar[5]arene for inhibition of biofilm formation. In this continuous study, the experimental results testified that both positive-charge cooperativity and pillar platform were essential for their antibiofilm activity [81]. In 2017, Pisagatti, Notti and coworkers reported CP[5]A as the carrier for amikacin, i.e., a classical aminoglycoside antibiotic and antibacterial property toward Staphylococcus aureus. The drug release could be realized by weakening the host-guest interaction, indicating the potential of pillarenes as carriers for antibacterial theranostics [82].
According to above mentioned examples, pillarenes with appropriate modification exhibited efficient host-guest interactions toward typical molecules that are important for physiological process, enabling them to construct diagnostic or therapeutic agents for various disease. Pillarene derivatives can also recognize some other molecules for biological applications. In 2016, Li and coworkers developed the protein adsorption switch, utilizing the host-guest recognition of butoxy pillar[5]arene and adipic acid [83]. Peptides are the basic building blocks to fabricate proteins. Hu, Schmuck and coworkers developed the first pillarene functionalized with a guanidiniocarbonyl pyrrole (GCP)-conjugated short peptide segment. The obtained amphiphilic peptide could finally self-assemble into "pearl necklace" shape and serve as gene transfection vector based on the efficient host-guest interaction.[84] Adenosine triphosphate (ATP) is the intracellular mediator and energy source, playing a crucial role in biological process. Multidrug resistance (MDR) was usually caused by the decrease of the influx rate of drug, utilizing the energy generated from ATP hydrolysis. Huang and coworkers discovered the efficient recognition between cationic pillar[6]arene and ATP due to the hydrophobic cavity. Thus, the copolymer functionalized with folic acid as the targeted segment was introduced into PEGylate pillar[6]arene to fabricate the targeted drug delivery system. The energy source contributing to MDR was cut off by the host-guest complexation of pillar[6]arene and ATP, resulting in the enhancement of efficacy of the anticancer drug loaded in the supramolecular delivery system [85].
Schematic illustration of (A) the inhibition of human papillomavirus 16 L1 pentamer formation by CP[5]A, (B) the stages of biofilm formation, (C) the structures of the two forms of CP6A and SA and their host-guest binding behavior, and (D) the antimicrobial (S. aureus) assay of SA, 1:1 molar ratio of SA and CP6A, and control of 10 eq. CP6A. Reproduced with permission from [76] and [78], copyright 2014 and 2017 Royal Society of Chemistry. Reproduced with permission from [80], copyright 2016 American Chemical Society.
3.3 Pillarene-based supramolecular amphiphiles for controlled drug delivery
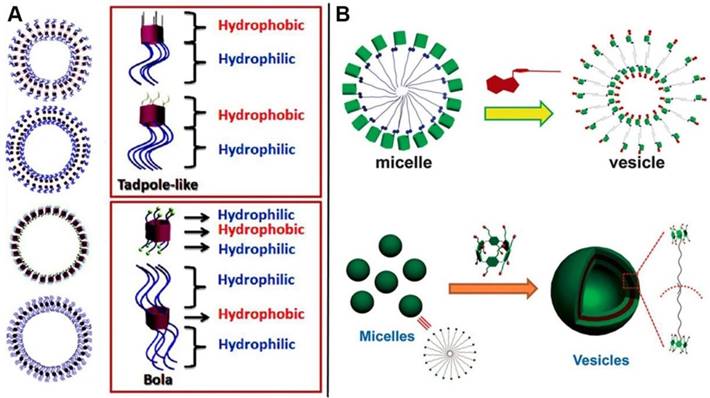
Amphiphiles, containing hydrophobic and hydrophilic portions on the different sides, can construct a variety of well-defined structures with unique properties. Supramolecular self-assemblies are the ordered architectures, where the building blocks are gathered together through non-covalent interactions. Representative assembled amphiphilic structure comes to lipid bilayer in biological membranes, indicating the significance of amphiphiles in physiological process. Synthetic amphiphiles, especially macrocyclic amphiphiles, have attracted much attention during the past decades due to their unique characteristics of structures and host-guest interaction [33]. A variety of supramolecular amphiphiles fabricated from pillarene-based assemblies have also been reported during the past decade. Pillarene-based supramolecular amphiphiles could mainly be constructed by two methods: (i) modification of the two sides of pillarenes with hydrophobic and hydrophilic chains by covalent bonds, respectively, for further self-assembly into different morphologies (Figure 12A); and (ii) formation of host-guest complexes from water-soluble pillarenes and guests with hydrophobic chains that could further self-assemble into typical architectures (Figure 12B). The supramolecular amphiphiles are promising candidates to construct drug delivery systems because the amphiphilic features make them easily enter into cells by endocytosis.
In 2012, Huang, Liu and coworkers reported the vesicle-like structures by host-guest complexation of pillar[6]arene and photo-responsive guest containing azobenzene [86]. Subsequently, five amphiphilic pillar[5]arene derivatives were synthesized to investigate the influence of chemical structures on their self-assembly morphologies [87]. Diao and coworkers synthesized a amphiphilic pillar[6]arene, which could complex with ATP and assemble into vesicles with enzyme-responsive property [88]. Xue and coauthors also reported a pillar[6]arene-based supra-amphiphile, which self-assembled into vesicles in water upon complexation with a sodium p-hydroxybenzoate derivative [89]. Besides pillar[5]arene and pillar[6]arene, pillar[7]arene [90] and pillar[10]arene [91] have also been used to construct supramolecular amphiphiles. The micelle or vesicle architectures were always constructed by the self-assembly of pillarene-based supramolecular amphiphiles, that can also be applied as microreactor [92], photochemical reaction manipulator [93], and drug delivery systems [94].
Depending on the supramolecular properties of pillarene-based host-guest interactions, supramolecular amphiphiles are also endowed with stimuli-responsive properties, indicating their potentials in theranostics. Vesicles are the main and classical morphologies fabricated from pillarene-based amphiphiles, having the ability to load different cargos be they dyes or anticancer drugs within the hollow cavity. The nanocontainers (Figure 13) can be endowed with the ability to respond to external stimuli such as pH variation [95], temperature [96], oxidation [97], biological agents [98], redox [98], chemical signals [99], magnetic field [100], light [101], and gas [102, 103].
Schematic illustration of (A) the fabrication of supramolecular micelles and vesicles self-assembled from amphiphilic modification of pillarenes; (B) Supramolecular micelles and vesicles assembled from host-guest complexation with respective modification. Reproduced with permission from [88] and [89], copyright 2014 Royal Society of Chemistry. Reproduced with permission from [104], copyright 2013 American Chemical Society.
Schematic illustration of supramolecular vesicles serving as drug delivery systems to release drug molecules upon activation by (A) magnetic field, (B) redox, and (C) pH, respectively. Reproduced with permission from [100], copyright 2018 American Chemical Society. Reproduced with permission from [104], copyright 2017 Royal Society of Chemistry. Reproduced with permission from [95], copyright 2015, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
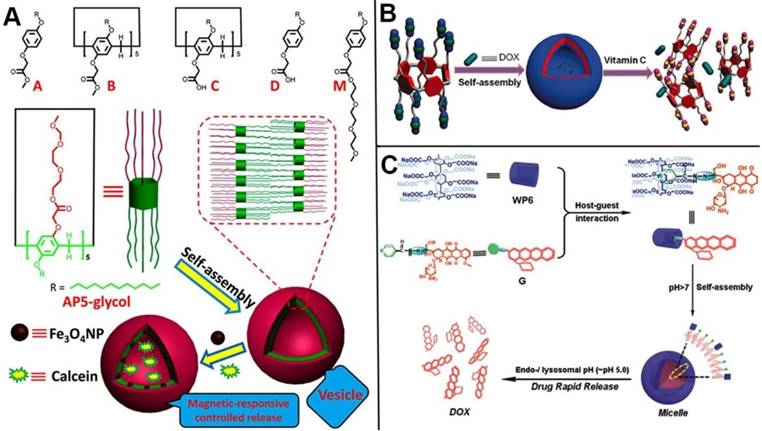
In 2013, Zhao and coworkers synthesized a series of bola or tadpole-like amphiphilic pillarenes with biocompatible property by introducing hydrophobic alkyl chains and hydrophilic ethylene glycols on respective sides, assembling into vesicles to carry fluorescent dyes and anticancer drugs for dual bioimaging and cancer therapy [104]. Huang and coworkers developed a series of pillarene-based supramolecular amphiphiles for drug delivery. In 2015, they reported the first redox-responsive amphiphilic macromolecular [2]pseudorotaxane based on pillar[5]arenes and paraquat derivative, which was used as drug delivery systems for the loading and controlled release of anticancer drugs [105]. Ji, Jin and coworkers used the DOX functionalized methyl viologen molecules as a guest. The host-guest complex of this guest and water-soluble pillar[5]arene assembled into prodrug micelles in acidic conditions, resulting in the enhancement of cancer therapy [106]. Liu and coworkers prepared a redox-responsive pillar[5]arene-based single-molecular-layer polymer nanocapsule for targeting and controlled drug delivery to inhibit tumor cells [107]. Du and coauthors synthesized the CP[6]A-based supramolecular vesicles that can encapsulate DOX and release drug molecules in response to five stimuli [108]. Pei and coworkers also constructed supramolecular amphiphiles based on pillarenes, for example, in 2014, they developed the first GSH-responsive cationic vesicles assembled from amphiphilic pillar[5]arenes, releasing anticancer drug and siRNA cooperatively to achieve the goal of cancer therapy and gene transfection [109], and in 2016, they designed a DNA interacting drug delivery vesicles constructed from the Trp-modified pillar[5]arene and its host-guest complex with guest. DOX as the anticancer drug was encapsulated within the vesicles and released at acidic environment [110].
Wang and coworkers constructed a variety of supramolecular vesicles assembled from amphiphilic host-guest complex based on pillarenes for targeted drug delivery, since they fabricated the pH-responsive supramolecular vesicles based on CP[6]A and ferrocene [111]. Subsequently, they developed drug delivery systems based on supramolecular amphiphiles, such as, multi-responsive supramolecular vesicles [112], chemo-photodynamic dual therapeutic vesicles [113], photo-responsive supramolecular vesicles based on azobenzene guest and pillar[6]arenes [114], DOX-based prodrug with pillar[6]arene [115], phosphate pillar[5,6]arene-based biocompatible vesicles [116], supramolecular vesicles assembled from pillar[5]arene and cationic polymer [117], GSH-responsive supramolecular vesicles based on D-galactose-modified pillar[5]arene [118], and glucose-responsive vesicles based on pillar[5]arenes and pyridylboronic acid [119]. The supramolecular vesicles mentioned above were mainly applied for anticancer or insulin drug delivery. Drugs were encapsulated in the vesicles assembled from supramolecular amphiphiles. The vesicle architectures collapsed upon triggering by external stimuli including pH, glucose, and GSH, resulting in drug release.
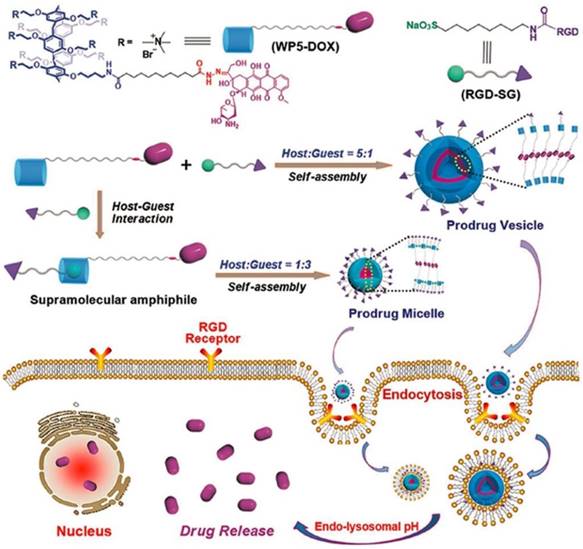
Recently, Schmuck, collaborated with Hu and other coworkers, fabricated tumor-targeting supramolecular vesicles with controllable size and morphology (Figure 14), based on DOX-modified pillar[5]arene (WP5-DOX) and Arg-Gly-Asp (RGD)-modified sulfonate guest (RGD-SG). In vitro and in vivo experiments demonstrated that the supramolecular vesicles could release DOX to cancer cells selectively, exhibiting an enhanced therapeutic effect including higher drug-loading capacity, targeting ability, and controllable morphology [120].
Schematic representation of controllable construction of supramolecular prodrug vesicles and micelles with tumor-targeting features and their applications for tumor-targeting drug delivery upon lowing pH. Reproduced with permission from [120], copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
It is worth mentioning pillarene-based photodynamic therapy (PDT) is also a promising area in supramolecular nanotheranostics. PDT is an improved treatment for cancer therapy, taking advantage of photosensitizers to absorb a specific wavelength of light to generate reactive oxygen species (ROS), which would damage tumor tissues with minimal invasiveness and lower side effects. Zhang and co-workers reported the pillarene-based redox-responsive supramolecular amphiphiles, with rapid drug release for enhanced PDT [121]. Because photosensitizers are crucial in the process of PDT, the same research group designed a mitochondria-targeting supramolecular photosensitizer based on pillar[5]arene, which could release and activate the photosensitizer in an acidic environment, accumulating in mitochondria for enhancing PDT [122]. Subsequently, they constructed supramolecular nanovesicles by the complexation of pyrophaeophorbide A (PPhA) and water-soluble pillar[5]arene for enhancing the efficacy of PDT. Besides, a biotin-pyridinium was introduced to endow the system with targeting ability towards cancer cells [123].
4. Conclusion and perspective
In conclusion, pillarenes have been extensively developed and attracted tremendous attention due to their unique characteristics in symmetric structure and host-guest properties. Astonishing advances of pillarenes and their derivatives have been achieved to enrich their applications in a great deal of fields including materials science, biological science, chemistry, and agricultural industry. In particular, pillarene-based supramolecular nanotheranostics have been widely exploited in recent years, taking advantage of their symmetric structures, versatile functionalities, and host-guest properties, which has been summarized in this review.
On reflection, we have classified pillarene-based supramolecular nanotheranostics into two main categories:
(i) Hybrid nanotheranostics. They are constructed by hybridizing pillarene-based supramolecular components with solid support materials, such as MSNs, MOFs, metal nanoparticles, and other inorganic platforms. In general, MSNs and MOFs could serve as the nanocarriers to load therapeutic agents and pillarene-based supramolecular switches anchored on the surface act as nanovalves to regulate the encapsulation and release of drugs, resulting in targeted drug delivery and on command drug release. These hybrid nanomaterials possess the integrated properties of each component, enabling the enhancement of the therapeutic effects.
(ii) Supramolecular nanotheranostics. They have been divided into three sub-categories, that is, (a) pillarene-based prodrugs or artificial channels that are significant for physiological process, constructed directly by pillarene functionalization; (b) pillarene-based host-guest complexes with therapeutic features; (c) supramolecular self-assembled architectures constructed from amphiphiles. These supramolecular materials usually possess good biocompatibility for being degradable in human body.
Owing to the superior characteristics of pillarenes such as ease of synthesis, diverse structural functionalization, and controllable host-guest interactions, great achievements have been made in such a short period. The reversible and stimuli-responsive properties of pillarene-based supramolecular nanotheranostics endow them with the abilities to design and prepare customer-oriented systems, which would fully satisfy the clinic demands in the future, contraposing the individual difference.
Despite these advantages, there are also some limitations to construct supramolecular nanotherapeutic systems from pillarene derivatives. According to the previous summary, many pillarene-based smart delivery systems have been developed for controllable delivery of small cargos. However, the carrier or delivery for large biomolecules is rarely developed and reported, since they are fatal elements for life process. Thus, researchers also have great opportunities to fabricate delivery systems for large biomolecules, especially in immunization therapy, which will further improve the therapeutic effects.
The investigation of pillarene-based supramolecular nanotheranostics is still in its infancy. Firstly, current systems are still very limited because almost all of them are based on pillar[5,6]arenes. With the improvement of synthetic methods, pillar[n]arenes with larger cavities, and other novel analogues such as extended pillarenes and leaning pillarenes [124-129] can be utilized to construct supramolecular nanotheranostic systems, which will expand the field remarkably. Secondly, most of the mentioned therapeutic systems were efficient in vitro, however, the ultimate goal of these researches is their therapeutic efficacy in real biological and clinical systems. Finally, hybrid materials with cooperative properties could be constructed to satisfy the clinical needs, for instance, materials possess diagnostic and therapeutic functions simultaneously, materials are used for combined drug delivery, etc.
In all, we strongly believe that pillarenes and their derivatives are very promising and strong candidates in building intelligent supramolecular nanotheranostics with enhanced therapeutic effects. Remarkable improvement and achievement will be made in this field and hopefully improvement of healthcare of human beings will benefit from the further development of pillarene-based nano- and biomaterials in the near future.
Abbreviations
ACh: acetylcholine; AH: adamantaneamine hydrochloride; AIDS: acquired immune deficiency syndrome; AIE: aggregation-induced emission; ATP: adenosine triphosphate; AuNPs: Au nanoparticles; AuNRs: Au nanorods; CB[7]: cucurbit[7]uril; CP[5]A: carboxylatopillar[5]arenes; CP[6]A: carboxylate-substituted pillar[6]arene; CT: charge-transfer; CVD: cardiovascular disease; DOX: doxorubicin; GA3: gibberellin acid; GCP: guanidiniocarbonyl pyrrole; GO: graphene oxide; HPV: human papillomavirus; LbL: layer-by-layer; MDR: multidrug resistance; MOFs: metal-organic frameworks; MRI: magnetic resonance imaging; MSNs: mesoporous silica nanoparticles; NIR: near-infrared; PDT: photodynamic therapy; PEI-Fa: folic acid conjugated polyethyleneimine; PPA[5]: phosphonated pillar[5]arenes; PPhA: pyrophaeophorbide A; PPy NPs: polypyrrole nanoparticles; RGO: reduced graphene oxide; SA: sanguinarine alkaloid; SC[4]A: sulfonatocalix[4]arenes; TPE: tetraphenylethene; UCNPs: upconversion nanoparticles.
Acknowledgements
This paper is dedicated to Prof. Yu Liu at Nankai University on the occasion of his 65th birthday. We thank the National Natural Science Foundation of China (51673084 and 21871108), the Jilin Province-University Cooperative Construction Project-Special Funds for New Materials (SXGJSF2017-3), and Jilin University Talents Cultivation Program for financial support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gravel J, Schmitzer AR. Imidazolium and benzimidazolium-containing compounds: from simple toxic salts to highly bioactive drugs. Org Biomol Chem. 2017;15:1051-71
2. Karimi M, Zangabad PS, Mehdizadeh F, Malekzad H, Ghasemi A, Bahrami S. et al. Nanocaged platforms: modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale. 2017;9:1356-92
3. Song N, Yang Y-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem Soc Rev. 2015;44:3474-504
4. Wen J, Yang K, Liu F, Li H, Xu Y, Sun S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem Soc Rev. 2017;46:6024-45
5. Nguyen KT, Zhao Y. Engineered hybrid nanoparticles for on-demand diagnostics and therapeutics. Acc Chem Res. 2015;48:3016-25
6. Yang Y-W, Sun Y-L, Song N. Switchable host-guest systems on surfaces. Acc Chem Res. 2014;47:1950-60
7. Dong R, Zhou Y, Huang X, Zhu X, Lu Y, Shen J. Functional supramolecular polymers for biomedical applications. Adv Mater. 2015;27:498-526
8. Zhou J, Yu G, Huang F. Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev. 2017;46:7021-53
9. Zhu H, Shangguan L, Shi B, Yu G, Huang F. Recent progress in macrocyclic amphiphiles and macrocyclic host-based supra-amphiphiles. Mater Chem Front. 2018;2:2152-74
10. Zhou X, Pathak P, Jayawickramarajah J. Design, synthesis, and applications of DNA-macrocyclic host conjugates. Chem Commun. 2018;54:11668-80
11. Shu X, Xu K, Hou D, Li C. Molecular recognition of water-soluble pillar[n]arenes towards biomolecules and drugs. Chem Eur J. 2018;58:1230-40
12. Cao D, Kou Y, Liang J, Chen Z, Wang L, Meier H. A facile and efficient preparation of pillarenes and a pillarquinone. Angew Chem Int Ed. 2009;48:9721-3
13. Chen Y, Cao D, Wang L, He M, Zhou L, Schollmeyer D. et al. Monoester copillar[5]arenes: synthesis, unusual self-inclusion behavior, and molecular recognition. Chem Eur J. 2013;19:7064-70
14. Hu WB, Hu WJ, Liu YA, Li JS, Jiang B, Wen K. Negative cooperativity in the binding of imidazolium and viologen ions to a pillar[5]arene-crown ether fused host. Org Lett. 2015;17:2940-3
15. Yang K, Pei Y, Wen J, Pei Z. Recent advances in pillar[n]arenes: synthesis and applications based on host-guest interactions. Chem Commun. 2016;52:9316-26
16. Kakuta T, Yamagishi T, Ogoshi T. Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes. Acc Chem Res. 2018;51:1656-66
17. Jie K, Zhou Y, Li E, Huang F. Nonporous adaptive crystals of pillarenes. Acc Chem Res. 2018;51:2064-72
18. Li ZY, Zhang Y, Zhang CW, Chen LJ, Wang C, Tan H. et al. Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior. J Am Chem Soc. 2014;136:8577-89
19. Strutt NL, Fairen-Jimenez D, Iehl J, Lalonde MB, Snurr RQ, Farha OK. et al. Incorporation of an A1/A2-difunctionalized pillar[5]arene into a metal-organic framework. J Am Chem Soc. 2012;134:17436-9
20. Song N, Chen D-X, Qiu Y-C, Yang X-Y, Xu B, Tian W. et al. Stimuli-responsive blue fluorescent supramolecular polymers based on a pillar[5]arene tetramer. Chem Commun. 2014;50:8231-4
21. Tan L-L, Li H, Tao Y, Zhang SX, Wang B, Yang Y-W. Pillar[5]arene-based supramolecular organic frameworks for highly selective CO2-capture at ambient conditions. Adv Mater. 2014;26:7027-31
22. Tian M-M Chen D-X, Sun Y-L, Yang Y-W, Jia Q. Pillarene-functionalized Fe3O4 nanoparticles as magnetic solid-phase extraction adsorbent for pesticide residue analysis in beverage samples. RSC Adv. 2013;3:22111
23. Wu L, Fang Y, Jia Y, Yang Y, Liao J, Liu N. et al. Pillar[5]arene-based diglycolamides for highly efficient separation of americium(III) and europium(III). Dalton Trans. 2014;43:3835-8
24. Strutt NL, Zhang H, Schneebeli ST, Stoddart JF. Functionalizing pillar[n]arenes. Acc Chem Res. 2014;47:2631-42
25. Wang K, Tan L-L, Chen D-X, Song N, Xi G, Zhang S.X-A. et al. One-pot synthesis of pillar[n]arenes catalyzed by a minimum amount of TfOH and a solution-phase mechanistic study. Org Biomol Chem. 2012;10:9405-9
26. Ke C, Strutt NL, Li H, Hou X, Hartlieb KJ, McGonigal PR. et al. Pillar[5]arene as a co-factor in templating rotaxane formation. J Am Chem Soc. 2013;135:17019-30
27. Li C, Chen S, Li J, Han K, Xu M, Hu B. et al. Novel neutral guest recognition and interpenetrated complex formation from pillar[5]arenes. Chem Commun. 2011;47:11294-6
28. Ogoshi T, Demachi K, Kitajima K, Yamagishi TA. Monofunctionalized pillar[5]arenes: synthesis and supramolecular structure. Chem Commun. 2011;47:7164-6
29. Ogoshi T, Demachi K, Kitajima K, Yamagishi TA. Selective complexation of n-alkanes with pillar[5]arene dimers in organic media. Chem Commun. 2011;47:10290-2
30. Sathiyajith CW, Shaikh RR, Han Q, Zhang Y, Meguellati K, Yang Y-W. Biological and related applications of pillar[n]arenes. Chem Commun. 2017;53:677-96
31. Feng W, Jin M, Yang K, Pei Y, Pei Z. Supramolecular delivery systems based on pillarenes. Chem Commun. 2018;54:13626-40
32. Wang Y, Xu JF, Chen YZ, Niu LY, Wu LZ, Tung CH. et al. Photoresponsive supramolecular self-assembly of monofunctionalized pillar[5]arene based on stiff stilbene. Chem Commun. 2014;50:7001-3
33. Jie K, Zhou Y, Yao Y, Huang F. Macrocyclic amphiphiles. Chem Soc Rev. 2015;44:3568-87
34. Xing P, Zhao Y. Supramolecular vesicles for stimulus-responsive drug delivery. Small Methods. 2018;2:1700364
35. Zhang H, Liu Z, Zhao Y. Pillarene-based self-assembled amphiphiles. Chem Soc Rev. 2018;47:5491-528
36. Feng W, Jin M, Yang K, Pei Y, Pei Z. Supramolecular delivery systems based on pillararenes. Chem. Commun. 2018;54:13626-40
37. Chen T, Yu H, Yang N, Wang M, Ding C, Fu J. Graphene quantum dot-capped mesoporous silica nanoparticles through an acid-cleavable acetal bond for intracellular drug delivery and imaging. J Mater Chem B. 2014;2:4979-82
38. Mal NK, Fujiwara M, Tanaka Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature. 2003;421:350-3
39. Sun Y-L, Yang Y-W, Chen D-X, Wang G, Zhou Y, Wang C-Y. et al. Mechanized silica nanoparticles based on pillar[5]arenes for on-command cargo release. Small. 2013;9:3224-9
40. Sun Y-L, Zhou Y, Li Q-L, Yang Y-W. Enzyme-responsive supramolecular nanovalves crafted by mesoporous silica nanoparticles and choline-sulfonatocalix[4]arene [2]pseudorotaxanes for controlled cargo release. Chem Commun. 2013;49:9033-5
41. Zhou Y, Tan L-L, Li Q-L, Qiu X-L, Qi AD, Tao Y. et al. Acetylcholine-triggered cargo release from supramolecular nanovalves based on different macrocyclic receptors. Chem Eur J. 2014;20:2998-3004
42. Huang X, Du X. Pillar[6]arene-valved mesoporous silica nanovehicles for multiresponsive controlled release. ACS Appl Mater Interfaces. 2014;6:20430-6
43. Ding C, Liu Y, Wang T, Fu J. Triple-stimuli-responsive nanocontainers assembled by water-soluble pillar[5]arene-based pseudorotaxanes for controlled release. J Mater Chem B. 2016;4:2819-27
44. Li Q-L, Sun Y, Sun Y-L, Wen J, Zhou Y, Bing QM. et al. Mesoporous silica nanoparticles coated by layer-by-layer self-assembly using cucurbit[7]uril for in vitro and in vivo anticancer drug release. Chem Mater. 2014;26:6418-31
45. Li H, Tan L-L, Jia P, Li Q-L, Sun Y-L, Zhang J. et al. Near-infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nanorods. Chem Sci. 2014;5:2804-08
46. Huang X, Wu S, Ke X, Li X, Du X. Phosphonated pillar[5]arene-valved mesoporous silica drug delivery systems. ACS Appl Mater Interfaces. 2017;9:19638-45
47. Yao Y, Wang Y, Zhao R, Shao L, Tang R, Huang F. Improved in vivo tumor therapy via host-guest complexation. J Mater Chem B. 2016;4:2691-96
48. Li X, Han J, Wang X, Zhang Y, Jia C, Qin J, Wang C, Wu J-R, Fang W, Yang Y-W. A triple-stimuli responsive hormone delivery system equipped with pillarene magnetic nanovalves. Mater Chem Front. 2019;3:103-10
49. Yang K, Yang K, Chao S, Wen J, Pei Y, Pei Z. A supramolecular hybrid material constructed from pillar[6]arene-based host-guest complexation and ZIF-8 for targeted drug delivery. Chem Commun. 2018;54:9817-20
50. Tan L-L, Li H, Qiu Y-C, Chen D-X, Wang X, Pan R-Y, Wang Y, Zhang S.X-A, Wang B, Yang Y-W. Stimuli-responsive metal-organic frameworks gated by pillar[5]arene supramolecular switches. Chem Sci. 2015;6:1640-44
51. Tan L-L, Li H, Zhou Y, Zhang Y, Feng X, Wang B. et al. Zn2+-triggered drug release from biocompatible Zirconium MOFs equipped with supramolecular gates. Small. 2015;11:3807-13
52. Tan L-L, Song N, Zhang S.X-A, Li H, Wang B, Yang Y-W. Ca2+, pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases. J Mater Chem B. 2016;4:135-40
53. Wu M-X, Gao J, Wang F, Yang J, Song N, Jin X. et al. Multistimuli responsive core-shell nanoplatform constructed from Fe3O4@MOF equipped with pillar[6]arene nanovalves. Small. 2018;14:1704440
54. Wu M-X, Yan H-J, Gao J, Cheng Y, Yang J, Wu J-R. et al. Multifunctional supramolecular materials constructed from polypyrrole@UiO-66 nanohybrids and pillarene nanovalves for targeted chemophotothermal therapy. ACS Appl Mater Interfaces. 2018;10:34655-63
55. Yao Y, Wang Y, Huang F. Synthesis of various supramolecular hybrid nanostructures based on pillar[6]arene modified gold nanoparticles/nanorods and their application in pH- and NIR-triggered controlled release. Chem Sci. 2014;5:4312-16
56. Zhu H, Liu J, Shi B, Wang H, Mao Z, Shan T, Huang F. Pillarene-based host-guest recognition facilitated magnetic separation and enrichment of cell membrane proteins. Mater Chem Front. 2018;2:1475-80
57. Li H, Wei R, Yan GH, Sun J, Li C, Wang H. et al. Smart self-assembled nanosystem based on water-soluble pillarene and rare-earth-doped upconversion nanoparticles for pH-responsive drug delivery. ACS Appl Mater Interfaces. 2018;10:4910-20
58. Li Q-L, Sun Y, Ren L, Wang X, Wang C, Li L. et al. Supramolecular nanosystem based on pillarene-capped CuS nanoparticles for targeted chemo-photothermal therapy. ACS Appl Mater Interfaces. 2018;10:29314-24
59. Zhang H, Ma X, Nguyen KT, Zeng Y, Tai S, Zhao Y. Water-soluble pillarene-functionalized graphene oxide for in vitro Raman and fluorescence dual-mode imaging. ChemPlusChem. 2014;79:462-9
60. Mao X, Liu T, Bi J, Luo L, Tian D, Li H. The synthesis of pillar[5]arene functionalized graphene as a fluorescent probe for paraquat in living cells and mice. Chem Commun. 2016;52:4385-88
61. Yu G, Yang J, Fu X, Wang Z, Shao L, Mao Z. et al. Supramolecular hybrid material constructed from graphene oxide and pillar[6]arene-based host-guest complex as a ultrasound and photoacoustic signals nanoamplifier. Mater Horiz. 2018;5:429-35
62. Si W, Chen L, Hu XB, Tang G, Chen Z, Hou JL. et al. Selective artificial transmembrane channels for protons by formation of water wires. Angew Chem Int Ed. 2011;50:12564-8
63. Hu XB, Chen Z, Tang G, Hou JL, Li ZT. Single-molecular artificial transmembrane water channels. J Am Chem Soc. 2012;134:8384-7
64. Chen L, Si W, Zhang L, Tang G, Li ZT, Hou JL. Chiral selective transmembrane transport of amino acids through artificial channels. J Am Chem Soc. 2013;135:2152-5
65. Si W, Li ZT, Hou JL. Voltage-driven reversible insertion into and leaving from a lipid bilayer: tuning transmembrane transport of artificial channels. Angew Chem Int Ed. 2014;53:4578-81
66. Chen JY, H WW, Zhang M, Wu G, Li ZT, Hou JL. A synthetic channel that efficiently inserts into mammalian cell membranes and destroys cancer cells. Faraday Discuss. 2018;209:149-59
67. Zhang F, Ma J, Sun Y, Boussouar I, Tian D, Li H, Jiang L. Fabrication of a mercaptoacetic acid pillar[5]arene assembled nanochannel: a biomimetic gate for mercury poisoning. Chem Sci. 2016;7:3227-33
68. Wang R, Sun Y, Zhang F, Song M, Tian D, Li H. Temperature-sensitive artificial channels through pillar[5]arene-based host-guest interactions. Angew Chem Int Ed. 2017;56:5294-8
69. Wanderlind EH, Liz DG, Gerola AP, Affeldt RF, Nascimento V, Bretanha LC. et al. Imidazole-functionalized pillar[5]arenes: highly reactive and selective supramolecular artificial enzymes. ACS Catalysis. 2018;8:3343-7
70. Li C, Ma J, Zhao L, Zhang Y, Yu Y, Shu X. et al. Molecular selective binding of basic amino acids by a water-soluble pillar[5]arene. Chem Commun. 2013;49:1924-6
71. Wei T-B, Chen J-F, Cheng X-B, Li H, Han B-B, Zhang Y-M. et al. A novel functionalized pillar[5]arene-based selective amino acid sensor for L-tryptophan. Org Chem Front. 2017;4:210-3
72. Yu G, Tang G, Huang F. Supramolecular enhancement of aggregation induced emission and its application in cancer cell imaging. J Mater Chem C. 2014;2:6609-17
73. Yu G, Wu D, Li Y, Zhang Z, Shao L, Zhou J. et al. A Pillar[5]arene-based [2]rotaxane lights up mitochondria. Chem Sci. 2016;7:3017-24
74. Schnurr M, Joseph R, Naugolny-Keisar A, Kaizerman-Kane D, Bogdanoff N, Schuenke P. et al. High exchange rate complexes of 129Xe with water-soluble pillar[5]arenes for adjustable magnetization transfer MRI. ChemPhysChem. 2019;20:246-51
75. Smolko V, Shurpik D, Evtugyn V, Stoikov I, Evtugyn G. Organic acid and DNA sensing with electrochemical sensor based on carbon black and pillar[5]arene. Electroanalysis. 2016;28:1391-400
76. Zheng D-D, Fu D-Y, Wu Y, Sun Y-L, Tan L-L, Zhou T. et al. Efficient inhibition of human papillomavirus 16 L1 pentamer formation by a carboxylatopillarene and a p-sulfonatocalixarene. Chem Commun. 2014;50:3201-3
77. Galanos N, Gillon E, Imberty A, Matthews SE, Vidal S. Pentavalent pillar[5]arene-based glycoclusters and their multivalent binding to pathogenic bacterial lectins. Org Biomol Chem. 2016;14:3476-81
78. Ping G, Wang Y, Shen L, Wang Y, Hu X, Chen J, er al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chem Commun. 2017;53:7381-4
79. Barbera L, Plano LMD, Franco D, Gattuso G, Guglielmino SPP, Lando G. et al. Antiadhesive and antibacterial properties of pillar[5]arene-based multilayers. Chem Commun. 2018;54:10203-6
80. Joseph R, Naugolny A, Feldman M, Herzog IM, Fridman M, Cohen Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J Am Chem Soc. 2016;138:754-7
81. Joseph R, Kaizerman D, Herzog IM, Hadar M, Feldaman M, Fridman M. Phosphonium pillar[5]arenes as a new class of efficient biofilm inhibitors: importance of charge cooperativity and the pillar platform. Chem Commun. 2016;52:10656-59
82. Barbera L, Franco D, De Plano LM, Gattuso G, Guglielmino SP, Lentini G. et al. A water-soluble pillar[5]arene as a new carrier for an old drug. Org Biomol Chem. 2017;15:3192-5
83. Xiao X, Nie G, Zhang X, Tian D, Li H. Protein adsorption switch constructed by a pillar[5]arene-based host-guest interaction. Chem Eur J. 2016;22:941-5
84. Hu XY, Ehlers M, Wang T, Zellermann E, Mosel S, Jiang H. et al. Formation of twisted beta-sheet tapes from a self-complementary peptide based on novel pillararene-GCP host-guest interaction with gene transfection properties. Chem Eur J. 2018;24:9754-9
85. Yu G, Zhou J, Shen J, Tang G, Huang F. Cationic pillar[6]arene/ATP host-guest recognition: selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem Sci. 2016;7:4073-8
86. Yu G, Han C, Zhang Z, Chen J, Yan X, Zheng B. et al. Pillar[6]arene-based photoresponsive host-guest complexation. J Am Chem Soc. 2012;134:8711-7
87. Yao Y, Wei P, Yue S, Li J, Xue M. Amphiphilic pillar[5]arenes: influence of chemical structure on self-assembly morphology and application in gas response and λ-DNA condensation. RSC Adv. 2014;4:6042-7
88. Zhou J, Chen M, Diao G. Synthesis of the first amphiphilic pillar[6]arene and its enzyme-responsive self-assembly in water. Chem Commun. 2014;50:11954-6
89. Yao Y, Li J, Dai J, Chi X, Xue M. A water-soluble pillar[6]arene: synthesis, host-guest chemistry, controllable self-assembly, and application in controlled release. RSC Adv. 2014;4:9039-43
90. Li Z, Yang J, Huang F. pH-Responsive host-guest complexation between a water-soluble pillar[7]arene and a 2,7-diazapyrenium salt and its application in controllable self-assembly. Chin J Chem. 2018;36:59-62
91. Chi X, Ji X, Shao L, Huang F. A multiresponsive amphiphilic supramolecular diblock copolymer based on pillar[10]arene/paraquat complexation for rate-tunable controlled release. Macromol Rapid Commun. 2017;38:1600626
92. Chen R, Jiang H, Gu H, Zhou Q, Zhang Z, Wu J. et al. Tubular structures self-assembled from a bola-amphiphilic pillar[5]arene in water and applied as a microreactor. Org Lett. 2015;17:4160-3
93. Xia D, Wang P, Shi B. Controlling the photochemical reaction of an azastilbene derivative in water using a water-soluble pillar[6]arene. Org Biomol Chem. 2017;15:7618-22
94. Xia D, Shangguan L, Xue M, Shi B. Dual-responsive self-assembly of a bola-type supra-amphiphile constructed from a new pillar[6]arene-based recognition motif in water and its application in controlled release. New J Chem. 2016;40:9890-4
95. Cao Y, Zou X, Xiong S, Li Y, Shen Y, Hu X. et al. Supramolecular prodrug micelles constructed by drug-drug conjugate with water soluble pillar[6]arene for controllable and rapid drug release. Chin J Chem. 2015;33:329-34
96. Wang S, Yao C, Ni M, Xu Z, Cheng M, Hu X-Y. et al. Thermo- and oxidation-responsive supramolecular vesicles constructed from self-assembled pillar[6]arene-ferrocene based amphiphilic supramolecular diblock copolymers. Polym Chem. 2017;8:682-8
97. Wu X, Li Y, Lin C, Hu X-Y, Wang L. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem Commun. 2015;51:6832-35
98. Zhou Y, Jie K, Huang F. A redox-responsive selenium-containing pillar[5]arene-based macrocyclic amphiphile: synthesis, controllable self-assembly in water, and application in controlled release. Chem Commun. 2017;53:8364-7
99. Rui L, Liu L, Wang Y, Gao Y, Zhang W. Orthogonal approach to construct cell-like vesicles via pillar[5]arene-based amphiphilic supramolecular polymers. ACS Macro Lett. 2016;5:112-7
100. Zhou J, Chen M, Diao G. Magnetic-responsive supramolecular vesicles from self-organization of amphiphilic pillar[5]arene and application in controlled release. ACS Appl Mater Interfaces. 2014;6:18538-42
101. Zhou Q, Jiang H, Chen R, Qiu F, Dai G, Han D. A triply-responsive pillar[6]arene-based supramolecular amphiphile for tunable formation of vesicles and controlled release. Chem Commun. 2014;50:10658-60
102. Zhou Y, Li E, Zhao R, Jie K. CO2-enhanced bola-type supramolecular amphiphile constructed from pillar[5]arene-based host-guest recognition. Org Lett. 2018;20:4888-92
103. Jie K, Zhou Y, Yao Y, Shi B, Huang F. CO2-responsive pillar[5]arene-based molecular recognition in water: establishment and application in gas-controlled self-assembly and release. J Am Chem Soc. 2015;137:10472-5
104. Zhang H, Ma X, Nguyen KT, Zhao Y. Biocompatible pillararene-assembly-based carriers for dual bioimaging. ACS Nano. 2013;7:7853-63
105. Chi X, Yu G, Ji X, Li Y, Tang G, Huang F. Redox-responsive amphiphilic macromolecular [2]pseudorotaxane constructed from a water-soluble pillar[5]arene and a paraquat-containing homopolymer. ACS Macro Lett. 2015;4:996-9
106. Wang Y, Du J, Wang Y, Jin Q, Ji J. Pillar[5]arene based supramolecular prodrug micelles with pH induced aggregate behavior for intracellular drug delivery. Chem Commun. 2015;51:2999-3002
107. Fu S, Zhang Y, Guan S, Huang Q, Wang R, Tian R. et al. Reductive-responsive, single-molecular-layer polymer nanocapsules prepared by lateral-functionalized pillar[5]arenes for targeting anticancer drug delivery. ACS Appl Mater Interfaces. 2018;10:14281-6
108. Jiang L, Huang X, Chen D, Yan H, Li X, Du X. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar[6]arenes that are responsive to five stimuli. Angew Chem Int Ed. 2017;56:2655-9
109. Chang Y, Yang K, Wei P, Huang S, Pei Y, Zhao W. et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed. 2014;53:13126-30
110. Yang K, Chang Y, Wen J, Lu Y, Pei Y, Cao S. et al. Supramolecular vesicles based on complex of trp-modified pillar[5]arene and galactose derivative for synergistic and targeted drug delivery. Chem Mater. 2016;28:1990-3
111. Duan Q, Cao Y, Li Y, Hu X, Xiao T, Lin C. et al. pH-responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery. J Am Chem Soc. 2013;135:10542-9
112. Cao Y, Hu XY, Li Y, Zou X, Xiong S, Lin C. et al. Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release. J Am Chem Soc. 2014;136:10762-9
113. Meng L-B, Zhang W, Li D, Li Y, Hu X-Y, Wang L. et al. pH-Responsive supramolecular vesicles assembled by water-soluble pillar[5]arene and a BODIPY photosensitizer for chemo-photodynamic dual therapy. Chem Commun. 2015;51:14381-4
114. Hu XY, Jia K, Cao Y, Li Y, Qin S, Zhou F. et al. Dual photo- and pH-responsive supramolecular nanocarriers based on water-soluble pillar[6]arene and different azobenzene derivatives for intracellular anticancer drug delivery. Chem Eur J. 2015;21:1208-20
115. Cao Y, Li Y, Hu X-Y, Zou X, Xiong S, Lin C. et al. Supramolecular nanoparticles constructed by DOX-based prodrug with water-soluble pillar[6]arene for self-catalyzed rapid drug release. Chem Mater. 2015;27:1110-9
116. Hu X-Y, Liu X, Zhang W, Qin S, Yao C, Li Y. et al. Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem Mater. 2016;28:3778-88
117. Guo S, Liang T, Song Y, Cheng M, Hu X-Y, Zhu J-J. et al. Supramolecular polymersomes constructed from water-soluble pillar[5]arene and cationic poly(glutamamide)s and their applications in targeted anticancer drug delivery. Polym Chem. 2017;8:5718-25
118. Liu X, Shao W, Zheng Y, Yao C, Peng L, Zhang D. et al. GSH-responsive supramolecular nanoparticles constructed by b-D-galactose-modified pillar[5]arene and camptothecin prodrug for targeted anticancer drug delivery. Chem Commun. 2017;53:8596-9
119. Gao L, Wang T, Jia K, Wu X, Yao C, Shao W. et al. Glucose-responsive supramolecular vesicles based on water-soluble pillar[5]arene and pyridylboronic acid derivatives for controlled insulin delivery. Chem Eur J. 2017;23:6605-14
120. Hu XY, Gao L, Mosel S, Ehlers M, Zellermann E, Jiang H. et al. From supramolecular vesicles to micelles: controllable construction of tumor-targeting nanocarriers based on host-guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small. 2018;14:1803952
121. Chen Y, Rui L, Liu L, Zhang W. Redox-responsive supramolecular amphiphiles based on a pillar[5]arene for enhanced photodynamic therapy. Polym Chem. 2016;7:3268-76
122. Rui L, Xue Y, Wang Y, Gao Y, Zhang W. A mitochondria-targeting supramolecular photosensitizer based on pillar[5]arene for photodynamic therapy. Chem Commun. 2017;53:3126-9
123. Wu J, Tian J, Rui L, Zhang W. Enhancing the efficacy of photodynamic therapy (PDT) via water-soluble pillar[5]arene-based supramolecular complexes. Chem Commun. 2018;54:7629-32
124. Gao B, Tan L-L, Song N, Li K, Yang Y-W. A high-yield synthesis of [m]biphenyl-extended pillar[n]arenes for an efficient selective inclusion of toluene and m-xylene in the solid state. Chem Commun. 2016;52:5804-7
125. Wu J-R, Wang C-Y, Tao Y, Wang Y, Li C, Yang Y-W. A water-soluble [2]biphenyl-extended pillar[6]arene. Eur J Org Chem. 2018;2018:1321-5
126. Wu J-R, Mu A, Li B, Wang C-Y, Fang L, Yang Y-W. Desymmetrized leaning pillar[6]arene. Angew Chem Int Ed. 2018;57:9853-8
127. Shetty D, Trabolsi A. Making pillar[6]arenes to lean: an art of tuning a supramolecular host. Sci China Chem. 2019;62:289-90
128. Wu J-R, Yang Y-W. New opportunities in synthetic macrocyclic arenes. Chem Commun. 2019;55:1533-43
129. Dai D, Li Z, Yang J, Wang C, Wu J-R, Wang Y, Zhang D, Yang Y-W. Supramolecular assembly-induced emission enhancement for efficient mercury(II) detection and removal. J Am Chem Soc. 2019;55:5736-9
Author contact
![]() Corresponding author: Lianjun Ma (horsejlmedu.cn), Hui Gao (hgaoedu.cn) and Ying-Wei Yang (ywyangedu.cn)
Corresponding author: Lianjun Ma (horsejlmedu.cn), Hui Gao (hgaoedu.cn) and Ying-Wei Yang (ywyangedu.cn)
 Global reach, higher impact
Global reach, higher impact