13.3
Impact Factor
Theranostics 2019; 9(26):8018-8025. doi:10.7150/thno.38587 This issue Cite
Review
Transcytosis - An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery
1. Division of Nanomedicine, Department of Medicine, University of California, Los Angeles, California 90095, United States
2. California NanoSystems Institute, University of California, Los Angeles, California 90095, United States
*The authors contributed equally to this work.
Received 2019-7-20; Accepted 2019-8-16; Published 2019-10-17
Abstract
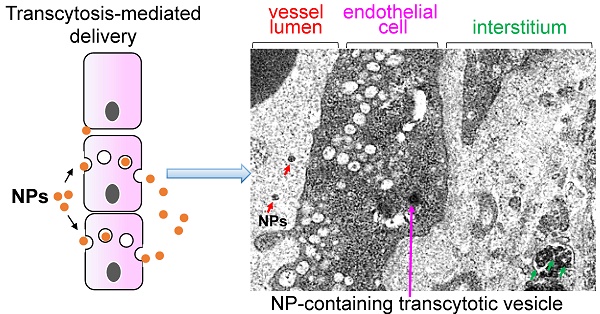
Numerous nano drug delivery systems have been developed for preclinical cancer research in the past 15 years with the hope for a fundamental change in oncology. The robust nanotherapeutic research has yielded early-stage clinical products as exemplified by the FDA-approved nano formulations (Abraxane® for paclitaxel and Onyvide® for irinotecan) for the treatment of solid tumors, including pancreatic ductal adenocarcinoma (PDAC). It is generally believed that enhanced permeability and retention (EPR) plays a key role in nanocarriers' accumulation in preclinical tumor models and is a clinically relevant phenomenon in certain cancer types. However, use of EPR effect as an across-the-board explanation for nanoparticle tumor access is likely over-simplified, particularly in the stroma rich solid tumors such as PDAC. Recently, ample evidences including our own data showed that it is possible to use transcytosis as a major mechanism for PDAC drug delivery. In this mini-review, we summarize the key studies that discuss how transcytosis can be employed to enhance EPR effect in PDAC, and potentially, other cancer malignancies. We also mentioned other vasculature engineering approaches that work beyond the classic EPR effect.
Keywords: Transcytosis, EPR effect, nano drug delivery
Introduction
Most nanocarriers currently being tested in clinical trials rely on passive delivery, which conceptually depends on the enhanced permeability and retention (EPR) effect[1, 2]. While, the EPR effect is often explained as the presence of “leaky” tumor vasculature, the size-controlled delivery of nanoparticles varies dramatically among different cancer types[3-5]. In cancer patients, it is encouraging to see the incremental evidences of EPR using liposome and polymeric nanocarriers, which suggests that EPR is a clinically relevant phenomenon in certain cancer types or patient populations[3]. However, another suggestion is that the abnormal vascular fenestrations and leakiness of highly vascular human xenografts in mice may represent an experimental artifact[6, 7]. Accordingly, “EPR effect in patients” and “targeting principle beyond EPR” become “hot” topics for research and translational nanomedicine study. In fact, there is a major debate on the effectiveness of EPR effect in cancer animal models and ultimately patients[3, 6, 8]. In a meta-analysis based on 10 years nanomedicine literature, the authors concluded that ~0.7% (median) of the injected nanoparticle dose accumulated in solid tumors[9]. Other experts indicated that the EPR effect of 0.7% was an unfair and unconventional calculation in determining nanomedicine tumor homing efficiency[10]. A recent literature further pointed out the key aspects that were overlooked in the aforementioned meta-analysis, such as sophisticated nanocarrier physicochemical properties, experimental errors from unstable particle labeling, neglected contributions from circulation half-life, tumor type, weight, interstitial fluid pressure (IFP), etc[8, 10]. In the setting of solid tumors with a thick dysplastic stroma, the putative EPR effect is governed by the factors that are way beyond enlarged tumor fenestrations because these tumor types (including PDAC) may develop vasculature that are structurally collapsed or obstructed by tumor-associated fibroblasts or pericytes that adhere tightly to the endothelial cells (Figure 1). The cellular and non-cellular heterogeneity in the tumor microenvironment (TME), which is incompletely understood, collectively determines the fate of nanoparticle tumor access. In this manuscript, we mainly focus on nano drug delivery in PDAC with a view to discuss transcytosis-mediated tumor targeting, an EPR-independent targeting approach without the need of tumor vasculature leakiness.
PDAC is the 4th leading cause of cancer death. According to the American Cancer Society's estimation, there were 56,770 new PDAC cases and 45,750 patient death cases in the US in 2019[11, 12]. This accounts for approximately 3% of all cancer cases in the US and about 7% of all cancer deaths[11, 12]. Unlike other cancer malignancies, chemotherapy is the most frequently used treatment for the majority PDAC patients because of the late diagnosis that leads to advanced disease. At this stage, the standard treatment includes gemcitabine (GEM) and classic or modified FOLFIRINOX (a 4-drug regimen). Recently, the FDA approved the use of GEM plus Albumin-bound paclitaxel nanocomplex (Abraxane) and liposomal irinotecan nanocarrier (Onivyde) plus fluorouracil for PDAC treatment, which lead to an improvement of overall survival of ~2 months[13, 14]. The lack of a robust response to these first-generation nano formulations is in part due to the unfavorable PDAC TME that prevents drug delivery. In addition to cancer cells, a major pathological feature is the presence of thick tumor stroma, which lowers the putative EPR effect and nanoparticle access. In PDAC, the stroma barrier contains a variety of cellular and non-cellular components, such as pericytes, endothelial cells, fibroblasts, tumor associate macrophage, T cells, NK cells, collagen deposition, etc[15, 16]. The complexity in the TME also comes from the biophysical components, such as acidity, hypoxia, high tumor IFP, etc.[15, 16]. Collectively, fibrotic stroma and abnormal vasculature negatively impact drug delivery in PDAC.
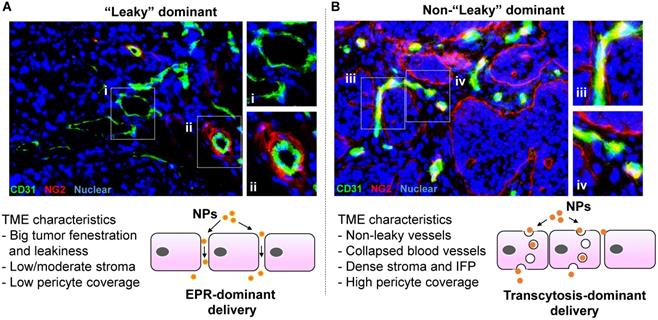
Representative solid tumor IHC staining to show “leaky” vs “non-leaky” tumor types. Human MCF-7 breast cancer (A) and BxPC3 pancreatic cancer (B) tissues were retrieved from our historical samples and OCT embedded for frozen section. Two-color immunohistochemistry staining was performed. The endothelial cell marker (CD31) was labeled in green (FITC), and the pericyte marker (NG2) was labeled in red (Alexa Fluor 594). Nuclear was labelled by Hoechst dye. Zoom in pictures to show the extent of pericyte coverage in each tumor type. While the blood vessels in MCF-7 tumor exhibit low pericyte coverage and round-like structure, we frequently observed structurally collapsed or obstructed blood vessels in BxPC3 tumor, which also contain high pericyte coverage. Accordingly, we assigned MCF-7 and BxPC3 tumors into “leaky” and “non-leaky” categories, respectively. The important tumor microenvironment (TME) characteristics are summarized. In our opinion, while EPR effect may play a key role in the “leaky” tumor (A, lower panel), transcytosis becomes more important in the “non-leaky” tumor type (B, lower panel).
Instead of relying the enlarged tumor fenestration that may not dominate in PDAC, we are asking how the nanoparticles can cross PDAC vasculature. This turns out to be a technically challenging question to answer for multiple reasons. First, due to logistic limitation, most preclinical studies rely on observations at empirically selected late time points. This experimental design leads to “endpoint” observations, which answer the question about whether the injected nanoparticle can reach the tumor site, but not necessarily how the nanoparticles reach the tumor site. Second, ultrastructural visualization of nano particulates in heterogeneous TME requires high operational standards in terms of sample preparation, imaging condition and equipment status. By considering these challenges, it would be ideal to make an in situ observation with ultrastructural resolution to answer the question about if the enlarged tumor fenestration is the solo factor that determines nanoparticle tumor access. Alternatively stated, does nanoparticle extravasation primarily rely on “leakiness” (Figure 1A, lower panel) or there is other mechanism that operates complementarily, i.e. through a transendothelial transport pathway (a.k.a. transcytosis) that assists nanoparticle tumor homing effectiveness (Figure 1B, lower panel).
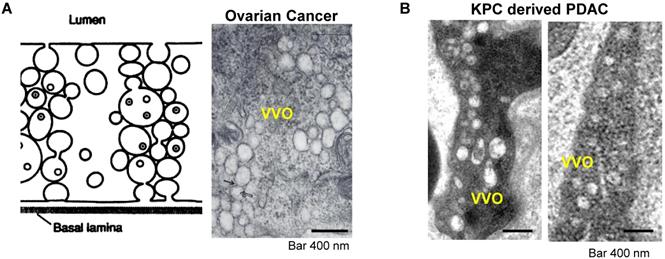
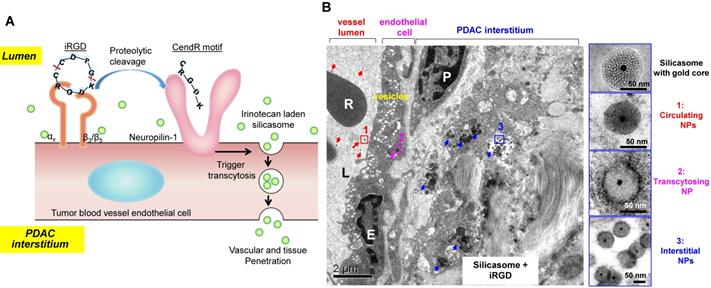
A very interesting observation that appeared in old ultrastructural literatures was the demonstration that ovarian tumor vascular endothelial cells displayed a network of tubular vesicles (a.k.a. the vesico-vacuolar organelle or VVO) (Figure 2A)[17-19]. VVOs were described as “grape-like” clusters of interconnecting vesicles and vacuoles, which span the entire thickness of vascular endothelium[17]. The authors suggested that these VVOs may provide a potential trans-endothelial path between the vascular lumen and the extravascular space, facilitating macromolecule transcytosis even without the need of vasculature leakiness[17]. Our own observation showed that VVO-like structures can be found in an orthotopic PDAC model using Kras mutant PDAC cells (derived from a spontaneous PDAC tumor from a transgenic KrasLSL-G12D/+Trp53LSL-R172H/+Pdx1-Cre mouse) (Figure 2B)[20]. Previous studies also showed an endocytic transcytosis pathway that can be therapeutically elevated by tumor-penetrating iRGD peptides (CRGD[K/R]GP[D/E]C)[21, 22]. iRGD is capable of homing to the tumor-specific integrins expressed on the endothelial cells on tumor vasculature (but not normal cells)[21, 22]. In iRGD, the CendR motif is not C-terminal, but an active CendR motif that can be generated through proteolytic cleavage (Figure 3A)[23, 24]. The exposed CendR motif interacts with a multifunctional, VEGF-binding, non-tyrosine kinase receptor, neuropilin-1 (NRP-1). NRP-1 binding triggers a mass transcytosis pathway that mimics macropinocytosis (except that NRP-1 receptor is involved), and is similar in concept to the VVO's[25-27]. Moreover, NRP-1 expression correlates with tumor progression and poor prognosis in various cancers, including PDAC[27]. Accordingly, the iRGD peptide is capable of promoting the penetration and tumor cell entry of a wide range of therapeutics in tumor models, including BxPC-3 and PC-09 PDAC models[27]. The therapeutics includes free drugs, macromolecules (dextran), dyes (Evans blue), peptide, antibodies, liposomes, and Abraxane[21, 22, 27, 28]. Recently, we showed that the anti-cancer efficacy of an irinotecan loaded silicasome nanocarrier can be significantly improved by the co-administration of free iRGD peptide even without the requirement of covalent attachment[20]. This led to a ~4-fold nanoparticle uptake increase at the orthotopic KPC PDAC site, leading to enhanced efficacy at primary and metastatic sites. Moreover, through the use of transmission electron microscopy (TEM), we obtained ultrastructural in situ evidence showing the appearance of grouped vesicles in PDAC endothelial cells, with the ability to carry gold nanoparticle labeled silicasomes from the blood vessel lumen to the PDAC matrix, without the requirement of tumor fenestration (Figure 3B)[20].
In addition, it was also demonstrated that albumin can mediate a transcytosis process in endothelial cells by targeting the 60-kDa glycoprotein (gp60) receptor, which binds to caveolin-1 by forming transcytotic vesicles[29-31]. Based on this caveolae-mediated transcytosis mechanism, many albumin-based drug delivery nanoparticles were developed for cancer treatment including PDAC[32-36]. One well-known example is the albumin-bound nanoparticle (nab) paclitaxel (Abraxane®), which achieved efficient delivery of the drug to the PDAC and other tumor sites by taking the advantage of caveolae-mediated transcytosis mechanism[35, 37]. Besides the albumin, another impressive example of receptor-mediated transcytosis of nanoparticles in PDAC is the development of urokinase plasminogen activator receptor (uPAR) targeting imaging nanoparticles[38]. The association of uPAR with caveolae first facilitates nanoparticles crossing the endothelium via transcytosis. This allows the particle entry into perivascular tumor areas, followed by the binding to uPAR-expressing PDAC tumor cells and tumor-associated stromal cells[37]. This approach has resulted in highly selective delivery of imaging probes into primary and metastatic pancreatic cancer lesions[38]. In addition to surface modification, recent data suggested that surface charge may also play an important role during the transcytosis process[39-43]. A macropinocytosis-mediated transcytosis was observed in cationic nanoparticles which exhibit efficient tumor penetration when compared to the treatment using anionic and neutral particles[39-42]. Zhou et al., designed a γ-glutamyl transpeptidase-responsive camptothecin-polymer conjugate. The idea was to use γ-glutamyl transpeptidase that was overexpressed on the endothelial cell membrane to cleave the γ-glutamyl moieties, leading to the positive primary amines on the particle surface[43]. The resulting cationic conjugate actively infiltrated throughout the tumor tissue through caveolae-mediated (not macropincytosis) endocytosis and transcytosis, which enable deep tumor penetration with effective anticancer drug delivery[43]. A brief summary of transcytosis mediated nanocarrier delivery examples in PDAC was provided in Table 1.
Transcytosis and vesiculo-vacuolar organelle (VVO). (A) Left: Schematic of VVO mediated transcytosis pathway; right: ultrastructural TEM view shows VVOs to consist of grape-like clusters of interconnecting vesicles and vacuoles in abluminal in a subcutaneous mouse ovarian tumor. Adapted with permission from ref.[17]. (B) Ultrastructural TEM shows the VVOs structures in an orthotopic KPC-derived PDAC tumor. Adapted with permission from ref.[20].
(A) Schematic of the iRGD-mediated transcytosis mechanism for silicasome nanocarrier delivery in PDAC tumor. (B) Ultrastructural TEM views show iRGD co-administration mediated silicasome transcytosis process in orthotopic KPC tumor. The TEM image shows gold core labeled silicasomes in (i) the lumen of a tumor blood vessel (red arrows), (ii) transport in the endothelial vesicles (pink arrow), and (iii) deposition in the tumor interstitium (blue arrows). High-magnification images of regions 1 through 3 are provided in the panels on the right. E, endothelial cell; P, pericyte. Scale bar: 2 μm (left panel); 50 nm (right panels). Adapted with permission from ref.[20].
Examples of transcytosis mediated nanocarrier delivery in PDAC
| Formulation | Transcytosis mechanism | Size | Zeta potential | Cancer model | Accumulation increased in tumor | ref |
|---|---|---|---|---|---|---|
| iRGD-conjugated lipid micelles | VVOs mediated | 15-25 nm | n/a | Orthotopic human MIA PaCa-2 xenograft | n/a | [22] |
| Lipid coated mesoporous silica nanoparticle (silicasome) Co-delivery with free iRGD peptide | VVOs mediated | ~130 nm | ~ -10 mV | Orthotopic murine pancreatic KPC-derived tumor Subcutaneous patient-derived xenograft (PDX) | 2~4-fold increase in KPC model, ~1.5-fold in PDX compared to without iRGD, | [20] |
| Urokinase plasminogen activator receptor (uPAR) targeting peptide modified iron oxide nanoparticles | caveolae mediated | 10 nm core | n/a | Orthotopic human MIA PaCa-2 xenograft | 3~4-fold increased signal compared to the free peptide | [38] |
| Albumin-bound curcumin nanoparticles | caveolar mediated | 130-150nm | ~ - 20 mV | Subcutaneous human MIA PaCa-2 xenograft | 2~10-fold increase at different time points | [32] |
| Transferrin conjugated doxorubicin-loaded human serum albumin nanoparticles | caveolae mediated | ~ 220 nm | ~ - 34.3 mV | In vitro human metastatic CAPAN-1 cells | n/a | [33] |
| Albumin-bound paclitaxel, ABI-007 (Abraxane) | caveolar mediated | ~130 nm | n/a | Subcutaneous human MIA PaCa-2 xenograft | Deeper penetration via intratumoral injection | [35] |
| Gemcitabine-loaded albumin nanoparticles | caveolar mediated | ~150 nm | ~ -10 mV | Subcutaneous human BxPC3 xenograft | n/a | [36] |
| γ-glutamyl transpeptidase-responsive camptothecin-polymer conjugate | caveolae mediated | ~9 nm | ~ -10 mV changed to ~ + 5 mV after γ-glutamyl cleavage | Subcutaneous and orthotopic human BxPC3 xenografts | ~2-fold increase compared to non-cleavage polymer | [43] |
Transcytosis is a type of transcellular transport across the interior of a cell which extensively studied initially for macromolecules delivery through the blood-brain barrier (BBB)[30, 44-54]. Actually, transcytosis-based mechanism has been used to achieve efficient delivery of drugs and genes by nanocarriers to various tissues[55-58]. In addition to PDAC, transcytosis is also involved in variety of solid tumor cancers, such as breast cancer[33, 37, 42, 59-61], lung cancer[37, 62], prostate cancer[37, 42], brain cancer[63-68], melanoma[42, 58], gastric cancer[69], colorectal cancer[32, 33, 37, 41, 42, 69] and other cancers[37]. One of the most recognized cases is drug delivery in brain cancers (e.g., glioma and glioblastoma) using transferrin (Tf) modification with the hope to across BBB[50, 68, 70, 71]. For example, Chang et al. demonstrated that Tf-coated PLGA-NPs entered massively within brain-developed F98 glioma tumors[64]. Porru et al. also developed zoledronic acid loaded nanoparticles with Tf-conjugation for transcytosis mediated BBB access and efficacy improvement in an orthotopic glioblastoma model[65]. Since tumor formation in brain may interfere BBB integrity, these observations, in our opinion, may be the combined effect of pathological vasculature leakiness and transcytosis-mediated nanoparticle brain access. Interestingly, Williams et al. found a type of PLGA-PEG mesoscale nanoparticles (~400 nm) selectively target to kidney via transcytosis mechanism across depend predominantly on size and surface functionalization (non-opsonizing surface) but is independent of moderate surface charges[72]. It becomes a very promising strategy of nanomedicines for kidney diseases[73]. Impressively, Leng et al. design a series of linear and branched histidine-lysine (HK) peptide carriers as nonviral vectors for gene and siRNA delivery, this type of HK polyplexes can target neuropilin-1 receptor on endothelial cells and tumor cells which mediated the transcytosis through the tumor endothelium and lead high tumor distribution and efficient transfection in MDA-MB-435 breast cancer model[74, 75]. The study further proved that the NRP-1 mediated transcytosis can be interfered by NRP-1 antibody blocking or enhanced using the approach of restricting nutrients with a glucose-transport inhibitor[74].
While the use of transcytosis to improve drug delivery is still not fully understood yet, ample evidences strongly suggested that physicochemical characteristics of nanocarriers may determine the effectiveness of transcytosis at tumor site. This includes the early-stage observations on particle chemical composition, size, shape, charge, surface modification, which may alter the rate and abundance of transcytosis[76-78]. However, it is too early to summarize a consensus to reproducibly activate transcytosis-mediated particle tumor access because these physicochemical parameters may collectively impact the process, which is further complicated by non-material factors, such as fibrotic status and IFP. Since favorable systemic circulation feature and intratumoral access may have different requirements with the respect to material properties, interesting strategies, such as the stimuli-responsive nanocarriers that may alter size/charge in circulation vs tumor have generated promising preclinical outcome[43, 76, 79-82].
Another example to broaden the EPR concept is increased stromal vascular access by reducing pericyte coverage in PDAC[83-87]. While pericyte interference exhibits distinct mechanism as compared to transcytosis activation, it provides an alternative approach to improve drug delivery in non-leaky tumor, which may function beyond EPR effect. This challenge was met by designing a PEI/PEG-coated mesoporous silica nanoparticle that can be used for attaching a small molecule TGF-β receptor kinase inhibitor, LY364947[87]. This drug interferes with the dominant signaling pathway for pericyte recruitment and adherence to endothelial cells. This carrier was derived through iterative design to achieve monodisperse particles of optimal size (~50 nm), stable copolymer attachment, maximum colloidal stability, and biocompatible polymer size selection (e.g., PEI 1.8 kD), and through choosing from a range of small molecule inhibitors to find a TGF-β inhibitor prototype with stable, pH-sensitive and high affinity binding. Ultimately, we obtained a nanocarrier containing high wt% LY364947. When tested in a human BxPC3 PDAC xenograft, this carrier could effectively interfere in pericyte adhesion to endothelial cells within 2 hours of intravenous injection. This allowed the development of a “two-wave” approach, in which the LY364947 nanocarrier was used as the 1st wave to open the stromal vascular gate, thereby allowing rapid tumor entry by 2nd wave drug carriers. This is in the line with nano-enabled engineered approach, which uses a multistage/multistep combination treatment to provide an impact on PDAC stroma, such as augmented blood vessel permeability, inhibition of drug inactivating enzymes and/or target specific biological factors[15, 88-91].
From the translational nanomedicine aspect, there is a clear agenda to predict and quantify the EPR effect in cancer patients. This notion holds true to explore new mechanism(s) such as transcytosis to facilitate nanocarriers' tumor access, especially in non-leaky tumor types. It is noteworthy to dissect the complexity of each cancer indication as well as the differences among each individual with the same cancer indication. In fact, MRI imaging (using fluorescently labeled iron oxide nanoparticles) followed by quantitative intravital fluorescence visualization can identify “responder” for nanomedicine therapy[92]. To effectively discern the heterogeneous nanoparticle tumor access in preclinical study, it is critical to consider stringent tumor models as compared to data generation using the convenient but highly artificial subcutaneous xenograft model. It is generally agreed upon in the field that new therapies (which is true for PDAC nano therapeutics) should be tested in the advanced models (Figure 4), which closely mimics disease characteristics[93]. This includes primary human patient-derived xenograft (PDX) models, which can be implanted subcutaneously and orthotopically in NSG mice immediately after surgical resection[94]. In this regard, the selection of a tumor PDX pair with differential NRP-1 expression on the tumor vasculature demonstrated differences in carrier uptake and irinotecan delivery during iRGD-mediated transcytosis activation. Our data indicated that it is necessary to contemplate the usage of a personalized approach to PDAC chemotherapy to enhance the efficacy of the irinotecan silicasome carrier by iRGD co-administration[20].
In summary, it is not surprising that major efforts are underway to study drug delivery using nano-enable approach for PDAC treatment. It becomes clear that transcytosis (perhaps with other unknown mechanisms) may coexist with the so-called EPR effect, which may not be a dominate factor in the case of PDAC. Further investigations are still required to fully understand this emerging approach, which may allow nanoparticle tumor targeting with major augment with respect to homing abundance, efficiency and time kinetics.
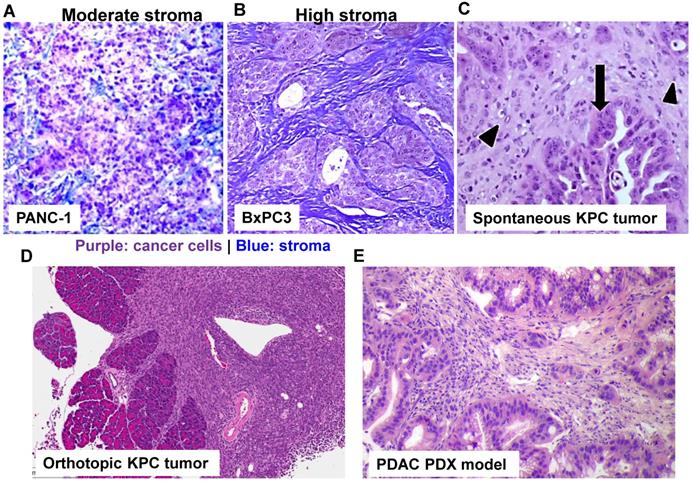
Use of stringent PDAC cancer models to study drug delivery using nanoparticle. With the rapid development of PDAC cancer biology, it has been possible by employing different PDAC mouse models to better understand the molecular mechanism underlying pancreatic cancer, including nanoparticle-mediated drug delivery. Trichrome staining of PANC-1 xenograft (A) and BxPC3 (B) in nude mice. While BxPC3 tumor (Kras WT) is usually regarded as stroma-rich, PANC-1 tumor (Kras mutated) contains moderate level of stroma content. With the recent success in the production of genetically engineered mouse models (GEMMs), it is theoretically possible to test nanotherapeutics in KPC model in which the conditional expression of mutant KrasG12D and Trp53R172H is governed by a pancreas-specific Cre. Without the involvement of Cre, a transcriptional and translational STOP cassette flanked by loxP sites silences the expression of mutant KrasG12D and Trp53R172H. In KPC tumor (C, adapted with permission from ref.[93]), substantial nuclear abnormalities occur and glands appear embedded in the tumor stroma (arrowheads) with completely random organization (arrows). However, a major pitfall using spontaneous KPC model is the variable growth characteristics of the spontaneous KPC model and the number of animal experiments that can be undertaken. Therefore, the variable tumor development and unfavorable logistics, precludes widespread use of KPC model. In order to perform robust experiment, we have established immortalized luciferase-transfected cell lines derived from spontaneous KPC tumors, and have used them to establish a surgical procedure for orthotopic tumors in immunocompetent, syngeneic B6/129 mice (D). We have confirmed that orthotopic implant in the pancreas leads to predictable tumor development within 1-2 weeks and mimic human PDAC characteristics such as local invasion of the G.I.T. and liver metastases after 3-5 weeks. Moreover, the availability of PDAC PDX model (E) allows the study of patient-specific response and personalized nanomedicine.
Acknowledgements
This work was supported by the U.S. Public Health Service Grant, 1U01CA198846.
Competing Interests
The authors declare the following competing financial interest(s): Huan Meng is the co-founder of Westwood Bioscience Inc and Nammi Therapeutics Inc. The remaining authors declare no conflict of interest.
References
1. Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136-51
2. Yi Y, Lin G, Chen S, Liu J, Zhang H, Mi P. Polyester micelles for drug delivery and cancer theranostics: current achievements, progresses and future perspectives. Mater Sci Eng C. 2018;83:218-32
3. Golombek SK, May J-N, Theek B, Appold L, Drude N, Kiessling F. et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17-38
4. Hansen AE, Petersen AL, Henriksen JR, Boerresen B, Rasmussen P, Elema DR. et al. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano. 2015;9:6985-95
5. Lee H, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX. et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin Cancer Res. 2017;23:4190-202
6. Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC. et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412-7
7. Bjornmalm M, Thurecht KJ, Michael M, Scott AM, Caruso F. Bridging bio-nano science and cancer nanomedicine. ACS Nano. 2017;11:9594-613
8. Grodzinski P, Kircher M, Goldberg M, Gabizon A. Integrating Nanotechnology into Cancer Care. ACS Nano. 2019;13:7370-6
9. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF. et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014
10. McNeil SE. Evaluation of nanomedicines: stick to the basics. Nat Rev Mater. 2016;1:16073
11. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
12. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34
13. Wang-Gillam A, Li C-P, Bodoky G, Dean A, Shan Y-S, Jameson G. et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-57
14. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-703
15. Dimou A, Syrigos KN, Saif MW. Overcoming the stromal barrier: technologies to optimize drug delivery in pancreatic cancer. Ther Adv Med Oncol. 2012;4:271-9
16. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-76
17. Dvorak A, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol. 1996;59:100-15
18. Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981-6
19. Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation. 1999;6:23-44
20. Liu X, Lin P, Perrett I, Lin J, Liao Y-P, Chang CH. et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J Clin Invest. 2017;127:2007-18
21. Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR. et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031-5
22. Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM. et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510-20
23. Liu X, Jiang J, Ji Y, Lu J, Chan R, Meng H. Targeted drug delivery using iRGD peptide for solid cancer treatment. Mol Syst Des Eng. 2017;2:370-9
24. Liu X, Jiang J, Nel AE, Meng H. Major effect of transcytosis on nano drug delivery to pancreatic cancer. Mol Cell Oncol. 2017;4:e1335273
25. Pang H-B, Braun GB, Friman T, Aza-Blanc P, Ruidiaz ME, Sugahara KN. et al. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat Commun. 2014;5:4904
26. Pang H-B, Braun GB, She Z-G, Kotamraju VR, Sugahara KN, Teesalu T. et al. A free cysteine prolongs the half-life of a homing peptide and improves its tumor-penetrating activity. J Control Release. 2014;175:48-53
27. Akashi Y, Oda T, Ohara Y, Miyamoto R, Kurokawa T, Hashimoto S. et al. Anticancer effects of gemcitabine are enhanced by co-administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin-1. Br J Cancer. 2014;110:1481
28. Hu C, Chen X, Huang Y, Chen Y. Co-administration of iRGD with peptide HPRP-A1 to improve anticancer activity and membrane penetrability. Sci Rep. 2018;8:2274
29. Schnitzer J. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol Heart Circ Physiol. 1992;262:H246-H54
30. Vogel SM, Minshall RD, Pilipović M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1512-L22
31. Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci U S A. 2004;101:7699-704
32. Kim TH, Jiang HH, Youn YS, Park CW, Tak KK, Lee S. et al. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403:285-91
33. Bae S, Ma K, Kim TH, Lee ES, Oh KT, Park E-S. et al. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials. 2012;33:1536-46
34. Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168-82
35. Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS. et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother Pharmacol. 2015;76:699-712
36. Yu X, Di Y, Xie C, Song Y, He H, Li H. et al. An in vitro and in vivo study of gemcitabine-loaded albumin nanoparticles in a pancreatic cancer cell line. Int J Nanomedicine. 2015;10:6825
37. Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A. et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317-24
38. Yang L, Mao H, Cao Z, Wang YA, Peng X, Wang X. et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology. 2009;136:1514-25 e2
39. Harush-Frenkel O, Rozentur E, Benita S, Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435-43
40. Miura S, Suzuki H, Bae YH. A multilayered cell culture model for transport study in solid tumors: Evaluation of tissue penetration of polyethyleneimine based cationic micelles. Nano Today. 2014;9:695-704
41. Suzuki H, Bae YH. Evaluation of drug penetration with cationic micelles and their penetration mechanism using an in vitro tumor model. Biomaterials. 2016;98:120-30
42. Wang H-X, Zuo Z-Q, Du J-Z, Wang Y-C, Sun R, Cao Z-T. et al. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today. 2016;11:133-44
43. Zhou Q, Shao S, Wang J, Xu C, Xiang J, Piao Y. et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019;14:799-809
44. Mostov KE, Simister NE. Transcytosis. Cell. 1985;43:389-90
45. Fishman J, Rubin J, Handrahan J, Connor J, Fine R. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J Neurosci Res. 1987;18:299-304
46. Milici A, Watrous N, Stukenbrok H, Palade G. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987;105:2603-12
47. Pardridge WM, Triguero D, Yang J, Cancilla PA. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. J Pharmacol Exp Ther. 1990;253:884-91
48. Pardridge WM, Kang Y-S, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res. 1995;12:807-16
49. Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871-932
50. Widera A, Norouziyan F, Shen W-C. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55:1439-66
51. Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420:32-8
52. Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci U S A. 1996;93:14164-9
53. Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3-14
54. Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54-61
55. Florence AT, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev. 2001;50:S69-S89
56. Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3:499
57. Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB. Delivery of nanoparticle-complexed drugs across the vascular endothelial barrier via caveolae. IUBMB life. 2011;63:659-67
58. Lu W, Xiong C, Zhang R, Shi L, Huang M, Zhang G. et al. Receptor-mediated transcytosis: a mechanism for active extravascular transport of nanoparticles in solid tumors. J Control Release. 2012;161:959-66
59. Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2:373-83
60. Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99
61. Abbasi S, Paul A, Shao W, Prakash S. Cationic albumin nanoparticles for enhanced drug delivery to treat breast cancer: preparation and in vitro assessment. J Drug Deliv. 2012;2012:686108
62. Lu H, Utama RH, Kitiyotsawat U, Babiuch K, Jiang Y, Stenzel MH. Enhanced transcellular penetration and drug delivery by crosslinked polymeric micelles into pancreatic multicellular tumor spheroids. Biomater Sci. 2015;3:1085-95
63. Gu G, Gao X, Hu Q, Kang T, Liu Z, Jiang M. et al. The influence of the penetrating peptide iRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials. 2013;34:5138-48
64. Chang J, Paillard A, Passirani C, Morille M, Benoit J-P, Betbeder D. et al. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29:1495-505
65. Porru M, Zappavigna S, Salzano G, Luce A, Stoppacciaro A, Balestrieri ML. et al. Medical treatment of orthotopic glioblastoma with transferrin-conjugated nanoparticles encapsulating zoledronic acid. Oncotarget. 2014;5:10446
66. Jiang X, Sha X, Xin H, Xu X, Gu J, Xia W. et al. Integrin-facilitated transcytosis for enhanced penetration of advanced gliomas by poly (trimethylene carbonate)-based nanoparticles encapsulating paclitaxel. Biomaterials. 2013;34:2969-79
67. Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR. et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7:8583-92
68. Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48:2967-3014
69. Simón-Gracia L, Hunt H, Scodeller P, Gaitzsch J, Kotamraju VR, Sugahara KN. et al. iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials. 2016;104:247-57
70. Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247:291-307
71. Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci U S A. 2013;110:8662-7
72. Williams RM, Shah J, Ng BD, Minton DR, Gudas LJ, Park CY. et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015;15:2358-64
73. Williams RM, Jaimes EA, Heller DA. Nanomedicines for kidney diseases. Kidney Int. 2016;90:740-5
74. Leng Q, Mixson AJ. The neuropilin-1 receptor mediates enhanced tumor delivery of H2K polyplexes. J Gene Med. 2016;18:134-44
75. Leng Q, Woodle MC, Mixson AJ. Targeted delivery of siRNA therapeutics to malignant tumors. J Drug Deliv. 2017;2017:6971297
76. Sun Q, Ojha T, Kiessling F, Lammers T, Shi Y. Enhancing tumor penetration of nanomedicines. Biomacromolecules. 2017;18:1449-59
77. Shi Y, Lammers T, Storm G, Hennink WE. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromol Biosci. 2017;17:1600160
78. Lu W, Tan Y-Z, Hu K-L, Jiang X-G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm. 2005;295:247-60
79. Liu X, Chen Y, Li H, Huang N, Jin Q, Ren K. et al. Enhanced retention and cellular uptake of nanoparticles in tumors by controlling their aggregation behavior. ACS Nano. 2013;7:6244-57
80. Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24:5476-80
81. Jin Q, Deng Y, Chen X, Ji J. Rational design of cancer nanomedicine for simultaneous stealth surface and enhanced cellular uptake. ACS Nano. 2019;13:954-77
82. Wang S, Huang P, Chen X. Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization. Adv Mater. 2016;28:7340-64
83. Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M. et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling. Proc Natl Acad Sci U S A. 2007;104:3460-5
84. Kano MR, Komuta Y, Iwata C, Oka M, Shirai Yt, Morishita Y. et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-β receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009;100:173-80
85. Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815
86. Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K. et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2012;109:16618-23
87. Meng H, Zhao Y, Dong J, Xue M, Lin Y-S, Ji Z. et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano. 2013;7:10048-65
88. Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2014;4:81
89. Adiseshaiah PP, Crist RM, Hook SS, McNeil SE. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol. 2016;13:750
90. Zhu L, Staley C, Kooby D, El-Rays B, Mao H, Yang L. Current status of biomarker and targeted nanoparticle development: The precision oncology approach for pancreatic cancer therapy. Cancer Lett. 2017;388:139-48
91. Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI. et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260-9
92. Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med. 2015;7:314ra183
93. Gopinathan A, Morton JP, Jodrell DI, Sansom OJ. GEMMs as preclinical models for testing pancreatic cancer therapies. Dis Model Mech. 2015;8:1185-200
94. Rückert F, Aust D, Böhme I, Werner K, Brandt A, Diamandis EP. et al. Five primary human pancreatic adenocarcinoma cell lines established by the outgrowth method. J Surg Res. 2012;172:29-39
Author contact
![]() Corresponding author: E-mail: menghuanucla.edu or hmengucla.edu
Corresponding author: E-mail: menghuanucla.edu or hmengucla.edu
 Global reach, higher impact
Global reach, higher impact