13.3
Impact Factor
Theranostics 2019; 9(12):3595-3607. doi:10.7150/thno.33100 This issue Cite
Research Paper
Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity
1. Institute of Modern Physics; Chinese Academy of Sciences; Lanzhou, China
2. Key Laboratory of Heavy Ion Radiation Medicine of Chinese Academy of Sciences; Lanzhou, China
3. Key Laboratory of Heavy Ion Radiation Medicine of Gansu Province; Lanzhou, China
4. University of Chinese Academy of Sciences; Beijing, China
5. Center of Mitochondria and Healthy Ageing; Yantai University; Yantai, China
6. National Institute of Radiological Sciences; National Institutes for Quantum and Radiological Science and Technology; Chiba, Japan.
*These authors contributed equally to this work.
Abstract
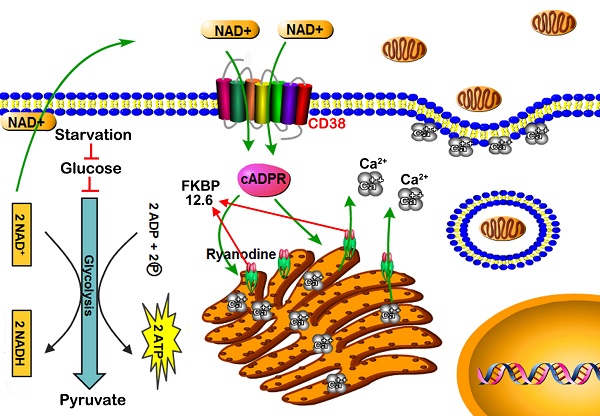
Emerging evidence indicates that reprogramming of energy metabolism involving disturbances in energy production from a defect in cellular respiration with a shift to glycolysis is a core hallmark of cancer. Alterations in cancer cell energy metabolism are linked to abnormalities in mitochondrial function. Mitochondrial dysfunction of cancer cells includes increased glycolysis, decreased apoptosis, and resistance to radiotherapy. The study was designed for two main points: firstly, to investigate whether exogenous functional mitochondria can transfer into glioma cells and explore the underlying molecular mechanisms from the perspective of endocytosis; secondly, to further verify whether the mitochondrial transplantation is able to rescue aerobic respiration, attenuate the Warburg effect and enhance the radiosensitivity of gliomas.
Methods: Mitochondria were isolated from normal human astrocytes (HA) and immediately co-incubated with starved human glioma cells (U87). Confocal microscopy and gene sequencing were performed to evaluate the ability of isolated mitochondria internalization into U87 cells. The interaction between endocytosis and isolated mitochondria transfer were captured by 3D tomographic microscopy and transmission electron microscopy. NAD+, CD38, cADPR and Ca2+ release were determined by commercial kits, western blot, HLPC-MS and Fluo-3 AM respectively. PCR array expression profiling and Seahorse XF analysis were used to evaluate the effect of mitochondrial transplantation on energy phenotypes of U87 cells. U87 cells and U87 xenografts were both treated with mitochondrial transplantation, radiation, or a combination of mitochondrial transplantation and radiation. Apoptosis in vitro and in vivo were detected by cytochrome C, cleaved caspase 9 and TUNEL staining.
Results: We found that mitochondria from HA could be transferred into starved U87 cells by simple co-incubation. Starvation treatment slowed the rate of glycolysis and decreased the transformation of NAD+ to NADH in U87 cells. A large amount of accumulated NAD+ was released into the extracellular space. CD38 is a member of the NAD+ glycohydrolase family that catalyzes the cyclization of extracellular NAD+ to intracellular cADPR. cADPR triggered release of Ca2+ to promote cytoskeleton remodeling and plasma membrane invagination. Thus, endocytosis involving isolated mitochondria internalization was mediated by NAD+-CD38-cADPR-Ca2+ signaling. Mitochondrial transfer enhanced gene and protein expression related to the tricarboxylic acid (TCA) cycle, increased aerobic respiration, attenuated glycolysis, reactivated the mitochondrial apoptotic pathway, inhibited malignant proliferation of U87 cells. Isolated mitochondria injected into U87 xenograft tumors also entered cells, and inhibited glioma growth in nude mice. Mitochondrial transplantation could enhance the radiosensitivity of gliomas in vitro and in vivo.
Conclusion: These findings suggested that starvation-induced endocytosis via NAD+-CD38-cADPR-Ca2+ signaling could be a new mechanism of mitochondrial transplantation to rescue aerobic respiration and attenuate the Warburg effect. This mechanism could be a promising approach for radiosensitization.
Keywords: mitochondrial transplantation, NAD+-CD38-cADPR-Ca2+ signaling, endocytosis, energy metabolism, radiation therapy, gliomas
 Global reach, higher impact
Global reach, higher impact