13.3
Impact Factor
Theranostics 2019; 9(13):3798-3811. doi:10.7150/thno.35331 This issue Cite
Research Paper
IL-21-based therapies induce clearance of hepatitis B virus persistence in mouse models
1. Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai 200040, People's Republic of China
2. Key Laboratory of Medical Epigenetics and Metabolism, Institutes of Biomedical Sciences, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, People's Republic of China
3. Key Laboratory of Medical Molecular Virology (Ministry of Education/National Health Commission/Chinese Academy of Medical Sciences), Department of Microbiology and Parasitology, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, People's Republic of China
4. Children's Hospital, Fudan University, Shanghai 201199, People's Republic of China
#: These authors contributed equally to this work.
Received 2019-3-29; Accepted 2019-5-6; Published 2019-5-31
Abstract
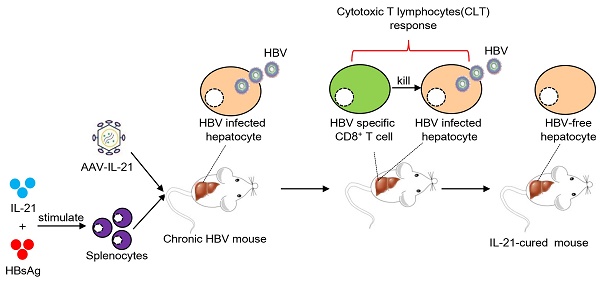
Chronic hepatitis B virus (HBV) infection causes hepatitis, liver cirrhosis and hepatocellular carcinoma. Covalently closed circular DNA (cccDNA) is the sole viral transcription template and not affected by current treatment options, constituting a key determinant of HBV persistence. Novel therapeutics with demonstrable effectiveness against cccDNA are required.
Methods: Previously, we established an HBV persistence mouse model using replicon plasmid derived from a clinical isolate (termed BPS) and identified IL-21 as a potent clearance-inducer. We also described another persistence mouse model based on cccDNA mimics produced in vivo termed recombinant cccDNA (rcccDNA). In this work, effectiveness of IL-21-based gene and cellular therapies was tested using these models.
Results: In both models of HBV persistence, single injections with adeno-associated virus (AAV) expressing murine IL-21 highly efficiently induced clearance of both HBV markers from serum, and more importantly, BPS DNA and rcccDNA from mouse liver. Mechanistically, IL-21-induced clearance was associated with activation and liver infiltration of CD8+ T cells, and CD8 antibody injections negatively affected AAV-IL-21 effectiveness. More notably, adoptive transfer of CD8+ T cells from AAV-IL-21-cured mice engendered clearance in acceptor HBV persistence mice. Furthermore, cured mice were protected against re-challenge with long-lived memory. Most significantly, infusion of splenocytes from treatment-naïve mice stimulated ex vivo with IL-21 protein and HBV antigen could also induce clearance in treatment-naïve mice.
Conclusion: These data demonstrate IL-21-based gene and cellular therapies as valid candidates for treating chronic HBV infections, with potential in removing cccDNA-harboring hepatocytes via activated CD8+ T cells accompanied by long-term protective memory.
Keywords: clearance, cellular immunity, hepatitis B virus, IL-21, mouse model
Introduction
Chronic infection of hepatitis B virus (HBV) affects more than 250 million people worldwide, and is a recognized major risk factor for liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [1, 2]. HBV is a small, non-cytopathic virus with a relaxed circular DNA (rcDNA) genome that takes human as the only natural host [2, 3]. Upon infection of hepatocytes, rcDNA genome is converted in the nucleus into covalently closed circular DNA (cccDNA), which then serves as the sole transcription template [2, 3]. Stability of cccDNA is an essential contributor to HBV persistence [3]. Ideally, curative treatments should result in elimination of intrahepatic cccDNA, removing any possibility of HBV surviving as occult infection or silent cccDNA waiting to be reactivated later, e.g. during immune suppression [4, 5]. Unfortunately, current treatment options, including interferon α and nucleos(t)ide analogues, do not affect stability or persistence of intrahepatic cccDNA. Consequently, the concept of 'functional cure', namely loss of serum HBV surface antigens (HBsAg) with or without the appearance of HBsAg antibodies (HBsAb), has been suggested as a more feasible therapeutic goal [6]. Even in the minority of treated patients that achieve functional cure, HBV reactivation remains a distinct risk. New therapeutics with demonstrable effectiveness in removing cccDNA need to be developed.
Previously, we described a novel mouse model of HBV persistence based on hydrodynamic injection (HDI) of HBV replicon plasmid derived from a clinical isolate (designated BPS) into immunocompetent mice, and showed that HDI of IL-21 expression plasmid could disrupt established BPS persistence [7]. However, to fully explore the potential of IL-21 as a chronic hepatitis B treatment option, a more clinically effective and safer means of delivery need to be tested. In addition, the mechanisms of IL-21-mediated anti-HBV activity in mouse models need to be investigated. Most importantly, whether IL-21-based intervention has any effect on clearance of HBV cccDNA must be addressed. For as yet unknown reasons, mouse hepatocytes apparently do not support cccDNA formation in vivo [8]. To circumvent this problem, we recently developed another mouse model of HBV persistence based on recombinant cccDNA (rcccDNA) formed in vivo [9, 10], which closely mimics cccDNA thus enabling evaluation of cccDNA clearance in immunocompetent mouse.
In this work, we show that recombinant liver-specific serotype 8 [11] adeno-associated virus expressing IL-21 (AAV-IL-21) efficiently induced clearance of both serum HBV markers and intrahepatic BPS DNA and rcccDNA in respective models. Mechanistically, AAV-IL-21 treatment resulted in marked activation and liver infiltration of CD8+ T cells. Antibody blocking and adoptive transfer experiments confirmed that activated CD8+ T cells were responsible for causing HBV clearance. Most significantly, IL-21-based cellular therapy, in which splenocytes from treatment-naïve mice were stimulated ex vivo with IL-21 plus HBsAg and then infused into other treatment-naïve mice, also induced clearance of BPS persistence. Furthermore, HBV persistence mice cured by AAV-IL-21 or by adoptive transfer of CD8+ T cells displayed long-term protection from re-challenge.
Results
AAV-IL-21 cleared persisting HBV replicon DNA from mouse liver
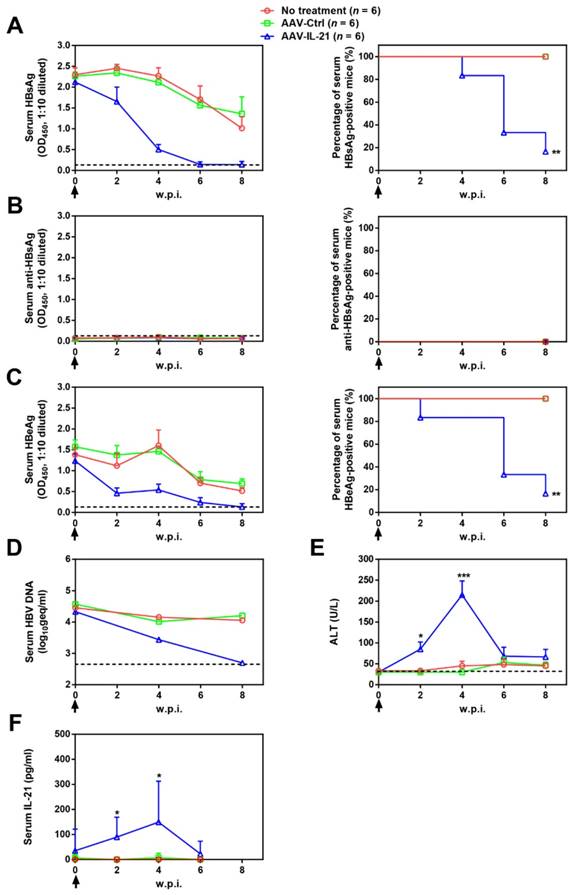
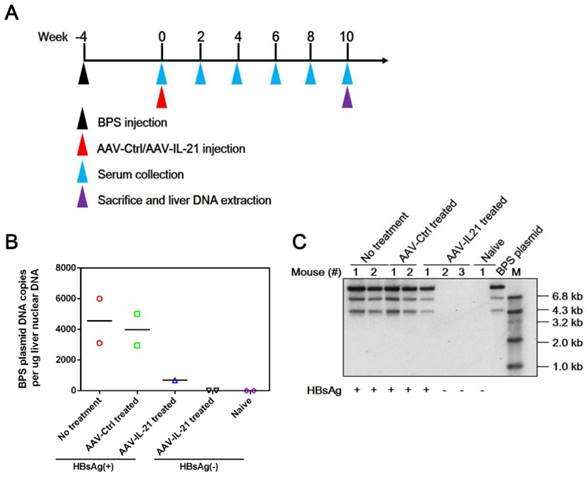
To investigate whether recombinant AAV expressing mouse IL-21 (AAV-IL-21, Figure S1) could clear BPS persistence, BALB/c mice harboring pre-established BPS persistence (serum HBsAg persistently positive > 4 weeks post HDI) were subjected to single-dose AAV injection. As shown in Figure 1A and C, AAV-IL-21 injection caused serum HBsAg and HBeAg (HBV e antigen) levels to decrease much more quickly compared to mice receiving no treatment or AAV-Ctrl injection, and become undetectable in 5 out of 6 mice by 8 w.p.i., while both markers persisted in all untreated and AAV-Ctrl-treated mice. Similarly, HBV DNA level in pooled sera started to decrease following AAV-IL-21 treatment and dropped below detection limit by 8 w.p.i., while it remained largely unchanged in the other two groups (Figure 1D). AAV-IL21-induced clearance was not accompanied by HBsAb development (Figure 1B), but correlated with serum ALT elevations peaking at 4 w.p.i. and reverting to baseline levels by 8 w.p.i (Figure 1E), and a similar episode of serum IL-21 elevation and reversion (Figure 1F). No serum ALT or IL-21 changes were detected in mice receiving no treatment or AAV-Ctrl (Figure 1E-F). Serum bilirubin and creatine kinase levels remained generally normal in all groups (Figure S2). BPS DNA in mouse liver was then measured using quantitative realtime PCR and Southern blot to confirm true clearance. As shown in Figure 2, BPS DNA was undetectable by 10 w.p.i. in AAV-IL-21-treated mice that cleared serum HBsAg, and at markedly lower levels in AAV-IL-21-treated mice that did not achieve clearance, but persisted at high levels in untreated and AAV-Ctrl-treated mice. Expectedly, viral replication intermediates were also undetectable in AAV-IL-21-cured mice (Figure S3). In longer follow-up experiments, 6 of 6 AAV-IL-21-cured BPS persistence mice remained negative for serum HBV antigens and liver BPS DNA at 20 w.p.i. (Figure S4). These results indicated that AAV-IL-21 could efficiently and safely induce HBV clearance in BPS persistence model.
AAV-IL-21 treatment induced HBV clearance in BPS persistence mice. BPS HDI mice remaining positive for serum HBV antigens for 4 weeks were injected (solid arrows) with 2 × 1011 geq of AAV-IL-21 or AAV-Ctrl, or left untreated. Sera collected at indicated time points were analyzed for HBsAg (A), HBsAb (B), HBeAg (C), HBV DNA (pooled sera, D), ALT (E) and IL-21 (F). Means and SEMs within group are presented with the group size (n) indicated. Group positivity percentage data are presented for HBsAg, HBsAb and HBeAg (A-C, right panels). Dotted lines represent cut-off thresholds (A-C, left panels), quantification lower limit (D) or pre-treatment baseline (E). Data are compared to that of the untreated group and statistical significance calculated using log-rank (Mantel-Cox) test (A-C, right panels) and unpaired two-tailed t-test (E and F). *p < 0.05; **p < 0.01; ***p < 0.001. w.p.i., weeks post injection of AAV. geq, genome equivalents.
AAV-IL-21 cleared persisting HBV rcccDNA from mouse liver
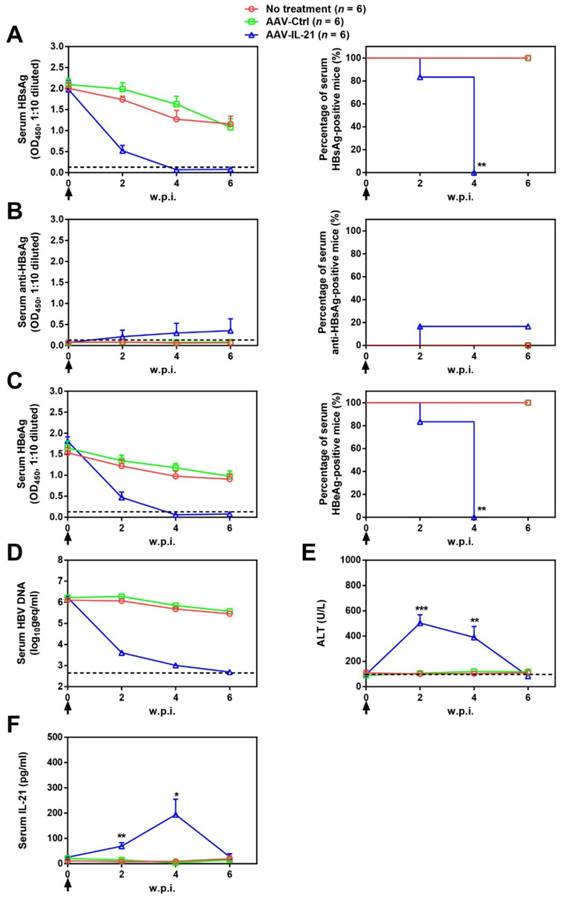
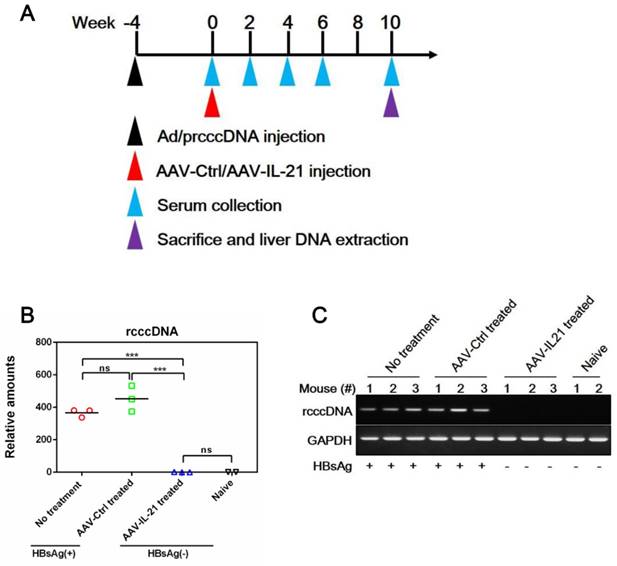
Since BPS replicon plasmid is different from cccDNA in both size and sequences, we tested AAV-IL-21 in another mouse model of HBV persistence based on recombinant cccDNA (rcccDNA), which is produced from a precursor (prcccDNA) through Cre-mediated recombination and highly similar to wild type cccDNA except a small extraneous intron that is removed upon transcription [9, 10]. Recombinant adenovirus carrying prcccDNA (Ad/prcccDNA) was injected into C57BL/6 mice transgenic for liver-specific Cre recombinase [10], and mice with persistently positive serum HBsAg (> 4 weeks) were used for AAV injection. Compared to BPS mice, adenovirus-mediated delivery of HBV resulted in much higher transfection of hepatocytes (Figure S5), yet AAV-IL-21 showed comparable effectiveness in this model in inducing clearance of serum HBsAg, HBeAg and HBV DNA (Figure 3A, C-D). Similar to BPS model, AAV-IL-21-induced clearance in rcccDNA mice was not associated with HBsAb development (Figure 3B), but coincided with transient serum ALT and IL-21 elevations (Figure 3E-F). Most importantly, quantitative realtime PCR showed that by 10 w.p.i., intrahepatic rcccDNA was undetectable in AAV-IL-21-treated mice that had achieved serum HBsAg clearance, but persisted in untreated and AAV-Ctrl treated mice (Figure 4). In longer follow-up, 6 of 6 AAV-IL-21-cured rcccDNA persistence mice remained negative for serum HBV antigens and liver rcccDNA at 22 w.p.i. (Figure S6). Clearly, AAV-IL-21 could efficiently induce HBV clearance in rcccDNA persistence mice as well.
AAV-IL-21-induced HBV clearance correlated with activated CD8+ T cell response
Since AAV-IL-21 induced clearance with concurrent ALT elevations but not HBsAb development (Figure 1B and E, Figure 3B and E), most likely HBV-targeting cell-mediated immunity developed in response to IL-21 and resulted in attack and removal of HBV-harboring hepatocytes, eventually leading to loss of HBV replicon DNA and rcccDNA (Figure 2 and Figure 4). Indeed, AAV-IL-21-treated BPS mice had higher levels of HBsAg-specific IFN-γ-producing splenocytes compared to control groups (Figure S7), indicating activated CD8+ T cell responses. Liver sections from untreated, AAV-Ctrl- and AAV-IL-21-treated BPS mice at 2 and 6 w.p.i were then subjected to H&E and anti-CD8 staining. At 2 w.p.i., before serum HBsAg was cleared (Figure 1), marked infiltration, mainly of CD8+ T cells, was observed in AAV-IL-21-treated mice but not the control groups (Figure S8). By 6 w.p.i., however, infiltration by CD8+ T cells was no longer observable, regardless of whether clearance had been achieved. Such liver infiltration by CD8+ T cells generally coincided with transient serum ALT elevations in AAV-IL-21 treated mice (Figure 1E and Figure 3E), strongly suggesting attack of BPS-harboring hepatocytes by CD8+ T cells, possibly cytotoxic T lymphocytes (CTL).
AAV-IL-21 treatment cleared intrahepatic BPS DNA from BPS persistence mice. BPS HDI mice remaining positive for serum HBV antigens for 4 weeks were injected with 2 × 1011 geq of AAV-IL-21 (n = 3), AAV-Ctrl (n = 2), or left untreated (n = 2). Serum HBsAg was tested every two weeks. At 10 weeks post injection (w.p.i.) of AAV, mice were sacrificed and liver samples taken (A). Two AAV-IL-21 treated mice had displayed HBsAg negativity for over 4 weeks by the time of sacrifice. BPS plasmid in nuclear DNA extracted from liver tissue samples were measured using quantitative realtime PCR (B) and Southern blot (C) with serum HBsAg status indicated. Data from each mouse and group means are presented (B). Naïve, mice without BPS HDI (n = 2).
AAV-IL-21 treatment induced HBV clearance in rcccDNA persistence mice. Cre Tg C57BL/6 mice positive for serum HBV antigen for 4 weeks after Ad/prcccDNA injection were injected (solid arrows) with 2 × 1011 geq of AAV-IL-21, AAV-Ctrl, or left untreated. Sera collected at indicated time points were analyzed for HBsAg (A), HBsAb (B), HBeAg (C), HBV DNA (pooled sera, D), ALT (E) and IL-21 (F). Means and SEMs within group are presented with the group size (n) indicated. Group positivity percentage data are presented for HBsAg, HBsAb and HBeAg (A-C, right panels). Dotted lines represent cut-off thresholds (A-C, left panels), quantification lower limit (D) or pre-treatment baseline (E). Data are compared to that of the untreated group and statistical significance calculated using log-rank (Mantel-Cox) test (A-C, right panels) and unpaired two-tailed t-test (E and F). *p < 0.05; **p < 0.01; ***p < 0.001. w.p.i., weeks post injection of AAV. geq, genome equivalents.
IL-21 receptor (IL-21R) is expressed on CD8+ T cells and essential for their functionality [12]. To prove that CD8+ T cell response was stimulated during IL-21-mediated HBV clearance, splenic CD8+ T cells from AAV-Ctrl- and AAV-IL-21-treated BPS mice at 2 w.p.i. (during serum ALT elevations and liver CD8+ T cell infiltrations in AAV-IL-21 treated mice, see above) were subjected to comparative transcriptome sequencing (Table S1). GO enrichment analysis showed that all 27 T cell related biological process GO terms, including T cell activation (GO:0042110), differentiation (GO:0030217) and proliferation (GO:0042098), were significantly enriched in differentially expressed genes (Figure S9A), reflecting extensive effects of IL-21 on CD8+ T cells. IL-21-induced activation of JAK-STAT signaling is involved in CD8+ T cell activation [13, 14]. Transcriptome sequencing indeed revealed higher transcription of key genes involved in positive regulation of JAK-STAT cascade (GO:0046427) post AAV-IL-21 treatment (Figure S9A-B). Moreover, Western blot confirmed increased STAT1 and STAT3 phosphorylation levels in splenic CD8+ T cells from AAV-IL-21-treated mice (Figure S9C). Evidently, IL-21-mediated HBV clearance was accompanied by activation of CD8+ T cell response, resulting in liver infiltrations and serum ALT elevations.
IL-21-activated CD8+ T cells are required and sufficient for engendering HBV clearance
To determine the relation between IL-21-induced CD8+ T cell response and HBV clearance, BPS persistence mice were repeatedly injected intraperitoneally with CD8 blocking antibody [15, 16] immediately before and continually after AAV-IL-21 injection. As shown in Figure S10, CD8 blocking caused slower decrease of serum HBV antigen levels and lower ALT elevations in AAV-IL-21-treated mice compared to mice receiving normal IgG or no injection. However, when CD8 antibody were withdrawn at 6 w.p.i., serum HBV antigens were cleared within 2 weeks (Figure S10A and C), accompanied by a transient ALT surge (Figure S10D). Similar observations were made in AAV-IL-21-treated rcccDNA mice (Figure S11). Clearly, functional CD8+ T cells are required for AAV-IL-21-induced clearance.
AAV-IL-21 treatment cleared intrahepatic rcccDNA from rcccDNA persistence mice. Cre Tg C57BL/6 mice positive for serum HBV antigen for 4 weeks after Ad/prcccDNA injection were injected with 2 × 1011 geq of AAV-IL-21 (n = 3), AAV-Ctrl (n = 3), or left untreated (n = 3). Serum HBsAg were tested as indicated. At 10 weeks post injection (w.p.i.) of AAV, mice were sacrificed and liver samples taken (A). All AAV-IL-21 treated mice had been HBsAg-negative for over 4 weeks at sacrifice. RcccDNA in liver nuclear DNA was measured using quantitative realtime PCR and normalized against GAPDH genomic DNA in the same sample (B). Statistical significances are calculated using unpaired two-tailed t-test. ***p < 0.001. ns, not significant. Reaction mixtures from (B) were subjected to agarose electrophoresis for amplicon size check (C). Serum HBsAg status of respective mouse was indicated (B and C). naïve, Cre Tg C57BL/6 mice without Ad/prcccDNA injection (n = 2).
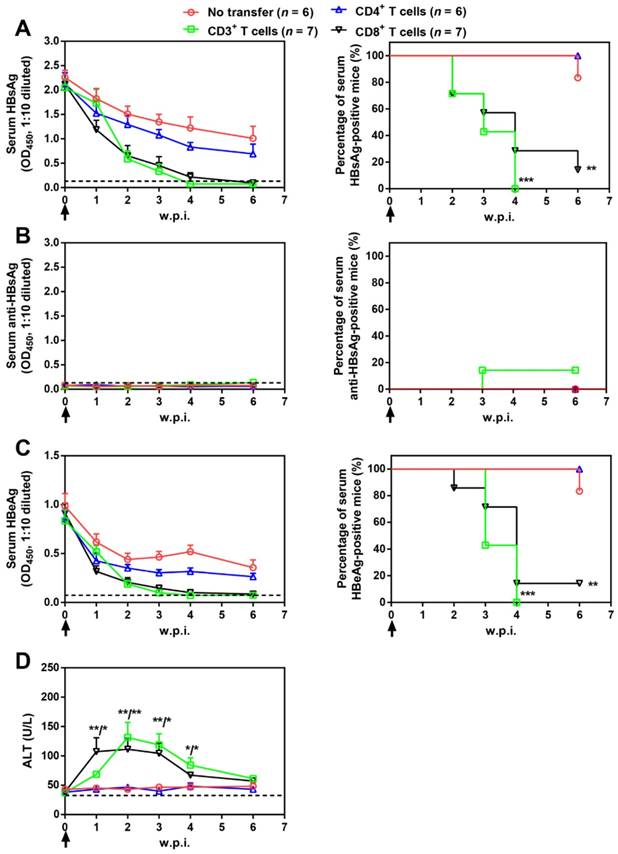
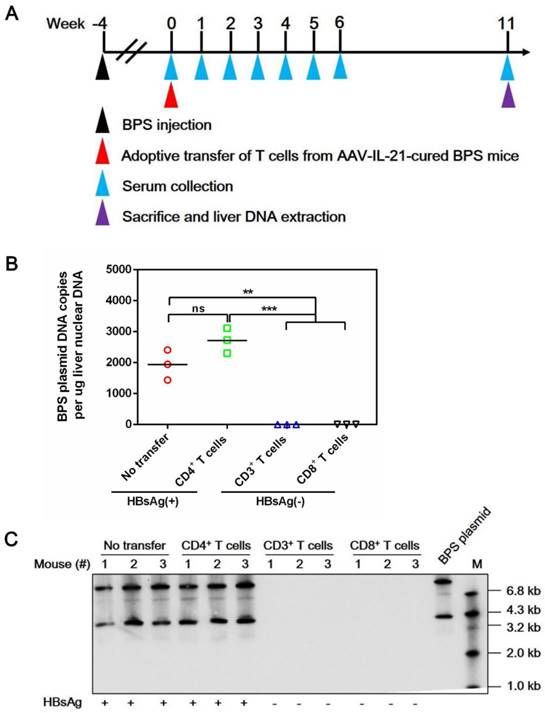
Splenic CD3+, CD4+, and CD8+ T cell subpopulations were then isolated from AAV-IL-21-cured mice and transferred into untreated BPS persistence mice. As shown in Figure 5, serum HBV antigens decreased more rapidly in mice receiving CD3+ or CD8+ T cells compared to those receiving no transfer or CD4+ T cells, and were eventually cleared in all CD3+ T cell recipients by 4 w.p.i. and in 6 out of 7 CD8+ T cell recipients by 6 w.p.i. of T cells. Similar to plasmid-delivered [7] and AAV-delivered (Figure 1B) IL-21 treatment, clearance in CD3+ and CD8+ T cell recipient mice was not associated with HBsAb development, but coincided with a course of serum ALT elevations (Figure 5D). In contrast, serum HBV antigens remained positive in all CD4+ T cell recipients and all but one untreated mice by 10 w.p.i. (Figure 5). BPS mice cured by transfer of CD3+ or CD8+ T cells from AAV-IL-21-cured BPS mice were negative for liver BPS DNA, proving true clearance (Figure 6). Collectively, antibody blocking and adoptive transfer results shown above unequivocally showed that IL-21-activated CD8+ T cells are responsible for causing HBV clearance.
Adoptive transfer of CD3+ and CD8+ T cells from AAV-IL-21 cured BPS persistence mice induced HBV clearance in treatment-naïve BPS persistence mice. BPS persistence mice were transferred with CD3+, CD4+, or CD8+ T cells from AAV-IL-21-cured BPS persistence mice (serum HBsAg negative for over 4 weeks), or left untreated. Sera collected at indicated time points were analyzed for HBsAg (A), HBsAb (B), HBeAg (C), and ALT (D). Means and SEMs within group are presented with the group size (n) indicated. Group positivity percentage data are presented for HBsAg, HBsAb and HBeAg (A-C, right panels). Dotted lines represent cut-off thresholds (A-C, left panels) or pre-transfer baseline (D). Data are compared to that of the untreated mice and statistical significance calculated using log-rank (Mantel-Cox) test (A-C, right panels) and unpaired two-tailed t-test (D). *p < 0.05; **p < 0.01; ***p < 0.001. w.p.i., weeks post injection of AAV.
Adoptive transfer of CD3+ and CD8+ T cells from AAV-IL-21 cured BPS persistence mice induced clearance of intrahepatic BPS DNA. BPS persistence mice were transferred with CD3+ (n = 3), CD4+ (n = 3), or CD8+ (n = 3) T cells from AAV-IL-21-cured BPS persistence mice (serum HBsAg negative for over 4 weeks), or left untreated (n = 3). Sera collected at indicated time points were analyzed for HBsAg. At 11 weeks post injection (w.p.i.) of T cells, mice were sacrificed and liver samples taken (A). All CD3+ and CD8+ T cell recipient mice had displayed HBsAg negativity for over 4 weeks by the time of sacrifice. BPS plasmid in liver nuclear DNA extracted was measured using quantitative realtime PCR (B) and Southern blot (C). Serum HBsAg status of mice are indicated (B-C). Data from each mouse and group means are presented (B). Statistical significances are calculated using unpaired two-tailed t-test. **p < 0.01; ***p < 0.001. ns, not significant.
IL-21-based cellular therapy cleared BPS persistence
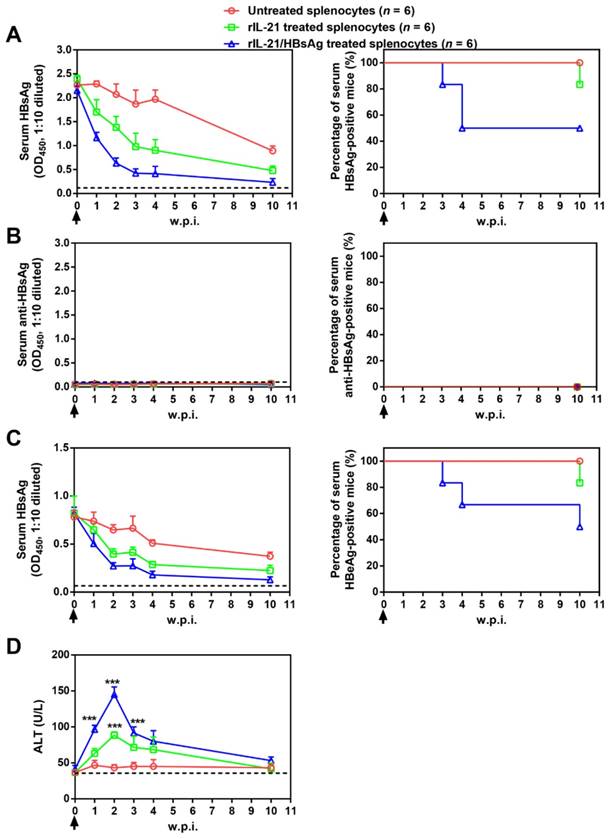
Adoptive transfer results shown above suggested the possibility of cellular therapy involving ex vivo IL-21 treatment and subsequent infusion of immune cells from chronically infected subjects. As a proof of concept, splenocytes from untreated BPS persistence mice were stimulated with recombinant IL-21 before injection into BPS persistence recipient mice. Compared to mice receiving untreated splenocytes, those receiving IL-21-treated splenocytes displayed faster decrease of serum HBsAg and HBeAg, accompanied by serum ALT elevations, but only 1 of 6 mice achieved BPS clearance by 10 w.p.i. (Figure 7).
Since HBV antigens are expressed predominantly in transfected liver cells in BPS HDI mice, ineffectiveness of IL-21-treated splenocytes in causing clearance might be due to the absence of HBV antigen during IL-21 stimulation. To test this hypothesis, IL-21 was supplemented with recombinant HBsAg during stimulation, and indeed, infusion of splenocytes treated with IL-21 plus HBsAg resulted in even quicker decrease of serum HBV markers, higher ALT elevations, and most importantly, viral clearance in half (3 of 6) of recipient mice by 10 w.p.i. (Figure 7).
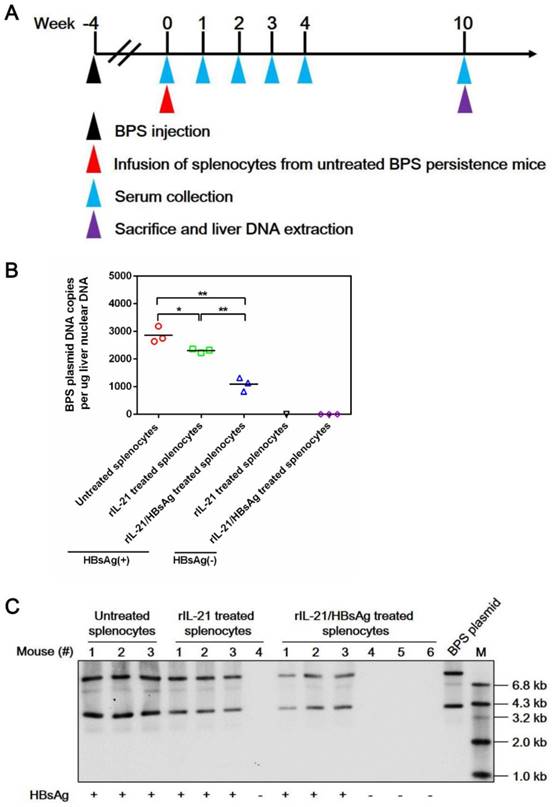
Mechanistically, infusion of both IL-21- and IL-21/HBsAg-treated splenocytes resulted in marked liver infiltration of CD8+ T cells (Figure S12), which coincided with serum ALT elevations (Figure 7D). Such infiltration by CD8+ T cells was no longer observable by 10 w.p.i., with concurrent reversion of serum ALT to normal levels, regardless of whether BPS clearance had been achieved (Figure S12). These results are nearly identical with what was observed in AAV-IL-21-treated BPS mice (Figure S8), indicating that ex vivo IL-21 treatment of splenocytes, with or without HBsAg, indeed activated HBV-targeting CD8+ T cells. Neither donor nor recipient mice in this cellular therapy experiment were ever exposed to AAV, proving that the observed effects were solely attributable to IL-21 and IL-21/HBsAg, respectively. Furthermore, clearance of serum HBV markers following infusion of IL-21/HBsAg-treated splenocytes correlated with disappearance of BPS DNA from mouse liver (Figure 8), proving true clearance.
Transfer of splenocytes stimulated with IL-21 and HBsAg induced HBV clearance in treatment-naïve BPS persistence mice. Splenocytes from untreated BPS persistence mice were cultured in the presence of 100 ng/ml recombinant mouse IL-21 (rIL-21) with or without 15 μg/ml recombinant HBsAg, or without stimulation. After 60 hours, 1 × 108 splenocytes were injected into each BPS persistence mice via tail vein. Sera collected at indicated time points were analyzed for HBsAg (A), HBsAb (B), HBeAg (C) and ALT (D). Means and SEMs within group are presented with the group size (n) indicated. Group positivity percentage data are presented for HBsAg, HBsAb and HBeAg (A-C, right panels). Dotted lines represent cut-off thresholds (A-C, left panels) or pre-transfer baseline (D). Data are compared to mice transferred with unstimulated splenocytes and statistical significance calculated using log-rank (Mantel-Cox) test (A-C, right panels) and unpaired two-tailed t-test (D). ***p < 0.001. w.p.i., weeks post splenocytes injection.
Transfer of splenocytes stimulated with IL-21 and HBsAg induced clearance of intrahepatic BPS DNA. Splenocytes from untreated BPS persistence mice were cultured in the presence of 100 ng/ml recombinant mouse IL-21 (rIL-21) with or without 15 μg/ml recombinant HBsAg, or without stimulation. After 60 hours, 1 × 108 splenocytes were injected into each untreated BPS persistence mice via tail vein. Sera collected at indicated time points were analyzed for HBsAg. At 10 weeks post injection (w.p.i.) of splenocytes, mice were sacrificed and liver samples taken (A). BPS plasmid in liver nuclear DNA extracted was measured using quantitative realtime PCR (B) and Southern blot (C). Serum HBsAg status of mice are indicated (B and C). Data from each mouse and group means are presented (B). Statistical significances are calculated using unpaired two-tailed t-test. **p < 0.01; *p < 0.05.
Successful IL-21-based therapies provided long-term protection
Next, we evaluated the protection of cured mice from HBV rechallenge. For AAV-mediated delivery, prolonged expression of IL-21 from potential chromosomally integrated AAV-IL-21 might interfere with evaluating such post-cure protection properly. In AAV-IL-21-treated mice, serum IL-21 levels reverted to baseline levels by 6-8 w.p.i. (Figure 1F), while AAV DNA became undetectable by PCR in liver DNA by 20 w.p.i. (Figure S13). Therefore, we re-challenged AAV-IL-21-cured BPS persistence mice at 20 w.p.i. or later, corresponding to 12-14 weeks after clearance (Figure 1). Serum HBV antigens became positive immediately after re-challenge but disappeared within 1 week in all 5 re-challenged mice, accompanied by serum ALT elevations, with HBsAb seroconversion in only one mouse (Figure S14). Furthermore, 4 weeks after serum HBV markers had become negative, no BPS DNA was detectable in mouse liver (Figure S15), indicating true clearance of re-challenged BPS. In contrast, similarly re-challenged untreated and AAV-Ctrl treated BPS mice displayed transient serum HBV antigen increases on top of persistent antigen levels, which reverted to pre-re-challenge levels within 1 or 2 weeks (Figure S14). These data confirmed that even in the absence of serum HBsAb or prolonged IL-21 expression, successful AAV-IL-21 treatment established long-term protection. Higher phosphorylation of STAT1 and STAT3 could be detected in splenic CD8+ T cells from AAV-IL-21-cured BPS persistence mice immediately after BPS re-challenge (Figure S16). Combined with the concurrent serum ALT elevations (Figure S14C), such activation of JAK/STAT signaling suggested that a memory CD8+ T cell response was likely responsible for the quick clearance of re-challenged BPS. Similar quick clearance of re-challenged HBV was also observed in BPS mice cured by adoptive transfer from AAV-IL-21-cured BPS mice and rcccDNA mice cured by AAV-IL-21 (Figure S17-19).
Discussion
In this study, we tested IL-21 based gene therapy (recombinant AAV serotype 8) and ex vivo cellular therapy in BPS as well as rcccDNA HBV persistence mouse models, and obtained remarkable IL-21-induced clearance (Figure 1-4; Figure S3-4 and Figure S6) and post-clearance protection (Figure S14-15 and Figure S18-19). Data from these diverse delivery and model systems unequivocally demonstrated a vital role of IL-21 in mediating HBV clearance, underpinning its therapeutic potentialoffering hope that cccDNA clearance is possibly attainable. To our knowledge, this is the first report demonstrating clearance of cccDNA, albeit recombinant cccDNA, through therapeutic intervention in mouse models. Since mice are not susceptible to HBV infection, these mouse models do not support viral spread or de novo cccDNA formation. Therefore, cautions need to be taken when extending the applicability of the therapies for treating human chronic HBV infection.
IL-21 is a pleiotropic regulator affecting multiple aspects of innate and adaptive immunity, including antibody production by B cells and cytotoxicity by CD8+ T cells [12, 13]. In chronic hepatitis B patients, serum IL-21 levels have been linked with HBeAg-to-HBeAb seroconversion during antiviral treatment [17]. In both BPS and rcccDNA mice, however, AAV-IL-21 treatment did not rectify the host's failure to mount an anti-HBsAg response (Figure 1B and Figure 3B). Instead, HBV clearance always coincided with a course of serum ALT elevations (Figure 1E and Figure 3E), suggesting immune attack of HBV-harboring hepatocytes. Relatively normal total bilirubin and creatine kinase levels in AAV-IL-21-treated mice (Figure S2) indicate that IL-21 administration did not cause general and non-specific inflammation, therefore removal of HBV-harboring hepatocytes was most likely an HBV-specific process.
Multiple mechanisms exist that could result in specific killing of pathogen-infected cells. Splenocyte ELISPOT, liver immunohistochemistry and splenic CD8+ T cell transcriptome sequencing results (Figure S7-9) strongly indicated activation of CD8+ T cells in AAV-IL-21-treated BPS mice. CD8 antibody blocking and adoptive transfer experiments subsequently proved that activated CD8+ T cells were required and sufficient to engender true HBV clearance (Figure S10-11; Figure 5-6). Further work is required to provide direct evidences showing that CTL targeting HBV-harboring hepatocytes mediates clearance. How IL-21 administration eventually translates into activated HBV-specific CD8+ T cells, e.g. whether there is de novo generation, and/or expansion or activation of pre-existing HBV-specific CD8+ T cells involved also remains to be addressed in detail.
Protection from BPS or Ad/prcccDNA re-challenge as late as 11-14 weeks after IL-21-induced clearance (Figure S14-15 and Figure S18-19) is both interesting and promising, considering that IL-21 did not facilitate HBsAg to HBsAb seroconversion (Figure 1B and Figure 3B). The fact that similar prolonged protection was observed in mice that cleared BPS persistence after receiving adoptive transfer of CD8+ T cells from AAV-IL-21-cured mice (Figure S17) indicated that IL-21-activated HBV-specific CD8+ T cells consisted of a memory component that can be re-activated upon re-exposure (Figure S16). In the context of human infections, given the possibility of transcriptionally silent cccDNA persisting indefinitely in hepatocytes, long-lasting CD8+ T cell memory probably constitutes a more desirable safeguard against possible future HBV reactivation compared to serum antibodies such as HBsAb. Clearly, whether IL-21-induced activation of HBV-targeting CD8+ T cells can be reproduced in humans warrants immediate attention.
Ability of CD8+ T cells from AAV-IL-21-cured mice to induce clearance upon adoptive transfer (Figure 5-6) revealed the possibility of IL-21-based cellular therapy for chronic HBV infections. By subjecting splenocytes from treatment-naïve mice to ex vivo stimulation with recombinant IL-21 and HBsAg, and infusing treated splenocytes into other treatment-naïve mice, we proved that such cellular therapy could indeed induce clearance of persisting BPS (Figure 7-8). Since no sorting was performed before or after IL-21/HBsAg stimulation, it is not yet clear what subsets of splenic immune cells are required and/or involved for therapy success, but the marked improvement to outcome as a result of including HBsAg during IL-21 stimulation strongly suggested the participation of antigen-presenting cells. Future work delineating cellular and molecular details in this process will no doubt allow establishment of more optimized protocols enabling further improved outcome.
Compared to systemic administration of IL-21 through protein injection or recombinant virus infection, IL-21-based cellular therapy demonstrated in this work provide multiple benefits regarding both effectiveness and safety through adjusting parameters like the number of injected lymphocytes and the injection times, etc. Since no in vivo use of extraneous IL-21 is involved, possible unintended effects of this pluripotent immune factor on immune or non-immune cells can be maximally avoided. Additional manipulations using other immune regulators aimed at improving antigen presentation and enhancing CD8+ T cell activation are also possible. Furthermore, ex vivo stimulated cells can be characterized, sorted and quantitated with regard to both HBV-specificity and cytocidal potency enabling finite and customizable dosage control. If results shown here eventually prove translatable to human, it can be envisioned that IL-21-based cellular therapy protocols using autologous immune cells such as PBMC for ex vivo stimulation followed by infusion would give rise to novel therapeutics with the potential for curing chronic HBV infection. Meanwhile, considering IL-21 induced clearance most likely depends on killing the HBV-infected hepatocytes, to avoid fulminant hepatitis, such therapies may require pre-treatment liver biopsies of patients to evaluate the percentage of HBV‐infected hepatocytes.
Materials and Methods
Plasmids and recombinant viruses
BPS replicon plasmid containing 1.3 fold over-length HBV genome derived from a clinical isolate (genotype B, GenBank Accession Number: AF100309.1) has been described [7]. Recombinant serotype 8 AAV co-expressing murine IL-21 (NM_021782.2) (AAV-IL-21) and EGFP, and control virus (AAV-Ctrl) expressing EGFP only was purchased from Obio Technology, China. Recombinant human adenovirus serotype 5 harboring precursor of recombinant cccDNA (Ad/prcccDNA) has been described [10].
Animal experiments
Male BALB/c mice aged 6-8 weeks were purchased from Experimental Animal Centre, Fudan University. Male C57BL/6 mice aged 6-8 weeks transgenic for Cre recombinase under the control of the albumin promoter (Cre Tg C57BL/6) have been described [10].
BPS persistence mice were prepared by injecting 10 μg BPS replicon plasmid via HDI through tail vein and selecting mice positive for serum HBsAg for over four weeks [7], and injected with 2 × 1011 genome equivalents (geq) of AAV-IL-21 or AAV-Ctrl in 200 μl PBS via tail vein, or left untreated. RcccDNA persistence mice were prepared by injecting 1.5 × 109 plaque-forming units of Ad/prcccDNA into each Cre Tg C57BL/6 mouse in 200 μl PBS via tail vein and selecting mice positive for serum HBsAg over four weeks [10], and injected with recombinant AAV as above. BPS and Ad/prcccDNA re-challenges were performed in the same way as first injection.
For CD8 blocking, mice were injected intraperitoneally with 10 μg anti-CD8 (#100735, BioLegend, USA) or mouse normal IgG four hours prior to AAV-IL-21 injection. Injections were repeated twice per week thereafter till 6 weeks post injection (w.p.i.) of AAV.
CD3+, CD4+ and CD8+ T cells were isolated from single-cell suspensions prepared from spleens of AAV-IL-21 treated BPS persistence mice displaying HBsAg negativity for over 4 weeks using MojoSortTM CD3 T, CD4 T and CD8 T Cell Isolation Kits (BioLegend, USA). For adoptive transfer, each treatment-naïve BPS persistence mouse was injected with 2.5 × 107 CD3+, 1.5 × 107 CD4+, or 1 × 107 CD8+ T cells via tail vein.
For ex vivo IL-21 stimulation, splenocytes from treatment-naive BPS persistence mice cultured in DMEM containing 2 mM L-glutamine, 50 U/ml penicillin, and 10% fetal bovine serum (all from Invitrogen, China) were stimulated with 100 ng/ml murine IL-21 (#210-21, PEPROTECH, USA), with or without 15 μg/ml recombinant HBsAg (kind gift from Prof. Di Qu of Fudan University), for 60 hours and 1 × 108 cells were injected into each treatment-naïve BPS persistence mice via tail vein.
Ethics Statement
Mouse handling procedures were approved by the Animal Ethics Committee of School of Basic Medical Sciences, Fudan University. The approval number was JS-028. Mouse sera were collected via retro-orbital sinus bleeding, and spleen and/or liver samples were taken when needed after sacrifice by cervical dislocation. Our animal care and use protocol adhered to 'REGULATIONS FOR THE ADMINISTRATION OF AFFAIRS CONCERNING EX-PERIMENTAL ANIMALS' coming from the “LAWS AND REGULATIONS OF THE PEOPLE'S REPUBLIC OF CHINA GOVERNING FOREIGN-RELATED MATTERS” which is compiled by the Brueau of Legislative Affairs of the State Council of the People's Republic of China.
Serum analyses
HBsAg, HBsAb and HBeAg were analyzed using ELISA (Kehua, China). HBV DNA, alanine aminotransferase (ALT), total bilirubin and creatine kinase were analyzed using commercial quantitative assays (Adicon, China). IL-21 was measured using Milliplex multiplex assay (Merck Millipore, China) or ELISA (EK0797, BOSTER, China).
Analysis of liver HBV DNA
Total cellular or nuclear DNA were extracted from liver samples of sacrificed mice and BPS plasmid DNA or rcccDNA was assayed using quantitative real-time PCR as previously reported [7, 10] using primers listed in Table S2. BPS plasmid and replication intermediates DNA were analyzed in Southern blot as previously described [7].
ELISPOT and immunohistochemistry
Splenocytes cultured in RPMI 1640 containing 2 mM L-glutamine, 50 U/ml penicillin, and 10% fetal bovine serum (all from Invitrogen, China) were stimulated with 15 μg/ml recombinant HBsAg for 18 hours. Gamma interferon (IFN-γ)-secreting cells were detected using IFN-γ ELISPOT assay (eBioscience, China). For immunochemistry, liver tissue sections were stained with anti-HBsAg (1:1000, R-0283-02, ChangDao, China) or anti-CD8 (1:1000, GB11068, Servicebio, China) antibodies in addition to hematoxylin and eosin (H&E) staining.
Transcriptome sequencing
RNA extracted from splenic CD8+ T cells of AAV-Ctrl- or AAV-IL-21-treated BPS persistence mice at 2 w.p.i. were subjected to library construction and deep sequencing on Illumina HiSeqTM 2500 by Gene Denovo Biotechnology Co. (China). Gene expression levels were normalized using FPKM (fragments per kilobase of transcript per million mapped reads) method and differentially expressed genes (DEG) with a fold change ≥ 2 and a false discovery rate (FDR) < 0.05 were identified using edgeR package (http://www.r-project.org/). Gene ontology (GO) terms significantly enriched in DEGs were defined by hypergeometric test (Table S1).
Western blot
Splenic CD8+ T cells were lysed directly in SDS-PAGE sample loading buffer, and total and phosphorylated STAT1 and STAT3 were detected using antibodies against STAT1 (1:1000, #14994), p-STAT1 (1:1000, #9167), STAT3 (1:1000, #8768), and p-STAT3 (1:1000, #9145) from Cell Signaling Technology (USA).
Statistical analysis
Means and standard errors (SEMs) from different treatment groups were subjected to unpaired two-tailed t-test. Group positivity percentages are plotted as Kaplan-Meier plot and subjected to log-rank (Mantel-Cox) test. GraphPad 5 was used for plotting and statistical tests.
Abbreviations
HBV: hepatitis B virus; HCC: hepatocellular carcinoma; rcDNA: relaxed circular DNA; cccDNA: covalently closed circular DNA; HBsAg: HBV surface antigens; HBsAb: HBsAg antibodies; HDI: hydrodynamic injection; AAV: adeno-associated virus; HBeAg: HBV e antigen; ALT: alanine aminotransferase; Ad: adenovirus; CTL: possibly cytotoxic T lymphocytes; rIL-21: recombinant IL-21; rHBsAg: recombinant HBsAg; w.p.i.: weeks post injection; d.p.i.: days post injection.
Supplementary Material
Supplementary table 1.
Supplementary figures and table 2.
Acknowledgements
This work was supported by NSFC (81701986, 31670166, 81672009, 81672034), National Key Project for Infectious Diseases of China (2017ZX10202202 and 2018ZX10301208), Chinese Academy of Medical Sciences (2018PT31044) and Shanghai Municipal Education Commission (2017-01-07-00-07-E00057). We thank Prof. Di Qu (Fudan University) for providing recombinant HBsAg.
Author Contributions
All authors listed have contributed to the work described in this article. Conceived and designed the experiments, and wrote the paper: J.L., Z.S., Y.X. Performed the experiments: Z.S., Y.Z., G.L., J.W., M.L. Data analyses: Z.S., J.L., J.W., Q.D., J.Z., Y.X.
Competing Interests
The authors have declared that no competing interest exists.
References
1. World Health Organization: Hepatitis B, Fact Sheet. Revised 18 July 2018. http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b
2. Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13-21
3. Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672-86
4. Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33:167-77
5. Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(Suppl 1):100-7
6. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. Hepatology. 2017;66:1296-313
7. Shen Z, Yang H, Yang S, Wang W, Cui X, Zhou X. et al. Hepatitis B virus persistence in mice reveals IL-21 and IL-33 as regulators of viral clearance. Nat Commun. 2017;8:2119
8. Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158-69
9. Qi Z, Li G, Hu H, Yang C, Zhang X, Leng Q. et al. Recombinant covalently closed circular hepatitis B virus DNA induces prolonged viral persistence in immunocompetent mice. J Virol. 2014;88:8045-56
10. Li G, Zhu Y, Shao D, Chang H, Zhang X, Zhou D. et al. Recombinant covalently closed circular DNA of hepatitis B virus induces long-term viral persistence with chronic hepatitis in a mouse model. Hepatology. 2018;67:56-70
11. Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854-9
12. Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379-95
13. Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688-98
14. Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135-42
15. Ledbetter JA, Seaman WE, Tsu TT, Herzenberg LA. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981;153:1503-16
16. Takahashi K, Nakata M, Tanaka T, Adachi H, Nakauchi H, Yagita H. et al. CD4 and CD8 regulate interleukin 2 responses of T cells. Proc Natl Acad Sci U S A. 1992;89:5557-61
17. Ma SW, Huang X, Li YY, Tang LB, Sun XF, Jiang XT. et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol. 2012;56:775-81
Author contact
![]() Corresponding authors: Dr. Youhua Xie (yhxieedu.cn) and Dr. Jing Liu (liujing212edu.cn), Postbox 228, 131 Dong'an Rd, Shanghai 200032, People's Republic of China; Phone: +86-21-54237972; Fax: +86-21-54237973; or to Dr. Jiming Zhang (jmzhangedu.cn), Department of Infectious Diseases, Huashan Hospital, 12 Middle Wulumuqi Road, Shanghai 200040, People's Republic of China; Phone: +86-21-52887965; Fax: +86-21-52887963
Corresponding authors: Dr. Youhua Xie (yhxieedu.cn) and Dr. Jing Liu (liujing212edu.cn), Postbox 228, 131 Dong'an Rd, Shanghai 200032, People's Republic of China; Phone: +86-21-54237972; Fax: +86-21-54237973; or to Dr. Jiming Zhang (jmzhangedu.cn), Department of Infectious Diseases, Huashan Hospital, 12 Middle Wulumuqi Road, Shanghai 200040, People's Republic of China; Phone: +86-21-52887965; Fax: +86-21-52887963
 Global reach, higher impact
Global reach, higher impact