13.3
Impact Factor
Theranostics 2020; 10(10):4557-4588. doi:10.7150/thno.38069 This issue Cite
Review
Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics
Department of Radiology, Center for Medical Imaging, and State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, No.17 South Renmin Road, Chengdu, 610041, China
Received 2019-7-1; Accepted 2020-2-24; Published 2020-3-15
Abstract
In recent years, much progress has been motivated in stimuli-responsive nanocarriers, which could response to the intrinsic physicochemical and pathological factors in diseased regions to increase the specificity of drug delivery. Currently, numerous nanocarriers have been engineered with physicochemical changes in responding to external stimuli, such as ultrasound, thermal, light and magnetic field, as well as internal stimuli, including pH, redox potential, hypoxia and enzyme, etc. Nanocarriers could respond to stimuli in tumor microenvironments or inside cancer cells for on-demanded drug delivery and accumulation, controlled drug release, activation of bioactive compounds, probes and targeting ligands, as well as size, charge and conformation conversion, etc., leading to sensing and signaling, overcoming multidrug resistance, accurate diagnosis and precision therapy. This review has summarized the general strategies of developing stimuli-responsive nanocarriers and recent advances, presented their applications in drug delivery, tumor imaging, therapy and theranostics, illustrated the progress of clinical translation and made prospects.
Keywords: nanoparticles, stimuli-responsive, tumor microenvironment, diagnosis, theranostics, clinical translation
Introduction
Since the discovery of the enhanced permeability and retention (EPR) effect and impaired lymphatic drainage of tumors [1], nanocarriers have been regarded as promising drug delivery vehicles to tumors [2-5]. In general, nanocarriers in the range of 10 to 200 nm are more likely to be accumulated in solid tumors by passively extravasation from the hyperpermeable tumor blood vasculature [6] and the dynamic openings [7]. Nanocarriers provide a versatile platform for loading a wide range of payloads, including imaging agents, nucleic acids, anticancer drugs, photosensitizers and antibodies, etc., to improve the diagnostic and therapeutic outcomes [8,9]. By incorporating bioactive compounds inside nanocarriers, it could avoid enzymatic degradation and undesired exposure to healthy organs, maintain drug activities, as well as alert the half-life in blood circulation, tumor accumulation and biological performance. Until now, several types of nanocarriers have been engineered for drug delivery in oncology [10, 11], including dendrimers, metal nanoparticles (e.g., iron oxide nanoparticles), polymeric micelles, liposomes, inorganic nanoparticles (e.g., silicon nanoparticles), and cell membrane-based nanoparticles etc. Currently, some nanocarriers have been approved for cancer treatment in clinic, for instance, the doxorubicin-incorporated PEGylated liposome (i.e., Doxil®) is approved for handling Kaposi's sarcoma and ovarian cancer.
Nanocarriers are supposed to deliver bioactive compounds (e.g., imaging or therapeutic agents) to tumor tissues or cancer cells for achieving improved diagnostic and therapeutic efficacy. However, it meets several barriers during circulation or in tumors [12], such as protein corona, degradation, burst release or leaking of cargos, and recognition and clearance by the reticuloendothelial system (RES) etc. Several strategies have been applied to address this, including applying PEG shell for achieving stealth effect [13], decorating with targeting moieties or charge conversion materials for improved cellular internalization [14], multistage drug delivery [15], introducing hydrophobic units or cross-link the core to increase the stability, adding specific molecules to escape from RES, etc. Although the PEGylated nanocarriers exhibited advantages in prolonged circulation, improved drug solubility and reduced side effects, the delivery efficacy of most nanocarriers is still quite low, which requires further improvement [16]. Therefore, strategies for tumor-specific drug delivery have been exploited, mainly including stimuli-responsive nanocarriers [17], and ligand- installed nanocarriers [2], while both were developed to improve the precision of drug delivery but with different focus. The stimuli-responsive nanocarriers are mainly functionalized to delivery, release and activate cargos in specific regions (e.g., tumor microenvironments or intracellular spaces of cancer cells) by responding to internal/external stimuli, e.g., pH, enzymes, etc. [18, 19], while the ligand-installed nanocarriers are mainly applied to promote the specific internalization between nanocarriers and specific cells, e.g., cancer cells, tumor vascular endothelial cells [2], etc. The stimuli-responsive nanocarriers could specifically delivery cargos into tumor microenvironment or cancer cells, while the ligand-installed nanocarriers could specifically target cancer cells that highly expressing receptors. From the application view, the stimuli-responsive nanocarriers have attracted broad attention, as the stimuli could be existed/generated in most of the tumors, while the cancer cell-specific receptors were reported to be expressed only on partial cancer cells (e.g., the expression of Her2/neu was only found in less than 25% of breast cancer patients) [20], which may require preselection of receptors for the application of ligand-installed nanocarriers. It is possible to develop nanocarriers with stimuli-responsive functions for controlled drug release, and with ligands on their surface for targeting cancer cell. In addition, nanocarriers have also be functionalized for cancer theranostics, as the combination of diagnostics and therapy was generally referred as “theranostics” [16, 21], which could be achieved by loading both diagnostic and therapeutic compounds inside the same nanocarriers [22].
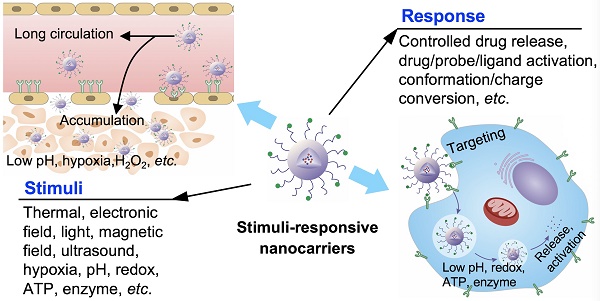
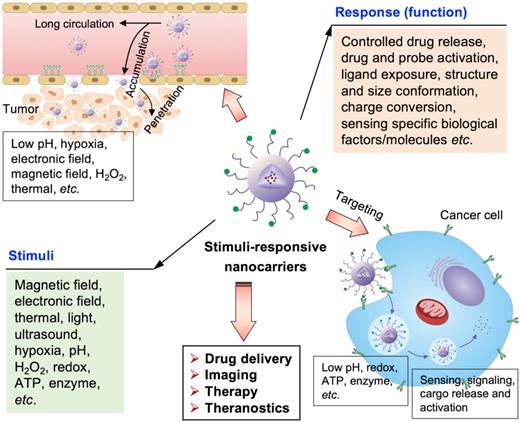
The stimuli-responsive nanocarriers have been rationally designed and developed by considering different pathological profiles in normal tissues, intracellular compartment and tumor microenvironment, to increase drug delivery specificity, efficacy and biological activities (Figure 1) [23-29]. In general, the nanocarriers could response to external stimuli, including magnetic field, temperature (i.e., thermal), ultrasound, light (e.g., laser) and electronic field, etc., and internal stimuli, including pH, ATP, H2O2, enzyme, redox-potential, and hypoxia etc., while the stimuli could be appeared in tumor microenvironment or inside cancer cells (Figure 1). The stimuli- sensitive functions facilitate on-demand or controlled drug release, promoted tumor accumulation, ligand exposure, drug or probe activation, nanoparticle structure or size conformation, charge conversion, as well as signaling in specific positions, sensing of special pathological factors/molecules, tumor-specific diagnosis and theranostics (Figure 1). Moreover, the external force (i.e., stimuli) could also alert the biological performances of nanocarriers, for example, the external magnetic field could increase the accumulation of magnetic nanocarriers in tumors. Furthermore, the stimuli could also be applied to provoke biological activities of certain prodrug- formulated nanocarriers in diseased regions/cells for precision therapy. In addition, the stimuli-responsive nanocarriers were reported to overcome multidrug resistance in cancer treatment [30].
This review has summarized recent progress and achievements in nanocarriers that responsive to external or internal stimuli, presented different stimuli-sensitive strategies and their applications in drug delivery, tumor imaging, therapy and theranostics. In the following sections, the clinical translation of stimuli-responsive nanocarriers has been illustrated, and finally the perspectives were made.
External-responsive nanocarriers
The external stimuli, mainly including thermal, magnetic field, electronic field, ultrasound and light, could affect the fate of nanocarriers inside the biological systems. With the external stimuli, it facilitates enhancing the accumulation of nanocarriers in desired regions with outer forces (e.g., magnetic field), controlled release, intracellular drug delivery, as well as activated imaging and therapy. There are several advantages of applying external-stimuli for drug delivery to tumors: (1) it could precisely control the location and intensity of given external stimuli (e.g., magnetic field, laser irradiation); (2) the external stimuli could be added or removed depending on the treatment requirement; (3) multiple external stimuli could be overlaid for achieving multifunction in cancer theranostics; (4) the possibility to provide multi-times or continuous (e.g., several hours or days) stimuli for drug delivery and therapy. However, the externally-directed triggers would be impractical for accessing and treating the metastatic lesions, when their location is uncertain. Here, the application of external stimuli-responsive nanocarriers will be discussed in this section.
Ultrasound-responsive nanocarriers
Ultrasound is a type of high-frequency sound waves, which could affect nanocarriers for controlled drug release at diseased sites (i.e., tumors). The intensity of ultrasound could be adjusted for different applications. At low ultrasound frequencies (< 20 kHz), it could be applied for imaging, while it could be applied for disrupting nanocarriers to release cargos or enhancing the permeability of cancer cell membrane at high ultrasound frequencies (> 20 kHz) [31]. Until now, several microbubbles have been commercialized, such as Albunex, Optison, Definity, Imagent, Levovist and Sonazoid etc [32]. However, the large size (1-10 μm), short half-life and low stability of microbubbles limit their access to vascular compartments in tumor tissues and deep penetration. Several size switchable microbubbles (i.e., from microbubbles to nanobubbles) [33], or nanocarriers have been engineered for ultrasound imaging [34], ultrasound- triggered drug delivery [35-37], and ultrasound- triggered cancer theranostics (Table 1), including nanobubbles [38], calcium carbonate (CaCO3) nanoparticles [39], liposome [40], nanodroplets [41], vesicles [42] and nanoparticles [43], etc. Generally, the ultrasound-sensitive nanocarriers are incorporating gas or contrast agents [44], including air, N2 and perfluorocarbons, etc., or generating gas in biological environment [45-47], such as CaCO3 nanoparticles [39].
The stimuli-responsive nanocarriers for drug delivery to tumors towards precision imaging, effective therapy and theranostics. The nanocarriers could accumulate and penetrate tumors, and target cancer cells for achieving different applications and functions by responding to the external and internal stimuli.
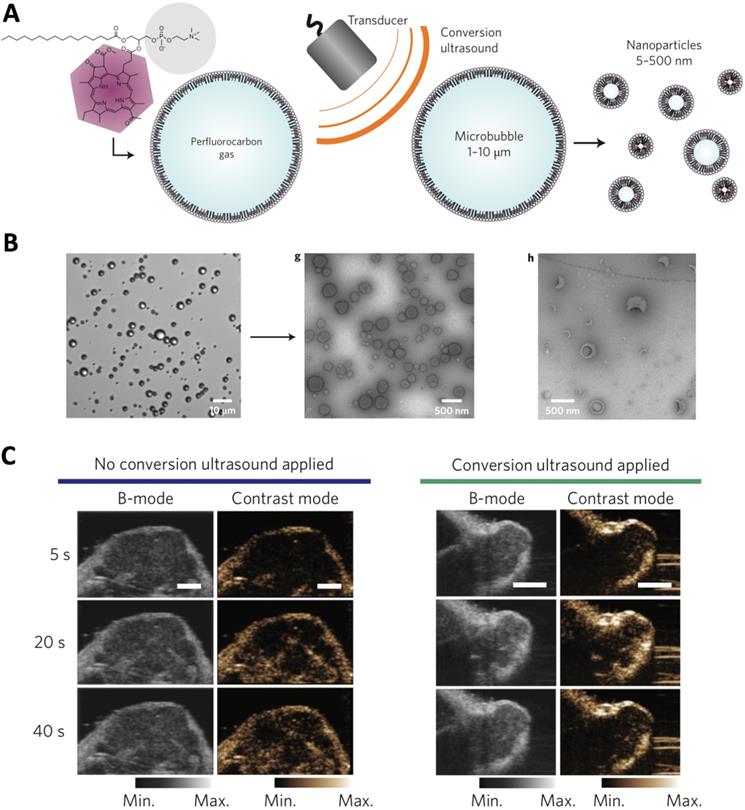
The ultrasound-responsive nanocarriers could be applied for tumor ultrasound imaging, which is safe, low cost and widely applied in clinic, and providing images with high spatial resolution. The gas and contrast agent (e.g., perfluoropentane) incorporated nanocarriers [48], as well as nanoparticles that could generate gas (e.g., CO2) in biological environment [34, 49], have demonstrated tumor-specific imaging at high resolution and intensity. In another strategy, the porphyrin microbubbles (1-10 μm) could be converted into nanobubbles (5-500 nm) for tumor ultrasound imaging (Figure 2) [33]. Besides, ultrasound could also be applied for triggering controlled release of cargos (e.g., imaging probes, anticancer agents) from nanocarriers at desired tumor sites [42, 50]. For example, the phase changeable, polymeric nanodroplets could be generated for tumor imaging and doxorubicin release due the collapse of microbubbles when responding to the low-intensity focused ultrasound [41]. Moreover, the ultrasound-responsive property could be applied for enhancing the tumor accumulation and intracellular delivery of bioactive compounds (e.g., siRNA, DNA) [51]. Because ultrasound could increase gap in tumor vasculature wall (i.e., disrupting of vascular integrity) to facilitate extravasation of drug delivery systems to malignant tissues, as well as enhance cellular uptake by cancer cells [52-54]. However, the large size of ultrasound-sensitive nanocarriers may limit their penetration across tumor tissues, due to the weak penetration of large nanocarriers [6]. In addition, the drug- loaded, ultrasound-sensitive nanocarriers could further be applied for cancer therapy [55], imaging- guided therapy [56-58], and theranostics [39, 59].
Thermal-responsive nanocarriers
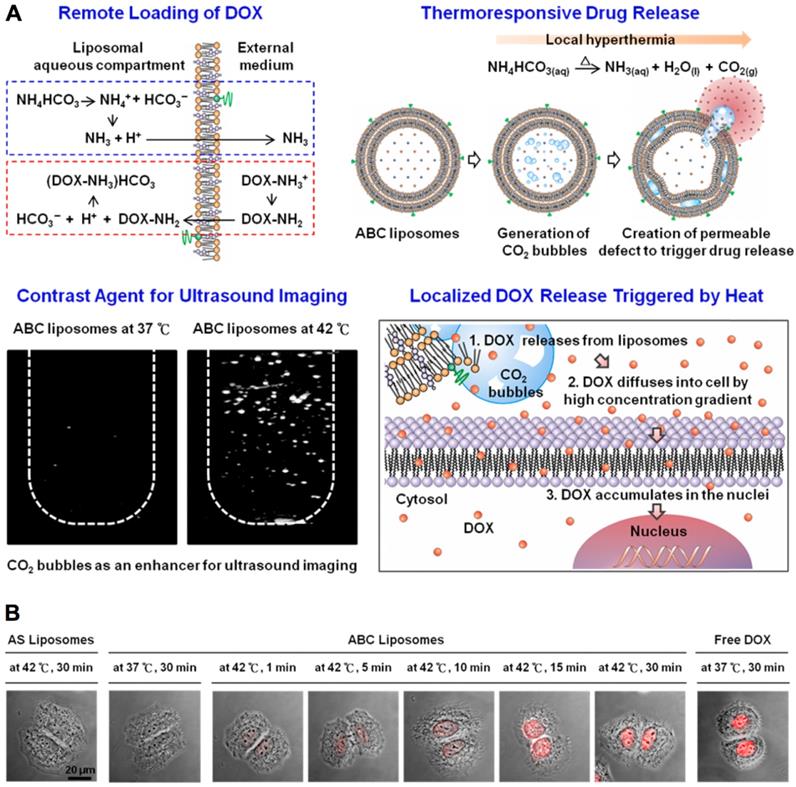
The temperature-sensitive nanocarriers have also been widely applied for drug delivery and dealing with cancer. Generally, the nanocarriers are designed to be stable in normal regions with temperature up to 37 °C and sensitive to higher temperature (> 40 °C) with significantly changes in their properties by responding to the narrow temperature shift. Until now, several thermal-sensitive nanocarriers have been formulated (Table 2), including liposomes [63-65], polymeric micelles [66-70], nanocomposites [66, 71], nanocapsules [72], nanogels [73-76] and vesicles [77, 78], etc. The thermal-sensitive nanocarriers is generated with materials that could undergo physicochemical properties variation associating with temperature change [71, 79]. The temperature- sensitive materials are mainly including poly(N- isopropylacrylamide) (PNIPAM) [80, 81], poly(N-inyl isobutyramide) (PAMAM) [82], poly(2-oxazoline) (POxs) [83], and poly [2-(2-methoxyethoxy) ethyl methacrylate] [PMEOMA] [84], etc. Besides, another strategy for achieving thermal-sensitivity is to incorporate thermal-unstable materials inside nanocarriers. For instance, the NH4HCO3 incorporated liposome could generate CO2 after giving local hyperemia (42°C) to make liposome swollen and collapse [64], leading to drug release for efficient intracellular drug delivery (Figure 3).
The ultrasound-triggered conversion of microbubbles to nanoparticles for multimodality tumor imaging. (A) Illustration of ultrasound-triggered conversion of porphyrin microbubbles to nanobubbles. (B) Confirmation of the conversion of microbubbles to nanobubbles with ultrasound stimuli by microscopy. (C) Ultrasound imaging of tumors by using no conversion ultrasound (left) and by administration of conversion nanoparticles (right). Adapted with permission from ref. [33], copyright 2015 Springer Nature Publishing AG.
Thermal-sensitive nanocarriers for drug delivery. (A) Thermal-sensitive liposomes (i.e., ABC liposomes) for molecular imaging, drug delivery and controlled drug release. (B) Cellular uptake of thermal-sensitive liposomes, control liposomes (i.e., AS liposomes) and free doxorubicin. Adapted with permission from ref. [64], copyright 2013 American Chemical Society.
Representative ultrasound-responsive nanocarriers
| Nanocarriers | Ultrasound-sensitive strategy/materials | Cargos | Applications | Ref. |
|---|---|---|---|---|
| Converting microbubbles | Converting porphyrin microbubbles to nanoparticles by ultrasound | Porphyrin and perfluorocarbon gas | Ultrasound imaging | [33] |
| CaCO3 nanoparticles | The CaCO3 could generate CO2 in the acidic tumor microenvironment | Doxorubicin | Tumor ultrasound imaging, drug release and tumor therapy | [39] |
| Nanobubbles | CO2 gas-generating polymeric nanoparticles | - | Ultrasound Imaging | [34] |
| Liposome | Perfluorocarbon for ultrasound-sensitive | Doxorubicin, gold nanospheres | Cancer imaging, photothermal-chemotherapy | [60] |
| Liposome | Containing NH4HCO3 to generate gas in tumors | Docetaxel and NH4HCO3 | Dual ligand targeted triplex therapy, and ultrasound imaging | [61] |
| Nanorattles | Perfluoropentane for ultrasound-sensitive | Perfluoropentane | Ultrasound and photoacoustic imaging, photothermal therapy | [48] |
| Nanodroplets | Perfluorocarbon | ZnF16Pc, IR dye, perfluorocarbon | Tumor multimodal imaging and therapy | [62] |
| Gas vesicles | Genetically encoded gas nanostructures from microorganisms | Gas | Ultrasound and multimodal imaging, molecular biosensors | [44] |
Representative thermal-responsive nanocarriers
| Nanocarriers | Thermal-sensitive strategy/materials | Cargos | Applications | Ref. |
|---|---|---|---|---|
| Liposomes | The incorporated NH4HCO3 could response to local hyperemia for drug release | Doxorubicin, NH4HCO3 | Temperature-controlled drug release | [64] |
| Nanoscale vesicles | The temperature-sensitive leucine zipper peptide in the wall of vesicles could open pores for cargo release | Doxorubicin | Temperature-triggered drug release | [87] |
| Micelles | PMEEECL-b-POCTCL diblock copolymer displays phase transition at temperature above its LCST for cargo release | Nile Red, doxorubicin | Thermal-triggered drug release, efficient drug delivery to cancer cells | [67] |
| Nanogels | PNIPAM grafted chitosan nanogels response to temperature for drug release | Curcumin | Temperature-triggered drug release, intracellular drug delivery | [73] |
| siRNAsome | With siRNA-SS-PNIPAM to form vesicles responding to temperature higher than LCST | Doxorubicin, siRNA | Against multi-drug resistant cancer cells | [78] |
| Polymersomes | Thermal-sensitive PNIPAM gel in side pH-sensitive polymersomes | Doxorubicin | Dual-thermal, pH-responsive drug release, tumor therapy | [88] |
| Complexes | PEI-g-PMEOMA-b-PHEMA) polymers for temperature sensitive gene delivery | pDNA | Gene therapy of tumors | [84] |
| Nanocapsules | Forming Pluronic/PEI with high temperature to load siRNA, which could be released inside cancer cells with cold shock | siRNA | Enhanced intracellular siRNA delivery to HeLa cancer cells | [72] |
The thermal-sensitivity nanocarriers could be applied for gene and drug delivery by using thermal- sensitive polymeric materials [63, 85, 86], which could shift from hydrophilic to hydrophobic for forming nanocarriers. In a recent study, the siRNA-SS- PNIPAM conjugates could form siRNAsomes by self- assembly at higher temperature (> 32°C) than the lower critical solution temperature (LCST) for phase transition [78]. In another study, the nanocarriers with PNIPAM on the surface formed micellar networks (i.e., aggregates) at temperature higher than LCST, while disassociated to each other at low temperature [75]. In this way, the thermal-sensitive nanocarriers could also be applied for plasmid DNA (pDNA) condensation [84], folding proteins [77], and incorporating hydrophobic anticancer drugs (e.g., doxorubicin) [66]. Besides, it could be applied for controlled releasing cargos in diseased regions with local hyperemia [64, 67, 85]. For instance, the doxorubicin could be released from the lipid-peptide vesicle by responding to mild hyperemia [87], as the peptides in the wall of vesicles could open pores at high temperature (42.5°C). In another case, the Nile Red and doxorubicin could be release from the polymeric micelles by responding to the thermal-stimuli, where the poly(γ-2-(2-(2-methoxyethoxy)ethoxy)ethoxy-ε- caprolactone)-b-poly(γ-octyloxy-ε-caprolactone) (PMEEECL-b-POCTCL) diblock copolymer displayed phase transition at temperature above its LCST (38 °C) [67]. The thermal-sensitive polymeric micelles displayed higher cellular uptake at high temperature (42.5°C) than at normal temperature (37 °C), as well as lower survival than free doxorubicin as tested on MCF-7 cancer cells. Although with much advances in developing temperature-sensitive nanocarriers, only limited thermal-sensitive materials are existed, which requires further development. The thermal-sensitive temperature of some materials and nanocarriers was neither in the range of biological systems (e.g., 37- 42°C), nor could be simply shifted to another desired temperature. It further has to point out that some thermal-responsive nanocarriers were developed with non-biodegradable polymers (e.g., PNIPAM), which may be difficult for clinical translation. Thus, development of biodegradable and thermal-sensitive materials would be a future direction. In addition, the accumulation of nanocarriers in tumors is still critically important for achieving pinpoint thermal- triggered drug release and therapy.
Magnetic-responsive nanocarriers
The magnetic-responsive nanocarriers have been engineered, as the magnetic nanoparticles has intrinsic tropism to magnetic field for tumor targeting, while it also could generate local hyperthermia under an alternating magnetic field for triggering drug release and tumor ablation. Until now, several magnetic-responsive nanocarriers have been formulated (Table 3), including magnetic nanoparticles [89, 90], liposomes [91], superparamagnetic iron-oxide nanoparticles (SPIONs) [92], polymeric micelles [93], albumin nanocapsules [94], magnetic nanocarriers [95, 96] and magnetic nanogels [97], etc. Generally, nanocarriers are incorporating magnetic materials for achieving magnetic-sensitivity, which are mainly including iron oxide nanoparticles (e.g., Fe3O4 nanoparticles) [98], iron oxide hybrid nanoparticles (e.g., graphene/Au/Fe3O4 hybrids) [99], and other magnetic nanomaterials (e.g., ZnFe2O4) [100]. The incorporated magnetic materials also could be applied for tumor imaging by magnetic resonance imaging (MRI) [92, 101, 102]. Besides magnetic materials, the contrast agents [103], anticancer drugs [101, 104], plasmids [100], antibodies [98] and photosensitizer [91], could also be incorporated inside the magnetic-sensitive nanocarriers for achieving multiple functions or multimodal therapeutic effects. Moreover, the nanocarriers could be engineered for passive tumor targeting through the EPR effect [105], as well as be installed with targeting moieties (e.g., folic acid) for active targeting cancer cells [94].
Representative magnetic-responsive nanocarriers
| Nanocarriers | Magnetic-responsive strategy/materials | Cargos | Applications | Ref. |
|---|---|---|---|---|
| Multifunctional magnetic nanocarriers | Magnetic field guided tumor targeting of SPIOs-loaded nanocarriers | SPIOs, doxorubicin | Tumor-targeted therapy | [95] |
| Albumin nanocapsules | Magnetic guided tumor targeting | Fe3O4, hydrophilic drugs | Targeting cervical cancer cells | [94] |
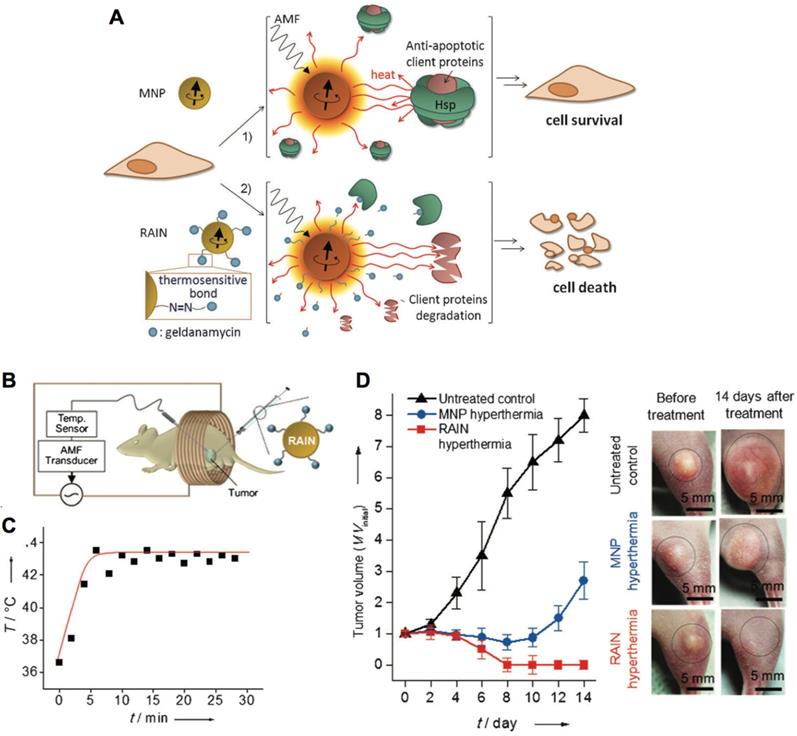
| Magnetic nanoparticles | Nanoparticles response to the alternating magnetic field for geldanamycin release and effective apoptotic hyperemia to kill cancer cells | Geldanamycin, amine-functionalized Zn0.4Fe2.6O4 | Nanoparticle-mediated resistance-free apoptotic hyperthermia for kill cancer cells | [89] |
| Mesoporous iron oxide nanoparticles | Burst gas generation and on-demand drug release upon high-frequency magnetic field exposure | Iron oxide nanoparticles, paclitaxel, perfluorohexane | Tumor active targeted thermos-chemo-therapy | [107] |
| Polymeric micelles | Generate magnetic hyperthermia and controlled drug release | La0.7Sr0.3MnO3, doxorubicin | Effective breast cancer theranostics | [93] |
| Multifunctional hybrid nanoparticle | Produce localized heat under an alternating magnetic field, which triggers the release of the loaded drug | Fe3O4, Au, carbon dots, doxorubicin | Photothermal therapy of melanoma tumor | [115] |
| Liposomes | Induce local hyperthermia by response to alternating magnetic field | Magnetic nanoparticles, rhodamine, photosensitizer | Ultimate hyperthermia and photodynamic therapy combined tumor ablation | [91] |
| Nanoparticles | Generate heat in response to an alternating current magnetic field | Fe3O4 nanoparticles, doxorubicin | Tumor active targeted therapy by magnetic hyperthermia and chemotherapy | [116] |
| Magnetic nanogels | Magnetic hyperthermia | Iron oxide nanoparticles, doxorubicin | Prostate cancer therapy by hyperthermia and chemotherapy | [97] |
| Porous magnetic microspheres | Produce thermal energy and trigger the vaporization of liquid perfluorohexane | Iron oxide nanoparticles, perfluorohexane | Tumor treatment by activating droplets vaporization | [103] |
| Magnetic nanoparticles | Localized hyperthermia kills tumor cell preferentially | Iron oxide nanoparticles | Treating primary and metastatic lung malignancies | [109] |
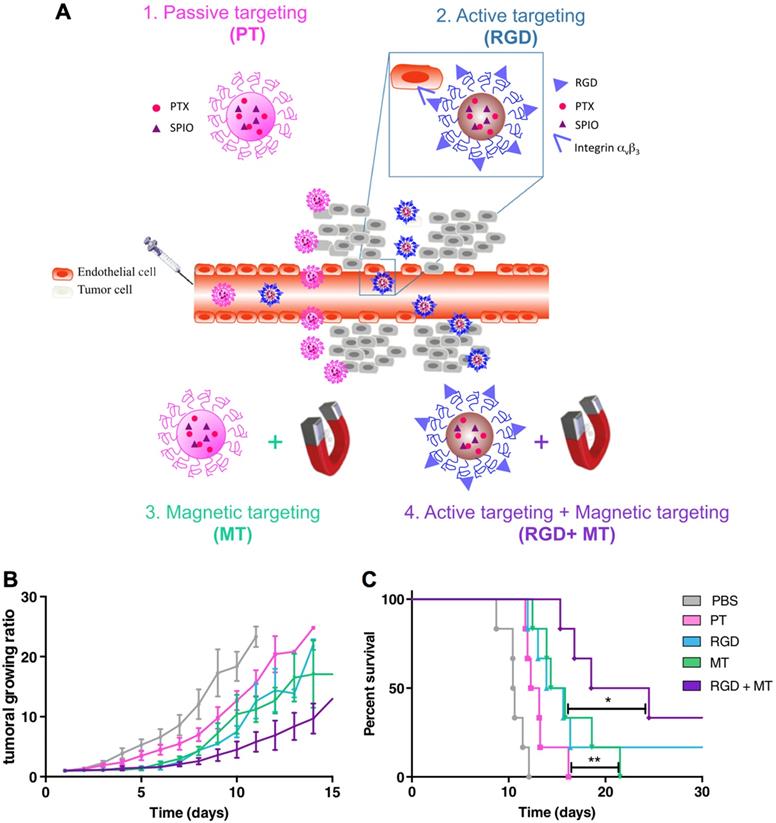
The interaction between magnetic nanocarriers and magnetic field facilitates the magnetic-guided accumulation of nanocarriers in tumors, while a typical approach is to locate a permanent magnetic field in malignant tissues after administration [94]. For example, much higher level of SPIONs and doxorubicin-loaded nanocarriers in tumors have been achieved with external magnetic field-guided tumor targeting, leading to effective tumor ablation [95]. In this way, it could be applied for promoting the accumulation of a myriad of bioactive compounds in tumors, including genes, anticancer drugs, and imaging probes [106]. Besides, the alternating magnetic field-triggered hyperthermia could induce on-demand release of cargos from the magnetic- sensitive nanocarriers in diseased regions (i.e., tumor or cancer cells) [105, 107, 108]. Using hyperthermia to cleave the thermosensitive bonds , the magnetic nanoparticles could release the heat shock protein inhibitors (i.e., geldanamycin), which could block the protective function of heat shock proteins to induce resistance-free apoptosis for effective tumor ablation (Figure 4) [89]. This magnetic-sensitive nanocarriers would facilitate treating tumors that resistant to hyperthermia therapy, and overcoming multi-drug resistant (MDR) of cancers. Moreover, the hyperthermia generated by magnetic-sensitive nanocarriers could further be applied for tumor ablation [90, 100], as hyperthermia could induce apoptosis of cancer cells. For example, the magnetic-responsive nanocarriers have been developed with ZnFe2O4 inside the core and decorated with cationic polymers of polyethyleneimine (PEI) to interact with plasmids on the surface [100]. It facilitated cellular uptake of plasmids by the adipose-derived mesenchymal stem cells (MD-MSCs), which could migrate to tumors guided by an alternating magnetic field for effective therapy. Besides primary tumors, the magnetic- responsive nanocarriers have also demonstrated high potential for treating metastatic tumors (e.g., lung metastasis) [109]. Furthermore, the magnetic-sensitive nanocarriers could be applied for tumor theranostics [110], as it could probe tumors by MRI or other imaging modalities, and remotely and non-invasively eradicate tumors with the generated hyperthermia in the alternating magnetic field [111]. For example, the PEGylated MoS2/Fe3O4 nanocomposites (MSIOs) made through a two-step hydrothermal method, have demonstrated high potential for tumor diagnosis by T2-weighted MR imaging and photoacoustic tomography (PAT) imaging, and magnetic-targeted effective photothermal ablation of tumors [112]. Meanwhile, it further allowed both T1- and T2-weighted MR imaging of tumors by doping Mn into the core of Fe3O4@MoS2 nanocomposites (i.e., multifunctional nanoflowers) [113]. Some other bioactive compounds, such as photosensitizer chlorin e6 (Ce6), could also be incorporated into the magnetic-sensitive nanocarriers for multi-functional cancer theranostics [96]. In addition, the superparamagnetic materials in magnetic-responsive nanocarriers could be extensively employed as a target moiety for improved tumor therapy, which is comparable to the decoration of active targeting moieties. As presented in a recent study, the paclitaxel (PTX) and SPIO-loaded poly(lactic-co-glycolic acid) (PLGA) nanocarriers have been engineered for tumor passive targeting by EPR effect, active targeting of αvβ3 integrins on cancer cells with RGD ligands (RGD), magnetic field (i.e., 1.1 T) guided tumor targeting (MT), and the combination of magnetic targeting and active targeting (RGD+MT) (Figure 5A) [114]. Accordingly, both RGD and magnetic targeting drastically exhibited much higher tumor accumulation (i.e., 8-fold increase) of nanocarriers than passive targeting, leading to effective tumor ablation and improved survival rates of colon CT26 tumor-bearing mice, while the combination of magnetic targeting and active targeting demonstrated the best performance in tumor ablation than other groups (Figure 5B,C). Notably, higher accumulation in tumors and lower deposition in livers/lungs have been achieved by magnetic field-guided targeting nanocarriers than the RGD-installed nanocarriers, demonstrating the promise of magnetic targeting approach. Overall, the magnetic field guided- targeting strategy requires tumor-specific drug delivery, as it may also affect normal organs/tissues that distributed with magnetic nanocarriers when exposed to the alternating magnetic field. In addition, the generation of hyperthermia requires high level of magnetic-sensitive nanocarriers in diseased regions, which should be located in the alternating magnetic field. This approach may facilitate treating tumors located in partial regions of the body (e.g., legs, feet and arms, etc.), due to safety consideration.
Magnetic-responsive nanocarriers for tumor therapy. (A) Schematic illustration of resistance-free apoptosis-inducing magnetic nanoparticles (RAIN) for cargo release and killing cancer cells. (B) Illustration of applying magnetic-sensitive nanocarriers for tumor treatment in an alternating magnetic field. (C) The temperature profiles in tumors. (D) The anti-tumor efficacy by magnetic-sensitive nanocarriers with hyperthermia. Adapted with permission from ref. [89], copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Nanocarriers for magnetic targeted tumor therapy. (A) Illustration of paclitaxel (PTX) and SPIO-loaded nanocarriers for tumor passive targeting (PT), active targeting of αvβ3 integrins with installed RGD ligands (RGD), magnetic field (1.1 T)-guided tumor targeting (MT), and combination of magnetic targeting and active targeting (RGD+MT). (B,C) The tumor growth ratio (B) and survival rates (C) of CT26-tumor bearing mice. Adapted with permission from ref. [114], copyright 2014 Elsevier B.V.
Light-sensitive nanocarriers
Nanocarriers that could responsive to light have also been extensively developed, as light is an attractive stimulus with the possibility to adjust the irradiation wavelength, power and affecting area [117]. In general, the light irradiation, such as UV-Vis and near-infrared light (NIR), could remotely affect the light-sensitive nanocarriers in biological systems (e.g., cancer cells, or tumors). Meanwhile, the light- triggered tumor therapy could be precisely conducted by control the range of irradiation to avoid or minimize potential harm to normal organs and tissues. Until now, several light-responsive nanocarriers have been exploited (Table 4), including polyion complex vesicles (PICsomes) [118], polyplexes [119, 120], nanoparticles [121, 122], polymeric micelles [123, 124], upconverting nanoparticles (UCNPs) [125,126], polymersomes [127,128], liposomes [129, 130], nanogels [131], nanorods [132], and nanorattles [48], etc. Meanwhile, the cargos/materials with light-response function could be applied for constructing light- sensitive nanocarriers, such as photosensitizers (e.g., IR780) [133], gold nanocomposites (gold nanoparticles) [134], UCNPs [123], organic molecules (e.g., azobenzene) [135], graphene [131], carbon nanotubes [136-138], and two-dimensional (2D) transitional metal nanomaterials (e.g., MoS2, WSe2 and WS2) [139, 140], etc. Nanocarriers could response to light for several activities: (1) alert the conformation of certain molecules, such as azobenzene, spiropyran, dithienylethene and diazonaphthoquinone etc. [141]; (2) cleave the light-sensitive chemical bonds for nanocarriers disassociation [123]; (3) trigger release of therapeutics from nanocarriers in diseased regions [130]; (4) light-activated imaging (e.g., photoacoustic imaging) or imaging-guided therapy [142-146]; (5) generate singlet oxygen (O21), also referred as reactive oxygen species (ROS) for photodynamic therapy (PDT) [147, 148], and photothermal effect for tumor ablation by photothermal therapy (PTT) [149, 150].
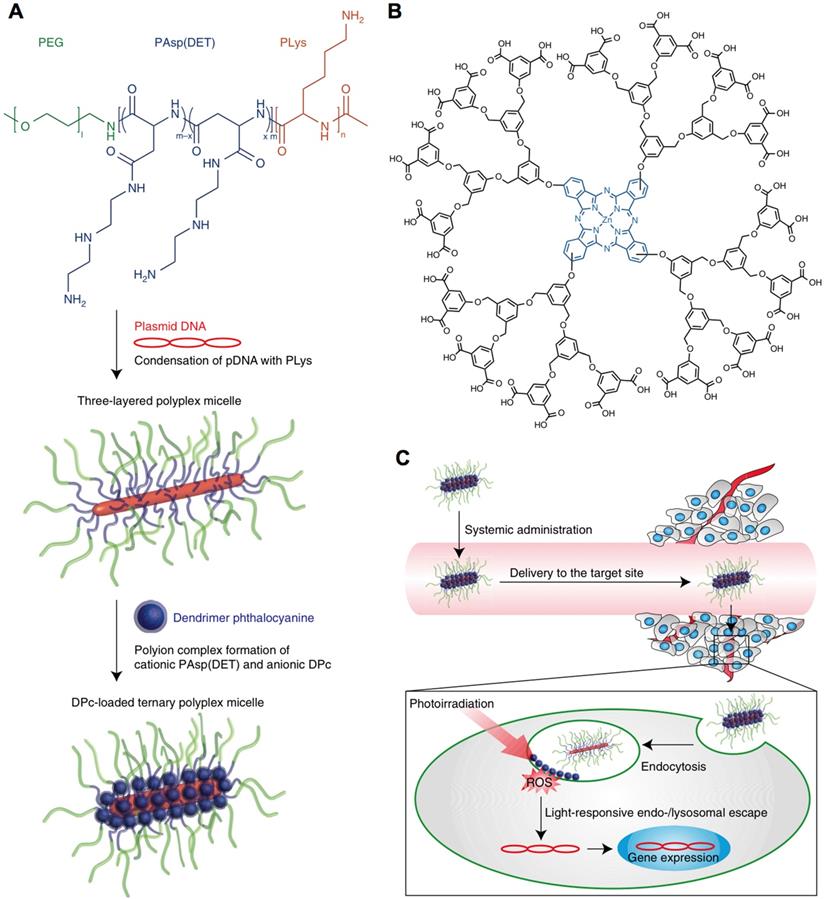
Nanocarriers could also be formed or assembled by responding to light, due to change the hydrophilic- hydrophobic balance or structure conversion of light-sensitive materials. Recently, the light-sensitive nanoparticles were formed by using 1,2-distearoyl-sn- glycero-3-phosphoethanolamine-N-carboxy(polyethylene glycol) (DSPE-PEG) to incorporate spiropyran in visible or dark conditions, and disassociated responding to UV irradiation due to the conversion of SP to merocyanine (MC) [121]. The photo-switching nanocarriers demonstrated high potential for loading different bioactive compounds for UV-Vis triggered drug release, including paclitaxel, docetaxel and doxorubicin etc., as well as for cancer therapy [151]. The light-switching function also could be applied for inducing reversible aggregation of nanoparticles (e.g., vesicles) [152]. However, the short wavelength of UV-Vis may limit their applications. Therefore, the NIR light-sensitive nanocarriers have also been engineered for controlled drug delivery [153], and penetrating into deep tissues [154]. For example, the IR-780-incorperated polymeric micelles could response to NIR for doxorubicin release [155]. Besides, the light-sensitive nanocarriers facilitate intracellular delivery of bioactive compounds, including genes [120], photosensitizers [118], and anticancer drugs [124], etc. In a recent study, the photosensitizer Al(III) phthalocyanine chloride disulfonic acid (AlPcS2a)- incorporated polyion complex vesicles (PICsomes) could sensitive to laser irradiation for endosome escape and drug release, exhibiting much stronger photocytotoxicity than that of AlPcS2a [118]. In another strategy, by co-administration of photofrin, it could also induce photochemical internalization (PCI) for achieving endosomal escape of nanocarriers to improve the therapeutic effects of camptothecin [124]. Moreover, the light-triggered endosome/lysosome escape also plays an important role in transferring genes into cytoplasm, as genes could be degraded in the late lysosomes to lose activity. For example, the light-responsive, three-layered polyplex micelles have been developed with polycationic polymers to condensate pDNA and load dendrimer phthalocyanine (i.e., photosensitizer), demonstrating efficient systemic gene transfection by light-triggered PCI for endosomal/lysosomal escape (Figure 6) [119].
Representative light-responsive nanocarriers
| Nanocarriers | Light-responsive mechanism/materials | Cargos | Applications | Ref. |
|---|---|---|---|---|
| Polyion complex vesicles (PICsomes) | Light-triggered release of photosensitizer, photochemical internalization | Al(III) phthalocyanine chloride disulfonic acid (AlPcS2a) | PDT of tumors, photoinduced cytoplasmic delivery of drugs | [118] |
| Three-layered polyplex micelles | Dendrimeric photosensitizer for light-responsive endo-/lysosomal escape | pDNA, photosensitizer | Light-induced systemic gene transfer for tumor therapy | [119] |
| Micelles | Using NIR light excitation of UCNPs to trigger dissociation of micelles | NaYF4:TmYb UCNPs | NIR light-triggered cargo release | [123] |
| Nanoparticles | Spiropyran for UV-Vis light responsive | Rhodamine B, coumarin 6, calcein, Cy5, paclitaxel, docetaxel, doxorubicin | Light-triggered drug delivery and tissue penetration | [121] |
| Nanoparticles | Photosensitizer Ce6 for light- triggered size reducing, and generation of O21 (ROS) | Camptothecin, Ce6 | Enhanced tumor penetration for combined therapy | [159] |
| Liposome | Porphyrin for light-responsive phototherapy | Doxorubicin, porphyrin | Chemotherapy and phototherapy of tumors | [129] |
| Lanthanide-doped UCNPs | Dithienylethene photo-responsive molecules | Er3+/Yb3+ and Tm3+/Yb3+ doped NaYF4 UCNPs | NIR light remote-control to drive the reversible photo-switching reactions | [125, 126] |
| Cell membrane-based nanocarriers | Indocyanine green (ICG) for photothermal therapy | Doxorubicin, ICG | NIR-triggered drug release and tumor active targeted photothermal and chemotherapy | [160] |
| Vesicle | The structure change of azobenzene makes disassociation with β-CD | β-CD, azobenzene | Mimic for cell aggregation | [152] |
| Nanogel | Graphene for light-triggered photothermal effects | Doxorubicin, graphene | Theranostics of lung cancer | [131] |
| Nanorods | Gold nanorods for thermal sensitivity | DNA, doxorubicin | Treatment of multidrug resistant cancer cells | [134] |
| Carbon nanotubes | Photothermal effects of carbon nanotubes | Doxorubicin | Photothermal and chemotherapy of tumor | [138] |
| 2D transitional metal nanomaterials | Photothermal effects of MoS2 | Doxorubicin | Photothermal and chemotherapy of tumor | [139] |
Schematic illustration of light-responsive nanocarriers for gene transfer. (A) Preparation of pDNA and photosensitizer-loaded nanocarriers. (B) Chemical structure of photosensitizer; (C) Light-triggered endo-/lysosomal escape for gene transfection inside cancer cells. Adapted with permission from ref. [119], copyright 2015 Springer Nature Publishing AG.
Furthermore, the light-sensitive nanocarriers could further be activated for imaging-guided tumor therapy [156, 157] and theranostics [60, 156], which could figure out the cut-edge of tumors for precisely irradiation by PTT or PDT. In addition, the light- sensitive nanocarriers could be applied for tumor ablation, as a result of light-triggered generation of ROS and photothermal effect [130, 156], or combined with other bioactive agents (e.g., anticancer drugs) for multimodal cancer theranostics [155, 158]. It has also demonstrated high efficacy for treating MDR cancers [134]. In general, the light- sensitive nanocarriers have demonstrated high potential for drug delivery, controlled drug release and cancer theranostics, especially tumors that could be accessed by light/laser due to the limitation of light penetration.
Internal stimuli-responsive nanocarriers
Specific biological factors in tumor microenvironment or inside cancer cells, such as enzymes, ATP, low pH, redox-potential and hypoxia, etc., could be specific triggers for controlled drug release, endosome/lysosome escape, prodrug activation, tumor specific imaging and therapy [161]. The internal triggers are intrinsically existed in tumor microenvironment or inside cancer cells. However, they usually show poor specificity and heterogenetic distribution in tumors, which may affect the efficacy of internal stimuli-sensitive nanocarriers. In this section, recent advances in nanocarriers responding to internal stimuli, mainly including pH, hypoxia, redox and enzymes, for tumor theranostics will be focused.
pH-responsive nanocarriers
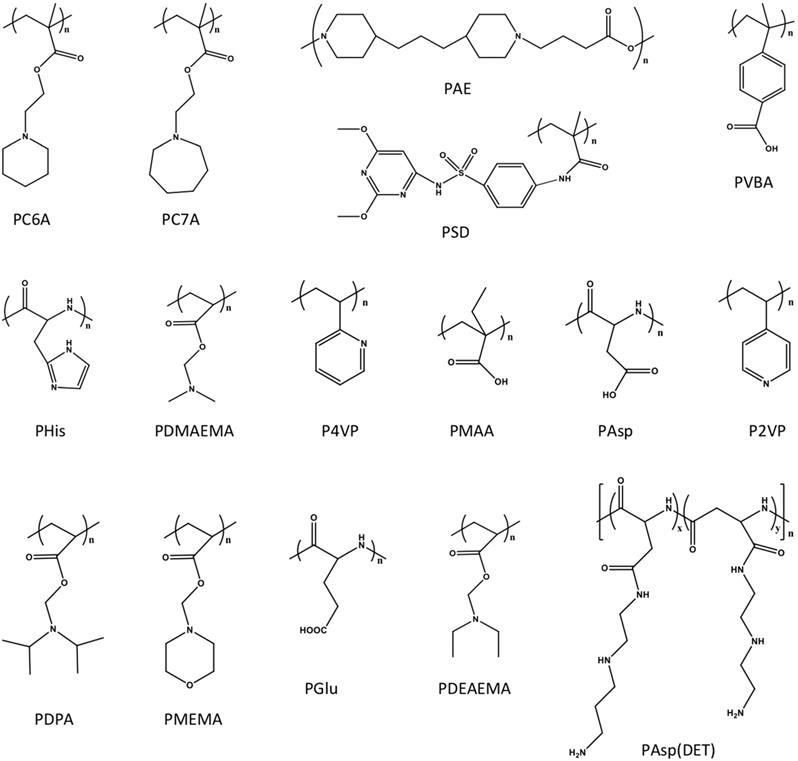
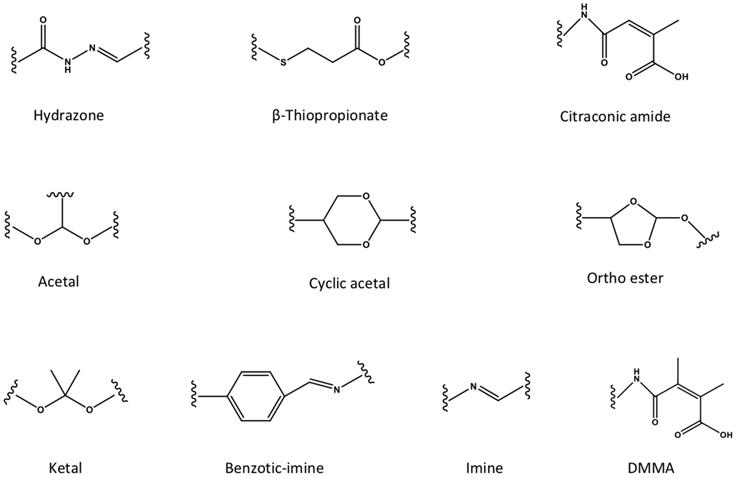
The pH-responsive nanocarriers have been extensively exploited, due to the nature of low pH inside the organelles (e.g., lysosomes and endosomes) of cancer cells and in tumor microenvironment. In general, the pH in cytoplasm, blood and normal tissues is almost around pH 7.0 to 7.4, while it exhibits approximately pH 6 to 4 in endosomal/lysosomal organelles, and pH 6.5 to 6.8 in tumor microenvironment [162]. Thus, the pH-responsive in tumor microenvironment could be applied for controlled drug release or prodrug activation, while keep the “stealth effect” of nanocarreirs in normal regions (e.g., in blood circulation) without leaking of cargos. This would decrease the risk of exposure normal organs (e.g., heart) to the toxic cargos (e.g., doxorubicin), and specifically deliver them to tumors for achieving high therapeutic performance. Until now, several types of pH-sensitive nanocarriers, including CaCO3 nanoparticles [163, 164], calcium phosphate (CaP) nanocarriers [165-167], inorganic nanoparticles or crystals [168-170], polymer-drug conjugates [171, 172], polymeric micelles [173-175], liposomes [176], polymersomes [177], nanogels [178- 180] and dendrimers [181], etc., have been exploited for imaging, intracellular drug delivery, charge conversion, and controlled drug release in tumor- microenvironment [172, 182]. Meanwhile, several pH-sensitive polymers have been synthesized for fabricating nanocarriers with pH-responsibility [183, 184], including poly(2- (pentamethyleneimino) ethyl methacrylate) (PC6A), poly(2-(hexamethyleneimino) ethyl methacrylate) (PC7A), poly(β-amino ester) (PAE), poly- sulfadimethoxine (PSD), poly(L-histidine) (PHis), poly(4-vinylbenzoic acid) (PVBA), 2,3-dimethylmaleic anhydride (DMMA), poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA), poly(N,N-diethylamino- 2-ethylmethacrylate) (PDEAEMA), poly(N'-(N-(2- aminoethyl)-2-aminoethyl) aspartamide) [PAsp (DET)], poly(2-diisopropylaminoethyl methacrylate) (PDPA), poly [(2-N-morpholino) ethyl methacrylate] (PMEMA), poly(4-vinylpyridine) (P4VP), poly (glutamic acid) (PGlu) [185], poly (methacrylic acid) (PMAA), poly(L-aspartic acid) (PAsp) and poly(2- vinylpyridine) (P2VP) (Figure 7). Meanwhile, certain pH-sensitive chemical bonds have also been applied for drug conjugation, confirmation/ size change and charge conversion, etc. (Figure 8), which facilitate pH-triggered drug release, and disassociation of nanocarriers inside cancer cells or in tumor microenvironment [186].
Compared to cytoplasm with an almost neutral pH (pH 7.2), the pH in endosomal/lysosomal organelles was around pH 6 to 4. Generally, nanocarriers enter into cancer cells through the pathway of endocytosis, which requires endosome/lysosome escape to avoid further degradation in late lysosomes with low pH. Currently, several intercellular pH-triggered nanocarriers have been engineered for liberating cargos inside cancer cells [187]. The pH-triggered charge conversion nanocarriers have also been engineered for intracellular drug delivery, where the neutral or negative charged nanocarriers could turn to be positively charged by responding to low pH in endosomes/lysosomes for disrupting endosomes/lysosomes, due to the protonation of the cationic materials [188, 189]. The pH-triggered charge conversion could be obtained with certain chemical groups, such as citraconic anhydride, 2,3-dimethylmaleic anhydride (DA), cis-aconitic anhydride, carboxy dimethylmaleic anhydride (CDM) and cis-4-cyclohexene-1,2-dicarboxinic anhydride, etc. The charge conversion strategy facilitates intracellular delivery of antibodies [190], proteins [189, 191], siRNA [192, 193], and DNA [194], as well as enhancing the tumor accumulation of nanocarriers [195], etc. As presented in a recent study, the pDNA- loaded nanocarriers (HA-NPs) were innovated by using PAsp(DET) for formulating cationic PAsp (DET)/pDNA condensates and endosome escape, as well as installing hyaluronic acid (HA) for active targeted gene therapy of cancer [196]. The HA-NPs could selectively internalize with CD44 receptors overexpressed on B16F10 melanoma cancer cells and tumor vascular endothelial cells to prompt preferential intracellular delivery of pDNA payloads, and block the CD44-angiogenic signaling for pursuit of inhibited tumorigenesis, leading to effective ablation of primary tumor and lung metastasis. Besides, the endocytosis procedures could be visible with probe-loaded, intracellular pH-sensitive nanocarriers. For example, the endocytic pH-sensitive nanoparticles has been reported, which could specifically probe early endosomes or late endosomes/lysosomes with different pH-sensitive groups [197, 198], and even probe early endosomes (pH 6.0) at single-organelle resolution [199]. Moreover, the intracellular pH could trigger controlled drug release from nanocarriers [200-203]. With one example, the cRGD-decorated polymeric micelles that self-assembled from epirubicin- conjugated block copolymers through hydrazide bonds, could specifically delivery and release epirubicin inside cancer cells for effective tumor ablation [204].
The intracellular or tumor microenvironment pH-responsive polymers have been applied for engineering pH-sensitive nanocarriers.
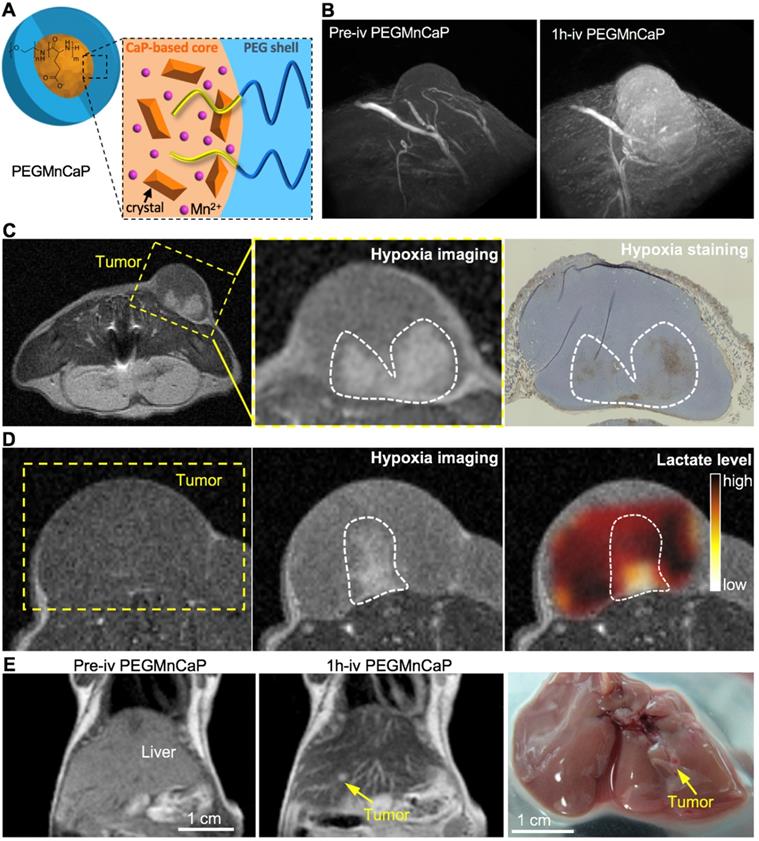
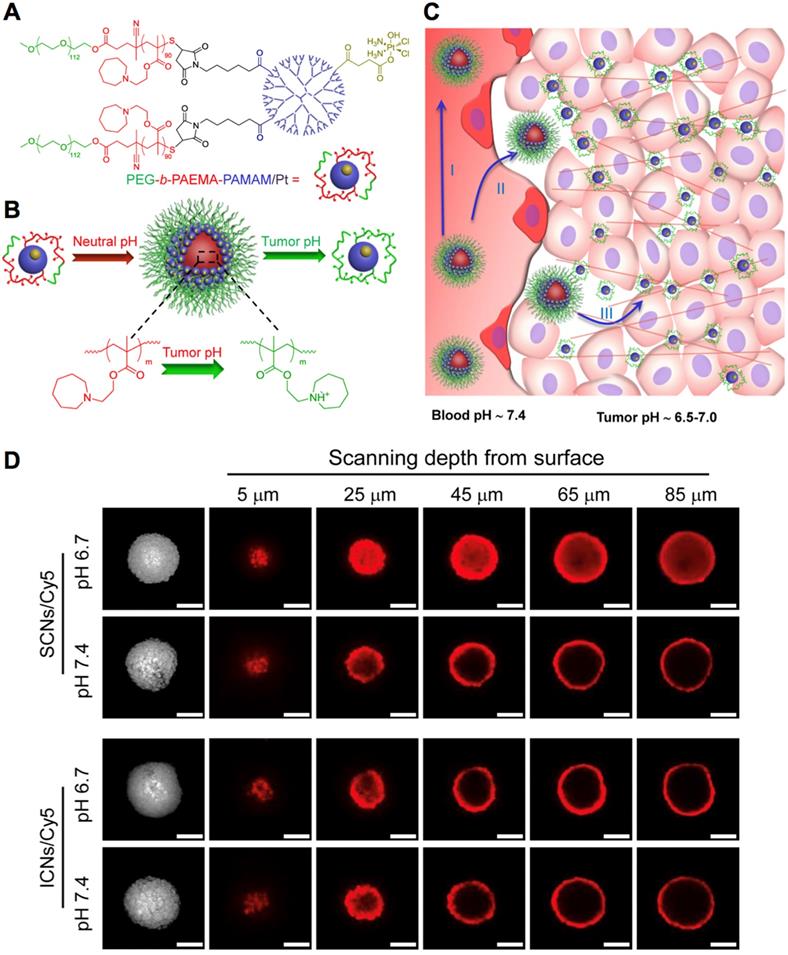
Functional nanocarriers could also response to the low pH in tumor microenvironment for cancer- specific theranostics. Firstly, the pH-sensitive nanocarriers could incorporate different types of imaging probes for tumor-selective imaging and diagnosis. For instance, the pH-sensitive polymeric micelles incorporating fluorescence dye could specifically probe several types of solid tumors, due to the specific exposure of dyes in tumors, while the diagnostic selectivity could be promoted higher by installing targeting moieties (i.e., cRGD) on the surface of micelles [20]. The nanocarriers could further be utilized for fluorescence imaging-guided surgical resection of tumors [206]. Considering the limited penetration of optical imaging, the pH-sensitive nanocarriers have been exploited for tumor imaging by MRI [207, 208]. For instance, the Mn2+-doped, polymer hybrid CaP nanocarriers (PEGMnCaP) have been developed with intratumoral pH-triggered contrast amplification for MR imaging of tumor malignancy (Figure 9A), as the released Mn2+ could bind to surrounding proteins to boost much higher relativities. It could specifically and sensitively amplify the contrast in tumors for accurate two- and three-dimensional MR imaging (Figure 9B). The PEGMnCaP could also distinguish hypoxia in tumors with even higher contrast enhancement than the surrounding tumor regions, as more Mn2+ were released in hypoxic regions with lower pH, while the hypoxia imaging was confirmed by immunostaining of hypoxia (Figure 9C) and checking the lactate level in the detected hypoxia regions (Figure 9D). It further accurately probed ultra-small liver metastasis (Figure 9E), which was difficult to be detected by conventional CAs. The pH-triggered MR imaging of solid tumors could be further applied for imaging-guided tumor neutron capture therapy [165]. For example, the pH-sensitive block copolymer hybrid CaP nanocarriers further demonstrated high performance in cancer theranostics by incorporating Gd-DTPA for tumor diagnosis and promoted gadolinium neutron capture therapy (GdNCT) [165, 208]. Besides, the intratumoral pH could also trigger size switching for improved penetration of nanocarriers [186, 209], as comparable large size of nanocarriers benefits long circulation, while small size benefits intratumoral penetration [6, 210]. For instance, the polymeric micelles have been self-assembled with platinum (Pt)-drug conjugated, pH-sensitive poly(ethylene glycol)-b-poly(2-azepane ethyl methacrylate)-modified polyamidoamine dendrimers (PEG-b-PAEMA-PAMAM/Pt) (Figure 10A). It could be disassociated into small size of polymer-drug conjugates by responding to tumor pH for deep penetration in tumors, exhibiting improved therapeutic efficacy (Figure 10B-D) [211]. Moreover, nanocarriers could response to pH for surface charge conversion in tumor microenvironment [212, 213], as neutral or negative charged nanocarriers holds the “stealth effect” during long circulation, while positive charged nanocarriers are more likely to internalize with cancer cells. Regarding this point, the surface of polymeric micelles were designed to switch from neutral charge at blood pH 7.4 to cationic at tumorous pH 6.5, which could maintain their “stealth effect” during circulation and increase internalization with cancer cells for improved tumor accumulation [195]. By tumor pH-triggered surface conversion, nanocarriers could also be applied for tumor-specific molecular imaging [214]. In addition, by conjugating ligands (e.g., biotin) to tumor pH-sensitive polymers, it was applied to hide the targeting ligands inside the PEG shell during circulation (i.e., pH 7.4) and present ligands in tumor microenvironment (i.e., pH <7.0) [215], to avoid unspecific internalization and uptake of ligands during circulation, as well as improve tumor active targeting efficacy [216]. The ligand- installed, pH-sensitive nanocarriers were reported to target tumors and spontaneous metastasis with effectively suppressed tumor growth [202].
The pH-responsive chemical bonds have been utilized for developing pH-sensitive nanocarriers.
The pH-responsive PEGMnCaP nanocarriers with contrast amplification ability have been developed for MR imaging of tumor malignancy. (A) The composition and characterization of Mn2+-doped PEGMnCaP. (B) PEGMnCaP specifically enhanced the contrast in C26 tumors for three-dimensional (3D) MR imaging. (C,D) PEGMnCaP probed hypoxia in tumors as confirmed by immune-staining of hypoxia (C) and chemical shift imaging (CSI) of lactate (D). (E) PEGMnCaP for precisely MR imaging of 1-2 mm ultra-small metastasis in liver. Adapted with permission from ref. [205], copyright 2016 Springer Nature Limited.
Hypoxia-responsive nanocarriers
The poorly vascularization inside solid tumors is likely to form hypoxia (low oxygen level), which plays an important role in cancer progression, such as locoregional spread and distant metastasis [217]. The promoted malignant phenotype by hypoxia has negative impact on prognosis and therapy and leads to resistance to standard therapy (e.g., radiotherapy, chemotherapy). Therefore, several strategies have been utilized for treating hypoxic tumors, mainly including increasing the oxygen level and using hypoxia activatable prodrugs, etc [218]. Until now, several types of nanocarriers have been engineered for drug delivery to hypoxic tumors (Table 5) [219], including liposomes [220], silica nanoparticles [221], upconversion nanoparticles (UCNPs), layer-by-layer nanoparticles [222], nanovesicles [128], polymeric micelles [223], polymersomes [224], albumin nanoparticles [225], cell membrane coated metal organic framework (MOF) [226], solid-state sensors [227], polymeric probes [228], and polymer hybrid CaP nanoparticles [205], etc. Meanwhile, different cargos could be loaded inside the hypoxia-activation nanocarriers, ranging from imaging agents (e.g., contrast agents), prodrugs (e.g., dihydrochloride (AQ4N)), anticancer drugs (e.g., doxorubicin), siRNA and photosensitizers (e.g., ICG), etc., demonstrating high performance in hypoxic tumor imaging and effective therapy by overcoming drug resistance [229].
The pH-responsive nanocarriers for tumor therapy. (A) The structure of pH-sensitive polymer-drug conjugates. (B) Illustration of pH-dependent self-assembly and disassociation of PEG-b-PAEMA-PAMAM/Pt nanocarriers (SCNs/Pt) at different pH. (C) Illustration of pH-triggered disassociation of SCNs/Pt nanocarriers in tumors. (D) The penetration of SCNs/Pt nanocarriers in BxPC3 pancreatic cancer spheroids. Adapted with permission from ref. [211], copyright 2016 American Chemical Society.
Representative hypoxia-responsive nanocarriers
| Nanocarriers | Magnetic-responsive strategy/materials | Cargos | Applications | Ref. |
|---|---|---|---|---|
| Liposomes | The prodrug of banoxantrone dihydrochloride (AQ4N) could be activated in hypoxic environment caused by PDT | Ce6, AQ4N | Cancer therapy | [230] |
| Silica nanoquencher | Azo monomer; cell-penetrating poly(disulfide)s (CPD) coated silica nanoquencher (BS-qNP) (CPD-protein@BS-qNP) | Antibody (Cetuximab), fluorescent dye | Hypoxia-triggered protein release and fluorescence imaging | [231] |
| Upconversion nanoparticles (UCNPs) | Oxygen indicator [Ru(dpp)3]2+Cl2 for hypoxia detection as UCNPs provided the excitation light of [Ru(dpp)3]2+Cl2 by upconversion process at 980 nm | [Ru(dpp)3]2+Cl2, UCNPs | Imaging hypoxic regions or oxygen changes in cells and zebrafish | [229] |
| Nanoparticles | The photosensitizer of ICG-mediated PTT induced hypoxia, which then activated the prodrug of TPZ | TPZ, ICG | Tumor therapy by PDT and chemotherapy | [232] |
| Nanoparticles | The shift from hydrophobic to hydrophilic of 2-nitroimidazole that grafted to polymers in light-activated hypoxia | Doxorubicin, light-sensitive polymer | Hypoxia-triggered drug release, tumor | [233] |
| Nanoparticles | PEG-azo(azobenzene)-PEI-DOPE block copolymer | siRNA | siRNA delivery and tumor RNAi | [234, 235] |
| Nanoparticles | Layer-by-layer nanoparticles with a pH-sensitive layer for targeting of tumor hypoxia | Sulfonated polystyrene beads or carboxylated quantum dots | Systemic tumor targeting | [222] |
| Cancer cell membrane coated MOFs | The porphyrinic MOFs could generate toxic ROS for PDT and cause hypoxic regions for activating TPZ | Porphyrinic metal organic framework, TPZ | Tumor targeted PDT and chemotherapy | [226] |
| Nanovesicles | The light irradiation of Ce6 induced hypoxia for oxidation bioreduction of 2-nitroimidazole in polymers and activation of TPZ | Ce6, TPZ | Tumor fluorescence imaging and therapy | [128] |
| Polymeric micelles | The metronidazole (MN) grafted in polymers could change hydrophobicity in hypoxic conditions for drug release | Doxorubicin | Tumor chemotherapy and radiotherapy | [236] |
| Polymersomes | The PLA (polylactic acid)-azobenzene-PEG is sensitive to hypoxia | Gemcitabine, hypoxia- sensitive dye “Image-iT” | Tumor imaging and drug delivery | [224] |
| Albumin nanoparticles | With hypoxia-sensitive azobenzene linker to covalently bridge photosensitizer Ce6-conjugated HSA and oxaliplatin prodrug-conjugated HSA | Oxaliplatin prodrug, Ce6 | Tumor chemotherapy and photodynamic therapy | [225] |
| Mesoporous silica nanoparticles | The Ce6-dopped mesoporous silica nanoparticles were decorated with PEG and glycol chitosan by hypoxia-sensitive azobenzene linker | Oligonucleotide (CpG), Ce6 | Cancer immunotherapy | [221] |
| Solid-state sensors | Iodide-substituted difluoroboron dibenzoylmethane-poly(lactic acid) (BF2dbm(I)PLA) solid-state sensor material | BF2dbm(I)PLA | Tumor hypoxia optical imaging | [227] |
| Polymeric probes | Poly(N-vinylpyrrolidone)-conjugated iridium-(III) complex (Ir-PVP) and poly(ε-caprolactone)-b-poly(N- vinylpyrrolidone) (PCL-PVP) nanoparticles | Iridium (III) complex | Optical imaging of tumor and metastasis | [228] |
| Polymer hybrid CaP nanoparticles | Tumor pH-triggered release of Mn2+ from CaP to boost higher contrast enhancement in hypoxic tumor regions | Mn2+ | MR imaging of solid tumors, hypoxia and metastasis | [205] |
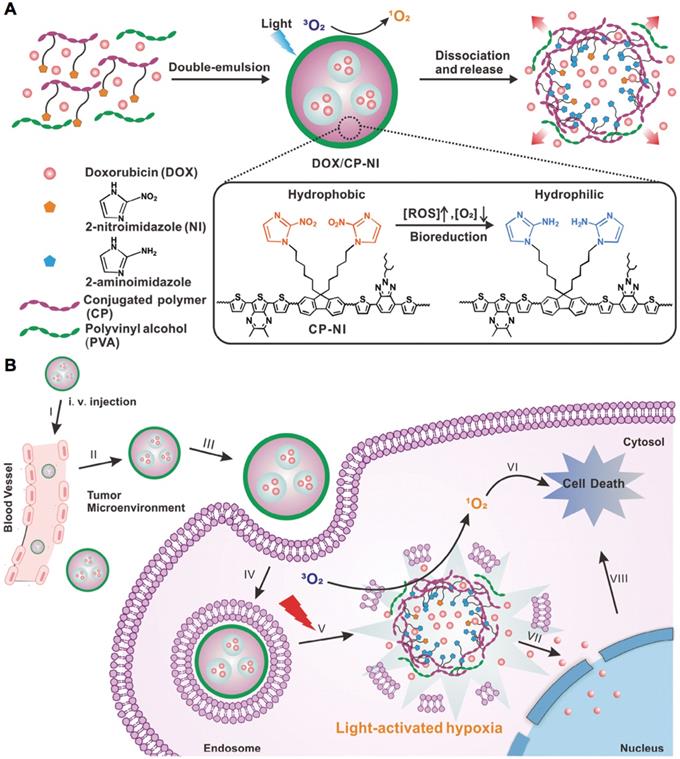
The tumor hypoxia could be targeted with hypoxia-responsive and some pH-sensitive nanocarriers, since hypoxic tumor regions are generally associated low pH due to the glycolysis of glucose and production of H+ and lactate [237]. The major strategy is utilizing hypoxia-sensitive nanocarriers, which are generally constructed with hypoxia- sensitive materials or derivates, e.g., 2-nitroimidazole [238-240], nitroimidazole [241-243], metronidazole [236], azobenzene [244-246], nitro-benzene derivatives [223] and iridium (III) complexes, etc. Hypoxia could trigger cargo release from the hypoxia-sensitive nanocarriers, e.g., the incorporated antibody (i.e., Cetuximab) could be released from the silica nanoparticles in hypoxic tumors due to the cleavage of the hypoxia-sensitive cross-linkers (i.e., Azo monomer) [231]. In another study, the nanocarriers were prepared with hypoxia-sensitive 2-nitroimidazole and light-sensitive conjugated polymers for generating ROS and local hypoxia after laser irradiation, to trigger doxorubicin release for enhanced synergistic anticancer efficacy (Figure 11) [233]. The hypoxia- sensitive nanocarriers also facilitate molecular imaging of tumors and metastasis. For example, the nanoscale probes with oxygen level-sensitive iridium (III) complexes have demonstrated high potential for optical imaging of tumors and metastatic lesions [228, 247]. Besides, some nanocarriers could delivery hypoxia-activatable prodrugs [e.g., tirapazamine (TPZ) and banoxantrone (AQ4N), etc.] to hypoxic tumors for enhanced therapy, while some photosensitizers could be co-loaded to generate hypoxia by laser irradiation for prodrug activation. For instance, the ICG and TPZ-incorporated liposomes with iRGD as targeting moieties could target both normoxic and hypoxic cancer cells, while the irradiation of ICG by NIR laser could produce extra hypoxia activate TPZ for enhanced therapy [232]. In another example, the vessel-disruptive agents (i.e. 5,6-dimethylxanthenone- 4-acetic acid) and TPZ incorporated, platelet membrane-coated nanoparticles could disrupt tumor blood vasculatures to promote drug accumulation for improved hypoxia-sensitive therapy [248]. In addition, some pH-sensitive nanocarriers have also be applied for treating tumor hypoxia [249], e.g., the pH-sensitive nanoparticles formed by layer-by-layer procedure could target hypoxic tumors for fluorescence imaging with the incorporated QDs [222]. So far, the hypoxia- sensitive nanocarriers have exhibited much progress in drug delivery to hypoxic tumor for molecular imaging and improved therapy. However, some underlying problems would be addressed in future studies, such as modulating hypoxic tumor microenvironment, increasing drug penetration and oxygen level, and clinical translation of hypoxia-responsive nanocarriers.
Redox-responsive nanocarriers
The redox-responsive nanocarriers have been widely applied for drug delivery due to the significantly different reduction potentials and capacities in tumors, e.g., the glutathione (GSH) level inside cancer cells (2-10 mM) is remarkable higher than that in normal regions (2-10 μM). Until now, several redox-sensitive nanocarriers have been engineered (Table 6), including nanocapsules [250], mesoporous silica nanoparticles [251], polymer-drug conjugates [252], polymersomes [253], polymeric vesicles [254], polymeric micelles [255-257], nanogels [258], gold nanoparticles [259] and hybrid nanoparticles [260], etc. The disulfide bonds could be cleaved into sulfhydryl groups by GSH [261], while the diselenide bonds (Se-Se) are also sensitive to redox potential [262], but with lower bond energy than that of disulfide bonds [263]. Moreover, the H2O2-responsive nanocarriers have also been developed for tumor therapy [264, 265], including for treating hypoxic tumors [266] and multidrug resistant tumors [267].
Schematic illustration of light-activated hypoxia-responsive nanocarriers. (A)Preparation of nanocarriers. (B)Nanocarriers generated ROS to induce local hypoxic environment, which triggered drug release to enhance the synergistic anticancer efficacy. Adapted with permission from ref. [233], copyright 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Redox-responsive nanocarriers for cancer theranostics
| Nanocarriers | Redox-responsive mechanism/materials | Cargos | Applications | Ref. |
|---|---|---|---|---|
| Nanocapsules | Disulfide bonds response to DTT) and GSH | Carboxyfluorescein | Redox-potential triggered drug release inside cancer cells | [250] |
| Mesoporous silica nanoparticles | Disulfide bonds | Fluorescence dye | Cell-specific targeting and redox-sensitive drug release | [251] |
| Mesoporous silica nanoparticles | Disulfide bonds | Doxorubicin | Controlled drug release and tumor active targeted therapy | [275] |
| Polymer-drug conjugates | Disulfide bonds | 10B-based sodium borocaptate | Efficient tumor targeted therapy, deep penetration, GSH-triggered drug release | [252] |
| Polymeric vesicles | Oxidation of the central-block sulphide moieties to sulphoxides and ultimately sulphones by H2O2 | - | The first example of use oxidative conversions to destabilize nanocarriers | [276] |
| Polymersomes | Disulfide bonds in poly (trimethylene carbonate-co-dithiolane trimethylene carbonate) | Doxorubicin | Lung cancer chemotherapy | [253] |
| Micelles | Disulfide bonds | Camptothecin | GSH-triggered drug release inside cancer cells for effective tumor therapy | [124] |
| Micelles | Se-Se bonds | Rhodamine B | GSH-triggered cargo release | [263] |
| Micelles | Disulfide bonds | siRNA | Cross-linked micelles with improved stability for siRNA delivery | [271] |
| Dendritic nanoparticles | Disulfide bonds | Cisplatin, fluorescence dye | Tumor theranostics | [277] |
| Cationic vesicles | Reduction of Fe3+ to Fe2+ by GSH | Anticancer drugs and siRNA | Redox‐responsive nanocarriers for drug/siRNA co‐delivery | [254] |
| Nanogels | Disulfide bonds | Camptothecin | Tumor therapy | [258] |
| Nanoparticles | Diselenide bonds | Paclitaxel | GSH-triggered drug release and tumor active targeted therapy | [278] |
| Nanoparticles | Catalase-response to H2O2 | Catalase, photosensitizer of methylene blue | Light-triggered, H2O2-responsive release of cargos for treating hypoxic cancer cells | [267] |
| Polyphosphazene nanoparticles | Cross-linking by disulfide bonds | Doxorubicin | Redox-responsive chemotherapy and photothermal therapy | [279] |
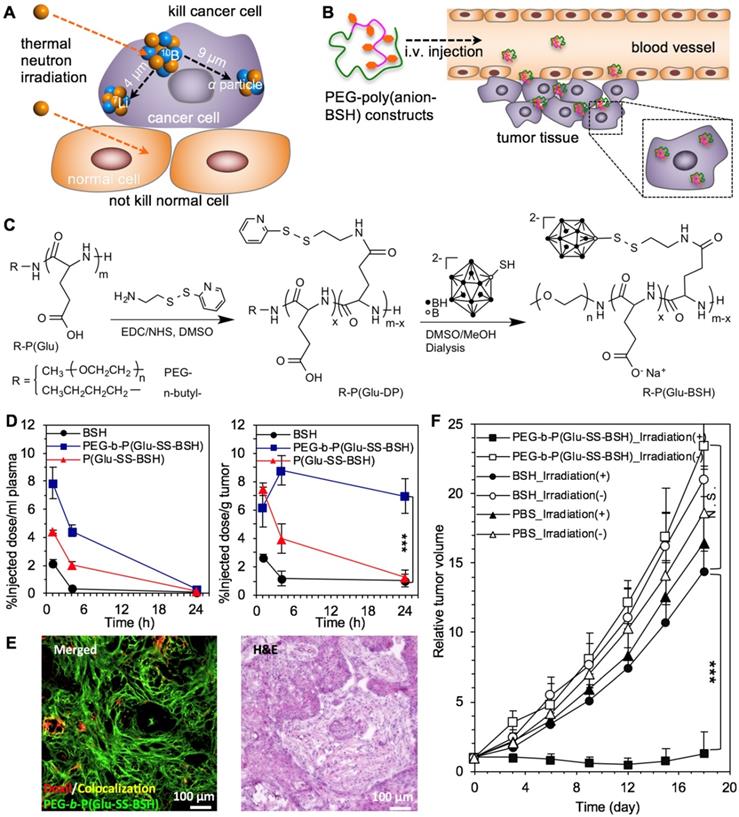
The redox-sensitive nanocarriers could trigger cargo release inside cancer cells [268], as some bioactive compounds were conjugated to nanomaterials through the disulfide bonds [252, 269] and the drug-loaded cavities in some nanocarriers (e.g., mesoporous silica nanoparticles) were sealed by disulfide bonds [251]. The redox-sensitive strategy could also be applied to detach the surface shell [270], and cross-link the core to increase the stability of nanocarriers [271, 272]. In another strategy, the cationic vesicles were formed by chelating of Fe3+ with amphiphilic piliararene, exhibiting GSH-triggered release of incorporated doxorubicin and siRNA from the collapse vesicles, as a result of GSH-induced reduction of Fe3+ to Fe2+ inside cancer cells [254]. Besides, the redox-responsive function could trigger the disassociation and degradation of nanocarriers inside cancer cells, as some nanocarriers were cross-linked by redox-sensitive bonds to increase the stability [271, 273]. The disulfide bonds cross-linked polymer nanocapsules could be disassociated by responding to GSH and dithiothreitol (DTT) [250]. Meanwhile, nanocarriers prepared by polymers with diselenide bonds (Se-Se) could also response to environmental redox-potential (i.e., GSH, H2O2) for controlled disassociation of nanoparticles and release of cargos [263]. Moreover, the redox-responsive nanocarriers facilitate intracellular delivery of bioactive compounds into cancer cells to overcome the cellular barriers, such as siRNA [254] and sodium borocaptate (BSH) [255], etc. For one example, the BSH-polymer conjugates have been engineered by conjugating with disulfide bonds for tumor boron neutron capture therapy (BNCT), because of the poor cellular uptake of clinically approved 10B-compounds (e.g., BSH) and the limited effective distance almost within diameter of cancer cells (Figure 12A-C) [252]. The BSH-polymer conjugates have significantly promoted the intracellular delivery of BSH, slightly extended the half-life in blood circulation and highly enhanced the tumor accumulation for deep penetration in tumor tissues and significant tumor therapy by BNCT (Figure 12D-F). Furthermore, the morphology of redox-sensitive nanocarriers may affect the intracellular delivery of cargos. Therefore, nanocarriers with different morphologies have been self-assembled with camptothecin and polymers through the disulfide bonds, including spheres, smooth disks, vesicles, and staggered lamellae [274], while the staggered lamellae ones demonstrated the most efficient cellular internalization than others. In addition, the redox-responsive nanocarriers demonstrated high potential for treating hypoxia tumors. For example, the Cy5.5-deoxybouvardin (RA-V) conjugates incorperated nanocarriers could target cancer cells by cRGD ligands, as well as release RA-V for intracellular fluorescence imaging and inducing apoptosis of cancer cells [266].
The redox-responsive nanocarriers for drug delivery to tumors toward effective therapy. (A,B) Illustration of boron neutron capture therapy (A) and nanocarriers for tumor BNCT (B). (C)The synthesis of redox-responsive polymeric nanocarriers. (D) Plasma clearance and tumor distribution of BSH and BSH-polymer conjugates. (E) The deep penetration of BSH-polymer conjugates in BxPC3 pancreatic tumors. (F) Boron neutron capture therapy of solid tumors with the polymer-boron cluster conjugates. Adapted with permission from ref. [252], copyright 2017 Elsevier B.V.
Enzyme-responsive nanocarriers
Enzymes play an important role in biological reactions, while the unregulated expression of certain enzymes in neoplastic conditions could be triggers for enzyme-responsive drug delivery. Several enzyme- responsive nanocarriers have been engineered for achieving controlled release of cargos in tumors and cancer cells [280, 281], prodrug/ligands activation, as well as morphology change, mainly including mesoporous silica nanoparticles [282, 283], dendrimers [284], magnetic nanoparticles [285, 286], polymeric micelles [287] and liposomes [288, 289] etc. As shown in Table 7, nanocarriers could response to several upregulated enzymes in tumor microenvironment and cancer cells [290], which are mainly including oxidoreductases (e.g., peroxidases) [291], transferases (e.g., creatine kinase) [289], and hydrolases, such as matrix metalloproteinases (MMPs) [292-294], human recombinant caspase 3 [295], proteinase K [60, 296], intestinal protease [286], cathepsin B [297] and trypsin [298, 299] etc.
Enzyme-responsive nanocarriers for cancer theranostics
| Bond type | Enzyme | Reaction | Occurrence | Materials | Cargo | Ref. | |
|---|---|---|---|---|---|---|---|
| Hydrolases | Peptide bonds | α- Chymotrypsin | Hydrolyze peptide amide bonds | Pancreas | Hollow mesoporous silica/poly(L-lysine) particles | Fluorescein and cytosine-phosphodiester-guanine oligodeoxynucleotide (CpG ODN) | [283] |
| Human recombinant caspase 3 | Hydrolyze peptide bonds only after an aspartic acid residue | Cytoplasm | Hyaluronic acid coating caspase 3 loaded pure drug nanoparticles | Paclitaxel | [295] | ||
| Cathepsin | Hydrolyze glycyl phenylalanyl leucyl glycine tetra-peptide | Lysosome | PEGylated lysine peptide dendrimer-gemcitabine conjugate | Gemcitabine | [284] | ||
| Hydrolyze tetrapeptide glycyl phenylalanyl leucyl glycine tetra-peptide | Lysosome | Amphiphilic biodegradable triblock N-(2-hydroxypropyl methyl) acrylamide copolymer-gadolinium- paclitaxel-Cyanine5.5 conjugates | Paclitaxel | [297] | |||
| Elastase | Hydrolyze peptide amide bonds of elastin | Tumor | PEGylated pDNA-nanoparticles | Nucleic acid | [306] | ||
| MMPs | Hydrolyze peptide amide bonds of extracellular matrix proteins | Participate in tissue remodeling and metastasis | Low molecular weight protamine and conjugated it to PEG-PCL nanoparticles | Paclitaxel | [307] | ||
| Hydrolyze peptide amide bonds of extracellular matrix proteins | Participate in tissue remodeling and metastasis | MSNs-Peptide-BSA-LA@DOX | Doxorubicin | [293] | |||
| Hydrolyze peptide amide bonds of extracellular matrix proteins | Participate in tissue remodeling and metastasis | Brush peptide-polymer amphiphiles composed fluorescent nanoparticle | Fluorescence dye | [294] | |||
| Hydrolyze peptide amide bonds of extracellular matrix proteins | Participate in tissue remodeling and metastasis | Micellar nanoparticles with a surface comprised of MMP-substrates and a hydrophobic paclitaxel core | Paclitaxel | [292] | |||
| Hydrolyze peptide amide bonds of extracellular matrix proteins | Participate in tissue remodeling and metastasis | Phenylboronic acid conjugated human serum albumin grafted mesoporous silica nanoparticles | Doxorubicin | [282] | |||
| Thrombin | Hydrolyze peptide amide bonds of fibrinogen | Participate in haemostasis, thrombosis, cell signaling, fibrinolysis and inflammation | Layer-by-layer assembly of poly(2-oxazoline)-based materials | Thrombolytic agent | [308] | ||
| Thermolysin | Hydrolyze peptide amide bonds containing hydrophobic amino acids. | Produced by Bacillus thermoproteolyticu | Poly(L-glutamic acid) star polypeptides using PPI dendrimers as initiators. | Rhodamine B | [309] | ||
| Trypsin | Hydrolyze peptide amide bonds | Pancreas | Bola-like cationic diphenylalanine nanocarriers | Doxorubicin | [298] | ||
| Hydrolyze peptide amide bonds at C terminal of lysine and arginine | Produced by the pancreas, activated in the small intestine | Protamine/ sulfatocyclodextrin supramolecular nanoparticles | Trisodium salt of 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) | [299] | |||
| Proteinase K | Hydrolyze peptide bonds | Candida albicans | Methotrexate-conjugated magnetic nanoparticles and glycine coated magnetic nanoparticles | Glycine and methotrexate | [296] | ||
| Hydrolyze peptide bonds | Candida albicans | Polytyrosine nanoparticles | Doxorubicin | [60] | |||
| Ester bonds | Acetylcholinesterase | Hydrolyze acetylcholine and other choline esters | Present in neuromuscular junctions | Poly(ethylene glycol)-block-poly(acrylic acid) with myristoylcholine chloride | Nile red | [310] | |
| Phospholipase | Hydrolyze lipids | Present in human digestive system, intracellular compartment and extracellular spaces | (R)-1-O-hexadecyl-2-palmitoyl-snglycero-3-phosphocholine | Antitumor ether lipids | [301] | ||
| Hydrolyze phosphoric acid monoester in peptide sequences | Participate in signal transduction and protein activity | ATP coated Ag nanoparticles | Silver nanoparticles | [311] | |||
| Glycosidic bonds | α-amylase | Cleaved α-1,4 glycosidic bond | Present in saliva | Hydroxyethyl starch based 10-hydroxy camptothecin (10-HCPT)-HES and 5-FU-HES conjugates | Paclitaxel | [312] | |
| β- Glucuronidase | Hydrolyze complex carbohydrates | Present in lysosome, necrotic tissue, and some solid tumor types | β-glucuronidase-responsive prodrugs with the potent monomethyl auristatin E linker | Monomethyl auristatin E | [313] | ||
| Oxidoreductases | Azo compounds | Azoreductase | Reductive azo compounds | Colon bacteria | Copolymers of 2-hydroxyethyl methacrylate (HEMA) and methyl methacrylate (MMA), and terpolymers of HEMA, MMA, and methacrylic acid | Ibuprofen | [291] |
| Transferases | Phosphorus-containing groups | Creatine kinase | Phosphorylate hydroxyl group in peptide sequences | Regulate cellular pathways | Liposome based DSPE-PEG2000-TAT | Paclitaxel | [289] |
The enzyme-sensitive nanocarriers could be utilized in the following aspects: (1) Activating prodrugs, probes and ligands by cutting the enzyme-sensitive bonds between the bioactive compounds and protective groups; (2) Degradation or disassociation of nanocarriers through enzyme- triggered cleavage of polymer backbones, charge conversion of nanomaterials and disassembly of nanoparticles; (3) Direct cleaving the conjugation between nanocarriers and drugs; (4) Enzyme- triggered physical disruption of nanocarriers; (5) Enzyme-triggered controlled release of cargos. For achieving enzyme-sensitive function, several factors should be considered for rational design nanocarriers: (1) The recognition and accessibility of enzymes to the sensitive groups/substrates in nanocarriers; (2) The threshold of the substrates that responding to enzymes, which should ensure the enzyme-triggered reaction; (3) the influence of physiological conditions and the physicochemical properties to the enzyme- sensitivity.
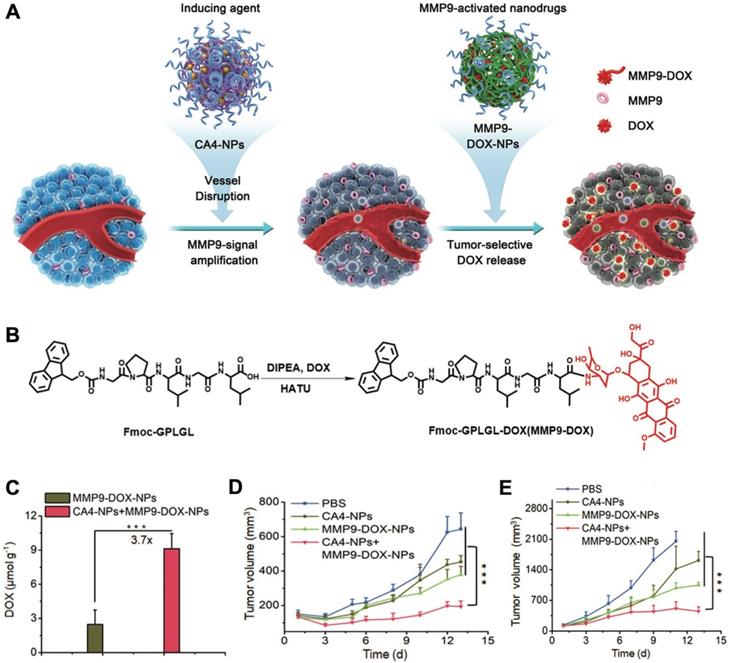
The specific enzyme-triggered cargo release allows drug delivery to tumors and avoids cargo exposure during circulation, which could maintain the activity of bioactive compounds, while avoid causing sides effects to normal organs/tissues. For enzyme-triggered drug release, the cathepsin could cleave the hydrolyze peptide bonds in gemcitabine- conjugated dendrimer nanocarriers inside lysosomes to liberate gemcitabine and cationic dendrimers, leading to lysosome escape and intracellular gemcitabine delivery [284]. In another study, the hyaluronic acid coated and prodrug-loaded nanoparticles could specifically release paclitaxel inside cancer cells by affecting the hydrolyze peptide bonds with human recombinant caspase 3 [295]. Besides, the prodrugs/ probes could be activated by enzymes in tumors, as the prodrug strategy is generally applied to protect the activity of drugs, probes and ligands during circulation to increase the diagnostic or therapeutic specificity [301]. In one example, the protease- activatable nanoprobes have been developed by combining fluorescent dye and Fe3O4 nanocrystals through MMP-9 [302], which could turn “ON” the fluorescence for tumor imaging when the peptide substrates linkers were cleaved by protease. In another case, the MMP9-activatable doxorubicin prodrug-loaded nanocarriers were developed (Figure 13A,B) [300], to combine with combretastatin A4 (CA4)-loaded nanocarriers for cancer synergistic treatment. The CA4-loaded nanocarriers could disrupt tumor blood vasculature and selectively enhance MMP9 expression in tumors to promote the accumulation of doxorubicin (Figure 13C), leading to effective treatment of 4T1 and C26 tumors (Figure 13D,E). Moreover, the enzyme-responsive nanocarriers could be applied for tumor specific imaging, e.g., the MMP-responsive iron oxide nanoparticles have specifically enhanced the T2-weighted contrast in tumors for diagnosis by MRI [285]. Furthermore, the enzyme could uncap the surface shell (e.g., peptides) of nanocarriers to improve their accumulation in tumors. For example, the nanocarriers self- assembled by paclitaxel- conjugated block copolymers and enzyme-recognition peptide shell, could change the morphology due to the cleavage of peptide shell by MMP, leading to high accumulation of the polymer-drug conjugates in tumors [292]. In addition, the enzyme-responsive function could be applied for disassociation of nanocarriers. The azobenzene-linked amphiphilic diblock copolymers have been applied to form polymeric micelles, and micellar architecture could be disrupted by responding to azoreductase and nicotinamide adenine dinucleotide phosphate (NADPH) [303]. It demonstrated high potential in the arena of colon-specific drug delivery, as azoreductase is existed in human intestine. The enzyme-triggered degradation of nanocarriers into small size structures would improve the penetration of drug delivery systems throughout the tumor's interstitial spaces. For instance, the 100 nm nanoparticles could be reduced to 10 nm by responding to proteases (i.e., MMP-2) in tumor microenvironment, which effectively enhanced the diffusion of drugs into the tumor's dense collagen matrix, while maintained long circulation for achieving EPR effect [304]. Overall, the enzyme- sensitive nanocarriers have demonstrated high potential in tumor diagnosis [285, 286], as well as treating primary and metastatic tumors [293, 294, 305].
Multimodal-responsive nanocarriers
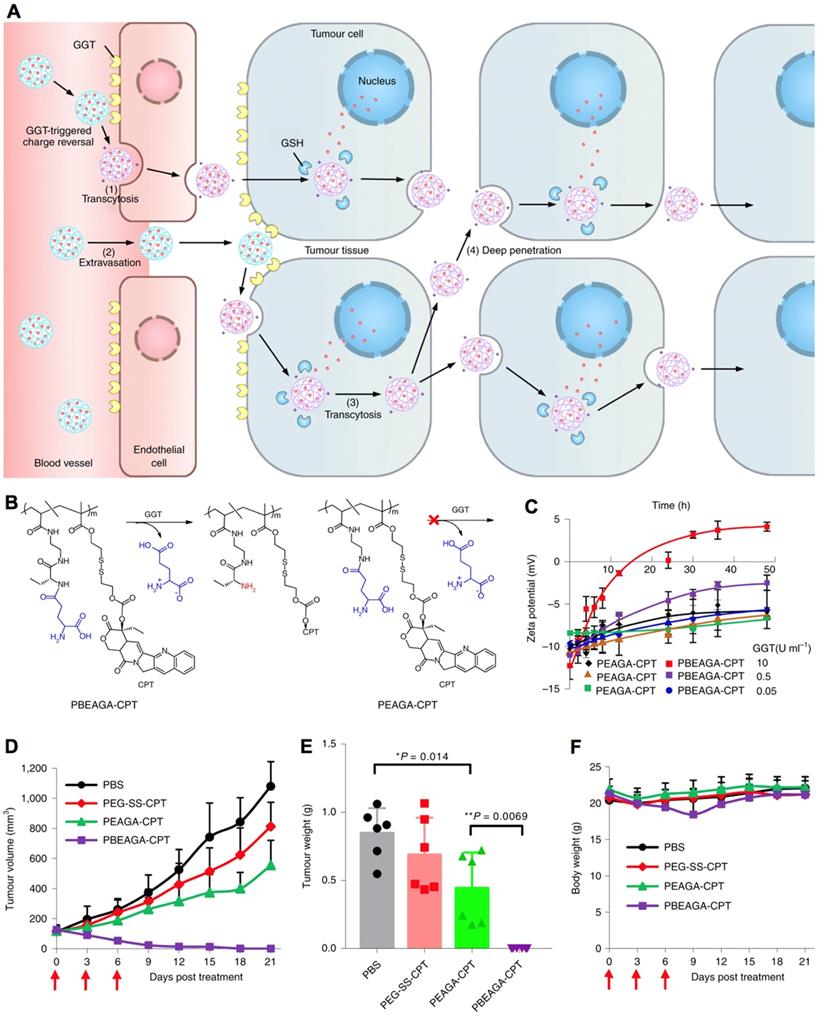
In addition, nanocarriers have also been engineered with multiple stimuli-responsive functions, facilitating multistage drug delivery, as well as achieving higher specificity and efficacy. For example, nanocarriers responding to both intracellular pH and GSH have been developed for promoted intracellular drug delivery [314]. In another study, the developed platinum drug delivery nanocarriers could response to intracellular GSH for disassociation, and response to intracellular low pH for controlled drug release [277]. Indeed, the multiple stimuli-responsive nanocarriers hold high potential in achieving long circulation, high tumor accumulation, deep penetration in tumor tissues, internalization with cancer cells and endosome escape, etc. Thus, several multiple stimuli-responsive nanocarriers have been engineered for delivery cargos to tumors [315-321]. In one example, the multiple stimuli- responsive nanocarriers could be discharged into small nanoparticles by responding to the low pH in tumor microenvironment, and then the platinum prodrugs in the small nanoparticles were activated by GSH for promoted penetrating and treating the poorly permeable pancreatic tumors [209]. In another example, the nanocarriers made by γ-glutamyl-based polymer-drug conjugates (PBEAGA-CPT) conjugates could response to both γ-glutamyl transpeptidase (GGT) and GSH have been developed [322], which could convert to be positive charged nanomaterials by responding to GGT for internalization with cancer cells and by responding to GSH inside cancer cells to release CPT (Figure 14A-C). The multimodal responsive polymer-drug conjugated nanocarriers have demonstrated high efficacy in transcytosis, extravasation, internalization with cancer cells and deep tumor penetration, leading to effective supression of subcutaneous HepG2 tumors (Figure 14D-F). In general, it is sophisticate for developing multiple stimuli-responsive nanocarriers, and also difficult to maintain the multiple functions in biological systems. Thus, nanocarriers with single or dual stimuli- responsive functions have been more focused [49, 323]. For instance, the polyphosphazene nanocarriers with pH- and redox-sensitivities have been engineered for tumor multimodal imaging- guided chemo-photodynamic therapy [324-326]. Here nanocarriers for multiple stimuli-triggered drug delivery were briefly introduced, as each stimuli- responsive function has already been discussed above.
Enzyme-responsive nanocarriers for cancer therapy. (A) Schematic illustration of nanocarriers incorporating combretastatin A4 nanodrug (CA4) plus MMP9-activatable doxorubicin prodrug for tumor therapy. (B) The chemical structure of MM9-activatable MMP9-activated doxorubicin prodrug. (C) The distribution of doxorubicin in tumors. (D,E) Tumor inhibition rate in 4T1 (D) and C26 (E) tumor models. Adapted with permission from ref. [300], copyright 2019 John Wiley & Sons, Inc.
Multimodal-responsive polymer-drug conjugated nanocarriers. (A) Illustration of the cationization-initiated transcytosis-mediated tumour penetration for transendothelial and transcellular transport of nanocarriers. (B) The structures of GGT-responsive cationizing PBEAGA-CPT conjugates and the non-GGT-responsive PEAGA-CPT conjugates. (C) The zeta potentials of the nanocarriers. (D-F) Antitumor efficacy of polymer-drug conjugated nanocarriers against subcutaneous HepG2 tumors, where the tumor growth rate (D), tumor weight (E) and bodyweight (F) were measured. Adapted with permission from ref. [322], copyright 2016 Springer Nature Limited.
Clinical translation of stimuli-responsive nanocarriers
| Stimulus | Nanocarriers | Cargo | Indications | Clinical status | Reference |
|---|---|---|---|---|---|
| Magnetic | Iron oxide magnetite | Iron oxide nanoparticles | Prostate cancer | Phase I | NCT02033447 |
| Iron and carbon (MTC-DOX) | Doxorubicin | Unresectable hepatocellular carcinoma | Phase II and III | NCT00034333 | |
| Hepatocellular carcinoma | Phase I and II | NCT00054951 | |||
| Liver metastasis | Phase I and II | NCT00041808 | |||
| Temperature | Liposomes (ThermoDox) | Doxorubicin | Recurrent regional breast cancer | Phase I and II | NCT00826085 |
| Liver tumor | Phase I | NCT02181075 | |||
| Pediatric refractory solid tumor | Phase I | NCT02536183 | |||
| Doxorubicin combined with high Intensity focused ultrasound (HIFU) | Painful bone metastases, breast carcinoma, non-small cell lung cancer, small cell lung cancer, adenocarcinoma | Phase II | NCT01640847 | ||
| Doxorubicin combined with standardized radiofrequency ablation | Hepatocellular carcinoma | Phase III | NCT02112656 | ||
| pH | Polymeric micelles (NC6300) | Epirubicin | Solid tumor, soft tissue sarcoma, metastatic sarcoma, sarcoma | Phase I and II | NCT03168061 |
| Secretory phospholipase A2 (sPLA2) | Liposomes (LiPlaCis) | Cisplatin | Advanced or refractory solid tumor, metastatic breast cancer, prostate cancer and skin cancer | Phase I and II | NCT01861496 |
Clinical translation of the stimuli-responsive nanocarriers
The advances in stimuli-responsive nanocarriers have led to clinical translation of several formulations. As shown in Table 8, there are six nanocarriers responding to magnetic, temperature, pH and secretory phospholipase A2 (sPLA2), are under clinical translation. Two magnetic-sensitive iron- based nanocarriers, iron oxide magnetite, and doxorubicin-loaded iron and carbon (MTC-DOX), are under clinical trial for treating cancers. The iron oxide magnetite was conducted Phase I clinical trial to evaluate safety, retention and distribution after injection, which final score is for treating prostate cancer in men by thermal ablation. Three clinical trials have been applied for MTC-DOX, including Phase II and III studying the safety, tolerance and efficacy (survival time) on treating unresectable hepatocellular carcinoma (NCT00034333); Phase I and II evaluation of prohibiting hepatocellular carcinoma progression after injection with external magnet (NCT00054951); and Phase I and II studying on liver metastasis (NCT00041808). Besides, the thermal-sensitive doxorubicin- incorporated liposomes (ThermoDox) have been applied for the following three clinical studies: Phase I and II studying the maximum tolerated dose, safety, pharmacokinetics and hyperthermia effects in patients with recurrent regional breast cancer (NCT00826085); Phase I investigation of doxorubicin release from liposome by focused ultrasound in liver tumors (NCT02181075); and MRI and high intensity focused ultrasound (HIFU) combined study to determine doxorubicin release in pediatric refractory solid tumor (NCT02536183). The clinical trial of ThermoDox has also be designed to evaluate the safety and efficacy by combining with HIFU on several tumors (Phase II, NCT01640847), e.g., painful bone metastases, breast carcinoma, non-small cell lung cancer, small cell lung cancer and adenocarcinoma; as well as study the efficacy on treating hepatocellular carcinoma combined with standardized radiofrequency ablation (Phase III, NCT02112656). Moreover, the pH-responsive, epirubicin-loaded polymeric micelles (NC6300) have entered Phase I and II study (NCT03168061) for evaluating the dose, activity and tolerability in patients with soft tissue sarcoma. In previous preclinical clinical study, NC6300 could reduce the cardiotoxicity of epirubicin by conjugating to polymers through pH-sensitive bonds (i.e., hydrazone) [327], and exhibited better therapeutic effect (10 mg/kg based on epirubicin) on treating hepatocellular carcinoma [328]. The preclinical evaluation has provided positive evidences for further clinical evaluation. In addition, the secretory phospholipase A2 (sPLA2)-sensitive, cisplatin- incorporated liposomes (LiPlaCis) have entered Phase I and II to study the safety, tolerability and sensitivity on patients with advanced breast cancer and metastatic breast cancer (NCT01861496). Although with progress, the clinical translation of stimuli- responsive nanocarriers still encountered several barriers: (1) the differences between animal tumor models and tumors in patients, as tumors in patients are more heterogeneity and complicated; (2) the toxicity, biosafety and biodegradability of nanocarriers should be addressed; (3) the stable stimuli-responsive function in vivo; (4) the tumor accumulation and therapeutic efficacy of stimuli- sensitive nanocarriers should be proved in clinical trial; (5) the factors that influence the stimuli- responsive properties in vivo should be clarified; (6) the right dose and administration way should be studied, e.g., intravenous injection (i.v.), intraperitoneal injection (i.p.). Therefore, future work would focus on clinical translation of the stimuli-sensitive nanocarriers, and optimizing the formulations from lessons of clinical trial.
Conclusion
The nanocarriers bring novel strategy for delivery bioactive compounds to tumors. The stimuli-sensitive nanocarriers provide high specificity and multiple functions in drug delivery, including controlled release, alerted tumor accumulation, switch “ON-OFF” activities, as well as promoted diagnostic and therapeutic accuracy and efficacy. Besides, the rational design of stimuli-nanocarriers has considered their biological manners in tumor microenvironment and cancer cells to maximize the efficacy and minimizing the adverse effects to normal organs and tissues. Until now, numerous external and internal stimuli-sensitive nanocarriers have been developed, exhibiting better outcomes than the conventional formulations. The stimuli-responsive systems could be widely applied for diagnosis, probing, sensing and therapy tumors and other diseases, such as cardiovascular diseases, etc. Moreover, maintaining the stimuli-sensitivity in large scale produced nanocarriers would be potential challenge. Furthermore, although with extensive studies on stimuli-sensitive nanocarriers, only a few formulations have entered clinical translation, which requires future extensive works on clinical translation. In addition, considering the heterogeneity of tumors, the molecular imaging would be applied for screening the stimuli-responsive nanocarriers in tumors and patients, to predict and study the sensitivity and responses [329]. Meanwhile, the stimuli-responsive nanocarriers may also be combined with antibodies for tumor immunotherapy [330, 331]. Overall, the development of nanocarriers responding to external and internal stimuli in diseased regions would promote the advent of “magic bullets” for tumor precision diagnosis and therapy in future.
Acknowledgements
This work was partially supported by the National Key R&D Program of China (2017YFA0207900), the National Young 1000 Talents Plan (D1424002A) and the Sichuan Science and Technology Program (2018RZ0134). The author would like to thank Dr. Yang Shi, Dr. Roy van der Meel, Dr. Twan Lammers and Dr. Xiaoyuan (Shawn) Chen for the invitation of this manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271-84
2. Mi P, Cabral H, Kataoka K. Ligand-installed nanocarriers towards precision therapy. Adv Mater. 2019. 1902 604
3. Yi Y, Lin G, Chen S, Liu J, Zhang H, Mi P. Polyester micelles for drug delivery and cancer theranostics: Current achievements, progresses and future perspectives. Mater Sci Eng C Mater Biol Appl. 2018;83:218-32
4. Chen HB, Gu ZJ, An HW, Chen CY, Chen J, Cui R. et al. Precise nanomedicine for intelligent therapy of cancer. Sci China Chem. 2018;61:1503-52
5. van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007-17
6. Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M. et al. Accumulation of sub-100nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815-23
7. Matsumoto Y, Nichols JW, Toh K, Nomoto T, Cabral H, Miura Y. et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11:533-8
8. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751-60
9. Mi P, Wang F, Nishiyama N, Cabral H. Molecular cancer imaging with polymeric nanoassemblies: from tumor detection to theranostics. Macromol Biosci. 2017;17:1600305
10. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37
11. Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844-92
12. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941-51
13. Zhu Y, Chen C, Cao Z, Shen S, Li L, Li D. et al. On-demand PEGylation and dePEGylation of PLA-based nanocarriers via amphiphilic mPEG-TK-Ce6 for nanoenabled cancer chemotherapy. Theranostics. 2019;9:8312-20
14. Cherukula K, Uthaman S, Park IK. "Navigate-dock-activate" anti-tumor strategy: Tumor micromilieu charge-switchable, hierarchically activated nanoplatform with ultrarapid tumor-tropic accumulation for trackable photothermal/chemotherapy. Theranostics. 2019;9:2505-25
15. Chen B, Dai W, He B, Zhang H, Wang X, Wang Y. et al. Current multistage drug delivery systems based on the tumor microenvironment. theranostics. 2017;7:538-58
16. Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2:17024
17. Cheng CA, Deng T, Lin FC, Cai Y, Zink JI. Supramolecular nanomachines as stimuli-responsive gatekeepers on mesoporous silica nanoparticles for antibiotic and cancer drug delivery. Theranostics. 2019;9:3341-64
18. Li Z, Song N, Yang Y-W. Stimuli-responsive drug-delivery systems based on supramolecular nanovalves. Matter. 2019;1:345-68
19. Song N, Lou XY, Ma L, Gao H, Yang YW. Supramolecular nanotheranostics based on pillarenes. Theranostics. 2019;9:3075-93
20. Wang YG, Zhou KJ, Huang G, Hensley C, Huang XN, Ma XP. et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13:204-12
21. Park SM, Aalipour A, Vermesh O, Yu JH, Gambhir SS. Towards clinically translatable in vivo nanodiagnostics. Nat Rev Mater. 2017;2:17014
22. Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics-application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660-77
23. Wang S, Huang P, Chen X. Stimuli-responsive programmed specific targeting in nanomedicine. ACS Nano. 2016;10:2991-4
24. Liu Y, Xu CF, Iqbal S, Yang XZ, Wang J. Responsive nanocarriers as an emerging platform for cascaded delivery of nucleic acids to cancer. Adv Drug Deliver Rev. 2017;115:98-114
25. Wang S, Huang P, Chen X. Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization. Adv Mater. 2016;28:7340-64
26. Kwon EJ, Lo JH, Bhatia SN. Smart nanosystems: bio-inspired technologies that interact with the host environment. Proc Natl Acad Sci U S A. 2015;112:14460-6
27. Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2017;2:16075
28. Ge Z, Liu S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem Soc Rev. 2013;42:7289-325
29. Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306-23
30. Huang P, Wang G, Su Y, Zhou Y, Huang W, Zhang R. et al. Stimuli-responsive nanodrug self-assembled from amphiphilic drug-inhibitor conjugate for overcoming multidrug resistance in cancer treatment. Theranostics. 2019;9:5755-68
31. Wang J, Mi P, Lin G, Wang YX, Liu G, Chen X. Imaging-guided delivery of RNAi for anticancer treatment. Adv Drug Deliv Rev. 2016:104 44-60
32. Son S, Min HS, You DG, Kim BS, Kwon IC. Echogenic nanoparticles for ultrasound technologies: Evolution from diagnostic imaging modality to multimodal theranostic agent. Nano Today. 2014;9:525-40
33. Huynh E, Leung BY, Helfield BL, Shakiba M, Gandier JA, Jin CS. et al. In situ conversion of porphyrin microbubbles to nanoparticles for multimodality imaging. Nat Nanotechnol. 2015;10:325-32
34. Kang E, Min HS, Lee J, Han MH, Ahn HJ, Yoon IC. et al. Nanobubbles from Gas-Generating Polymeric Nanoparticles: Ultrasound Imaging of Living Subjects. Angew Chem Int Ed. 2010;49:524-8
35. Wang L, Zhang M, Tan K, Guo Y, Tong H, Fan X. et al. Preparation of nanobubbles carrying androgen receptor siRNA and their inhibitory effects on androgen-independent prostate cancer when combined with ultrasonic irradiation. PloS ONE. 2014;9:e96586
36. Zhou Q-L, Chen Z-Y, Wang Y-X, Yang F, Lin Y, Liao Y-Y. Ultrasound-mediated local drug and gene delivery using nanocarriers. BioMed Res Int. 2014:963819
37. Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193-208
38. Delogu LG, Vidili G, Venturelli E, Menard-Moyon C, Zoroddu MA, Pilo G. et al. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proc Natl Acad Sci U S A. 2012;109:16612-7
39. Min KH, Min HS, Lee HJ, Park DJ, Yhee JY, Kim K. et al. pH-controlled gas-generating mineralized nanoparticles: a theranostic agent for ultrasound imaging and therapy of cancers. ACS Nano. 2015;9:134-45
40. Geers B, Dewitte H, De Smedt SC, Lentacker I. Crucial factors and emerging concepts in ultrasound-triggered drug delivery. J Control Release. 2012;164:248-55
41. Cao Y, Chen Y, Yu T, Guo Y, Liu F, Yao Y. et al. Drug release from phase-changeable nanodroplets triggered by low-intensity focused ultrasound. Theranostics. 2018;8:1327-39
42. Huang L, Yu C, Huang T, Xu S, Bai Y, Zhou Y. Ultrasound-responsive ultrathin multiblock copolyamide vesicles. Nanoscale. 2016;8:4922-6
43. Min HS, Son S, Lee TW, Koo H, Yoon HY, Na JH. et al. Liver-specific and echogenic hyaluronic acid nanoparticles facilitating liver cancer discrimination. Adv Funct Mater. 2013;23:5518-29
44. Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV. et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat Nanotechnol. 2014;9:311-6
45. Jin Z, Wen Y, Hu Y, Chen W, Zheng X, Guo W. et al. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale. 2017;9:3637-45
46. Gao H, Wen D, Tarakina NV, Liang J, Bushby AJ, Sukhorukov GB. Bifunctional ultraviolet/ultrasound responsive composite TiO2/polyelectrolyte microcapsules. Nanoscale. 2016;8:5170-80
47. Min HS, Kang E, Koo H, Lee J, Kim K, Park RW. et al. Gas-generating polymeric microspheres for long-term and continuous in vivo ultrasound imaging. Biomaterials. 2012;33:936-44
48. Li C, Zhang Y, Li Z, Mei E, Lin J, Li F. et al. Light-responsive biodegradable nanorattles for cancer theranostics. Adv Mater. 2018;30:1706150
49. Wang X, Niu D, Li P, Wu Q, Bo X, Liu B. et al. Dual-enzyme-loaded multifunctional hybrid nanogel system for pathological responsive ultrasound imaging and T2-weighted magnetic resonance imaging. ACS Nano. 2015;9:5646-56
50. Papa AL, Korin N, Kanapathipillai M, Mammoto A, Mammoto T, Jiang A. et al. Ultrasound-sensitive nanoparticle aggregates for targeted drug delivery. Biomaterials. 2017;139:187-94
51. Carson AR, McTiernan CF, Lavery L, Grata M, Leng X, Wang J. et al. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res. 2012;72:6191-9
52. Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics. 2012;2:1208-22
53. Paris JL, Manzano M, Cabanas MV, Vallet-Regi M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale. 2018;10:6402-8
54. Florinas S, Kim J, Nam K, Janat-Amsbury MM, Kim SW. Ultrasound-assisted siRNA delivery via arginine-grafted bioreducible polymer and microbubbles targeting VEGF for ovarian cancer treatment. J Control Release. 2014;183:1-8
55. Vandenbroucke RE, Lentacker I, Demeester J, De Smedt SC, Sanders NN. Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. J Control Release. 2008;126:265-73
56. Chen J, Ratnayaka S, Alford A, Kozlovskaya V, Liu F, Xue B. et al. Theranostic multilayer capsules for ultrasound imaging and guided drug delivery. ACS Nano. 2017;11:3135-46
57. Paris JL, de la Torre P, Victoria Cabanas M, Manzano M, Grau M, Flores AI. et al. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale. 2017;9:5528-37
58. Li W, Hou W, Guo X, Luo L, Li Q, Zhu C. et al. Temperature-controlled, phase-transition ultrasound imaging-guided photothermal-chemotherapy triggered by NIR light. Theranostics. 2018;8:3059-73
59. Wang Z, He Q, Zhao W, Luo J, Gao W. Tumor-homing, pH- and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J Control Release. 2017;264:66-75
60. Gu X, Qiu M, Sun H, Zhang J, Cheng L, Deng C. et al. Polytyrosine nanoparticles enable ultra-high loading of doxorubicin and rapid enzyme-responsive drug release. Biomater Sci. 2018;6:1526-34
61. Zhao F, Zhou J, Su X, Wang Y, Yan X, Jia S. et al. A Smart responsive dual aptamers-targeted bubble-generating nanosystem for cancer triplex therapy and ultrasound imaging. Small. 2017;13:1603990
62. Tang W, Yang Z, Wang S, Wang Z, Song J, Yu G. et al. Organic semiconducting photoacoustic nanodroplets for laser-activatable ultrasound imaging and combinational cancer therapy. ACS Nano. 2018;12:2610-22
63. Ho Y-J, Wu C-C, Hsieh Z-H, Fan C-H, Yeh C-K. Thermal-sensitive acoustic droplets for dual-mode ultrasound imaging and drug delivery. J Control Release. 2018;291:26-36
64. Chen K-J, Liang H-F, Chen H-L, Wang Y, Cheng P-Y, Liu H-L. et al. A Thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS Nano. 2013;7:438-46
65. Liu K-C, Arivajiagane A, Wu S-J, Tzou S-C, Chen C-Y, Wang Y-M. Development of a novel thermal-sensitive multifunctional liposome with antibody conjugation to target EGFR-expressing tumors. Nanomedicine: NBMS. 2019;15:285-94
66. Li W-S, Wang X-J, Zhang S, Hu J-B, Du Y-L, Kang X-Q. et al. Mild microwave activated, chemo-thermal combinational tumor therapy based on a targeted, thermal-sensitive and magnetic micelle. Biomaterials. 2017;131:36-46
67. Cheng Y, Hao J, Lee LA, Biewer MC, Wang Q, Stefan MC. Thermally controlled release of anticancer drug from self-assembled γ-substituted amphiphilic poly(ε-caprolactone) micellar nanoparticles. Biomacromolecules. 2012;13:2163-73
68. Araki T, Fuchi Y, Murayama S, Shiraishi R, Oyama T, Aso M. et al. Fluorescence tumor-imaging using a thermo-responsive molecule with an emissive aminoquinoline derivative. Nanomaterials. 2018;8:782
69. Rijcken CJF, Hofman J-W, van Zeeland F, Hennink WE, van Nostrum CF. Photosensitiser-loaded biodegradable polymeric micelles: preparation, characterisation and in vitro PDT efficacy. J Control Release. 2007;124:144-53
70. Shi Y, van Steenbergen MJ, Teunissen EA, Novo L, Gradmann S, Baldus M. et al. Pi-pi stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules. 2013;14:1826-37
71. Hervault A, Dunn AE, Lim M, Boyer C, Mott D, Maenosono S. et al. Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale. 2016;8:12152-61
72. Lee SH, Choi SH, Kim SH, Park TG. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: Swelling induced physical disruption of endosome by cold shock. J Control Release. 2008;125:25-32
73. Luckanagul JA, Pitakchatwong C, Ratnatilaka Na Bhuket P, Muangnoi C, Rojsitthisak P, Chirachanchai S. et al. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohyd Polym. 2018;181:1119-27
74. Almeida EAMS, Bellettini IC, Garcia FP, Farinácio MT, Nakamura CV, Rubira AF. et al. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohyd Polym. 2017;171:259-66
75. Wang C, Zhang G, Liu G, Hu J, Liu S. Photo- and thermo-responsive multicompartment hydrogels for synergistic delivery of gemcitabine and doxorubicin. J Control Release. 2017;259:149-59
76. Ruan C, Liu C, Hu H, Guo X-L, Jiang B-P, Liang H. et al. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem Sci. 2019;10:4699-706
77. Park WM, Champion JA. Thermally triggered self-assembly of folded proteins into vesicles. J Am Chem Soc. 2014;136:17906-9
78. Zheng M, Jiang T, Yang W, Zou Y, Wu HG, Liu XH. et al. The siRNAsome: a aation-free and versatile nanostructure for siRNA and drug co-delivery. Angew Chem Int Ed. 2019;58:4938-42
79. van Elk M, Deckers R, Oerlemans C, Shi Y, Storm G, Vermonden T. et al. Triggered release of doxorubicin from temperature-sensitive poly(N-(2-hydroxypropyl)-methacrylamide mono/dilactate) grafted liposomes. Biomacromolecules. 2014;15:1002-9
80. Barhoumi A, Wang WP, Zurakowsi D, Langer RS, Kohane DS. Photothermally targeted thermosensitive polymer-masked nanoparticles. Nano Lett. 2014;14:3697-701
81. Mi P, Ju XJ, Xie R, Wu HG, Ma J, Chu LY. A novel stimuli-responsive hydrogel for K+-induced controlled-release. Polymer. 2010;51:1648-53
82. Kono K, Murakami E, Hiranaka Y, Yuba E, Kojima C, Harada A. et al. Thermosensitive molecular assemblies from poly(amidoamine) dendron-based lipids. Angew Chem Int Ed. 2011;50:6332-6
83. Osawa S, Ishii T, Takemoto H, Osada K, Kataoka K. A facile amino-functionalization of poly(2-oxazoline)s' distal end through sequential azido end-capping and Staudinger reactions. Eur Polym J. 2017;88:553-61
84. Yang J, Zhang P, Tang L, Sun P, Liu W, Sun P. et al. Temperature-tuned DNA condensation and gene transfection by PEI-g-(PMEO2MA-b-PHEMA) copolymer-based nonviral vectors. Biomaterials. 2010;31:144-55
85. Kim SH, Tan JPK, Fukushima K, Nederberg F, Yang YY, Waymouth RM. et al. Thermoresponsive nanostructured polycarbonate block copolymers as biodegradable therapeutic delivery carriers. Biomaterials. 2011;32:5505-14
86. Li B, Smilgies D-M, Price AD, Huber DL, Clem PG, Fan H. Poly(N-isopropylacrylamide) surfactant-functionalized responsive silver nanoparticles and superlattices. ACS Nano. 2014;8:4799-804
87. Al-Ahmady ZS, Al-Jamal WT, Bossche JV, Bui TT, Drake AF, Mason AJ. et al. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano. 2012;6:9335-46
88. Oroojalian F, Babaei M, Taghdisi SM, Abnous K, Ramezani M, Alibolandi M. Encapsulation of thermo-responsive gel in pH-sensitive polymersomes as dual-responsive smart carriers for controlled release of doxorubicin. J Control Release. 2018;288:45-61
89. Yoo D, Jeong H, Noh SH, Lee JH, Cheon J. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew Chem Int Ed. 2013;52:13047-51
90. Yan H, Shang W, Sun X, Zhao L, Wang J, Xiong Z. et al. “All-in-One” nanoparticles for trimodality imaging-guided intracellular photo-magnetic hyperthermia therapy under intravenous administration. Adv Funct Mater. 2018;28:1705710
91. Di Corato R, Bealle G, Kolosnjaj-Tabi J, Espinosa A, Clement O, Silva AKA. et al. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano. 2015;9:2904-16
92. Smith CE, Ernenwein D, Shkumatov A, Clay NE, Lee JY, Melhem M. et al. Hydrophilic packaging of iron oxide nanoclusters for highly sensitive imaging. Biomaterials. 2015;69:184-90
93. Thorat ND, Bohara RA, Noor MR, Dhamecha D, Soulimane T, Tofail SAM. Effective cancer theranostics with polymer encapsulated superparamagnetic nanoparticles: combined effects of magnetic hyperthermia and controlled drug release. ACS Biomater Sci Eng. 2016;3:1332-40
94. Li ZF, Yang T, Lin CM, Li QS, Liu SF, Xu FZ. et al. Sonochemical synthesis of hydrophilic drug loaded multifunctional bovine serum albumin nanocapsules. ACS Appl Mater Inter. 2015;7:19390-7
95. Chiang CS, Shen YS, Liu JJ, Shyu WC, Chen SY. Synergistic combination of multistage magnetic guidance and optimized ligand density in targeting a nanoplatform for enhanced cancer therapy. Adv Healthc Mater. 2016;5:2131-41
96. Huang P, Li ZM, Lin J, Yang DP, Gao G, Xu C. et al. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials. 2011;32:3447-58
97. Cazares-Cortes E, Espinosa A, Guigner JM, Michel A, Griffete N, Wilhelm C. et al. Doxorubicin intracellular remote release from biocompatible oligo(ethylene glycol) methyl ether methacrylate-based magnetic nanogels triggered by magnetic hyperthermia. ACS Appl Mater Inter. 2017;9:25775-88
98. Zhang ZQ, Song SC. Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy. Biomaterials. 2017;132:16-27
99. Chen YT, Guo F, Qiu Y, Hu HR, Kulaots I, Walsh E. et al. Encapsulation of particle ensembles in graphene nanosacks as a new route to multifunctional materials. ACS Nano. 2013;7:3744-53
100. Yin PT, Shah S, Pasquale NJ, Garbuzenko OB, Minko T, Lee KB. Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials. 2016;81:46-57
101. Wang H, Wang K, Tian B, Revia R, Mu Q, Jeon M. et al. Preloading of hydrophobic anticancer drug into multifunctional nanocarrier for multimodal imaging, NIR-responsive drug release, and synergistic therapy. Small. 2016;12:6388-97
102. Yao X, Niu X, Ma K, Huang P, Grothe J, Kaskel S. et al. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small. 2017;13:1602225
103. Wang RH, Zhou Y, Zhang P, Chen Y, Gao W, Xu JS. et al. Phase-transitional Fe3O4/perfluorohexane microspheres for magnetic droplet vaporization. Theranostics. 2017;7:846-54
104. Wu F, Zhang M, Chen K, Mabrouk S, Pathak R, Tong Y. et al. Triple stimuli-responsive magnetic hollow porous carbon-based nanodrug delivery system for magnetic resonance imaging-guided synergistic photothermal/chemotherapy of cancer. ACS Appl Mater Inter. 2018;10:25604-13
105. Meikle ST, Pineiro Y, Banobre Lopez M, Rivas J, Santin M. Surface functionalization superparamagnetic nanoparticles conjugated with thermoresponsive poly(epsilon-lysine) dendrons tethered with carboxybetaine for the mild hyperthermia-controlled delivery of VEGF. Acta Biomater. 2016;40:235-42
106. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991-1003
107. Su YL, Fang JH, Liao CY, Lin CT, Li YT, Hu SH. Targeted mesoporous iron oxide nanoparticles-encapsulated perfluorohexane and a hydrophobic drug for deep tumor penetration and therapy. Theranostics. 2015;5:1233-48
108. Carregal-Romero S, Guardia P, Yu X, Hartmann R, Pellegrino T, Parak WJ. Magnetically triggered release of molecular cargo from iron oxide nanoparticle loaded microcapsules. Nanoscale. 2015;7:570-6
109. Stocke NA, Sethi P, Jyoti A, Chan R, Arnold SM, Hilt JZ. et al. Toxicity evaluation of magnetic hyperthermia induced by remote actuation of magnetic nanoparticles in 3D micrometastasic tumor tissue analogs for triple negative breast cancer. Biomaterials. 2017;120:115-25
110. Hayashi K, Sato Y, Sakamoto W, Yogo T. Theranostic nanoparticles for MRI-guided thermochemotherapy: “Tight” clustering of magnetic nanoparticles boosts relaxivity and heat-generation power. ACS Biomate Sci Eng. 2016;3:95-105
111. Cho MH, Lee EJ, Son M, Lee JH, Yoo D, Kim JW. et al. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat Mater. 2012;11:1038-43
112. Yu J, Yin WY, Zheng XP, Tian G, Zhang X, Bao T. et al. Smart MoS2/Fe3O4 nanotheranostic for magnetically targeted photothermal therapy guided by magnetic resonance/photoacoustic imaging. Theranostics. 2015;5:931-45
113. Jing XN, Zhi Z, Wang DQ, Liu J, Shao YP, Meng LJ. Multifunctional nanoflowers for simultaneous multimodal imaging and high-sensitivity chemo-photothermal treatment. Bioconjugate Chem. 2018;29:559-70
114. Schleich N, Po C, Jacobs D, Ucakar B, Gallez B, Danhier F. et al. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J Control Release. 2014;194:82-91
115. Wang H, Cao GX, Gai Z, Hong KL, Banerjee P, Zhou SQ. Magnetic/NIR-responsive drug carrier, multicolor cell imaging, and enhanced photothermal therapy of gold capped magnetite-fluorescent carbon hybrid nanoparticles. Nanoscale. 2015;7:7885-95
116. Hayashi K, Nakamura M, Miki H, Ozaki S, Abe M, Matsumoto T. et al. Magnetically responsive smart nanoparticles for cancer treatment with a combination of magnetic hyperthermia and remote-control drug release. Theranostics. 2014;4:834-44
117. Yan L, Li X. Biodegradable stimuli-responsive polymeric micelles for treatment of malignancy. Curr Pharm Biotechnol. 2016;17:227-36
118. Chen HB, Xiao L, Anraku Y, Mi P, Liu XY, Cabral H. et al. Polyion complex vesicles for photoinduced intracellular delivery of amphiphilic photosensitizer. J Am Chem Soc. 2014;136:157-63
119. Nomoto T, Fukushima S, Kumagai M, Machitani K, Arnida, Matsumoto Y. et al. Three-layered polyplex micelle as a multifunctional nanocarrier platform for light-induced systemic gene transfer. Nat Commun. 2014;5:3545
120. Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y. et al. Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat Mater. 2005;4:934-41
121. Tong R, Hemmati HD, Langer R, Kohane DS. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J Am Chem Soc. 2012;134:8848-55
122. Jin CS, Lovell JF, Chen J, Zheng G. Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano. 2013;7:2541-50
123. Yan B, Boyer JC, Branda NR, Zhao Y. Near-infrared light-triggered dissociation of block copolymer micelles using upconverting nanoparticles. J Am Chem Soc. 2011;133:19714-7
124. Yen HC, Cabral H, Mi P, Toh K, Matsumoto Y, Liu X. et al. Light-induced cytosolic activation of reduction-sensitive camptothecin-loaded polymeric micelles for spatiotemporally controlled in vivo chemotherapy. ACS Nano. 2014;8:11591-602
125. Boyer JC, Carling CJ, Gates BD, Branda NR. Two-way photoswitching ssing one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity. J Am Chem Soc. 2010;132:15766-72
126. Carling CJ, Boyer JC, Branda NR. Remote-control photoswitching using NIR light. J Am Chem Soc. 2009;131:10838-9
127. Ghoroghchian PP, Frail PR, Susumu K, Blessington D, Brannan AK, Bates FS. et al. Near-infrared-emissive polymersomes: Self-assembled soft matter for in vivo optical imaging. P Natl Acad Sci USA. 2005;102:2922-7
128. Qian C, Feng P, Yu J, Chen Y, Hu Q, Sun W. et al. Anaerobe-inspired anticancer nanovesicles. Angew Chem Int Ed. 2017;56:2588-93
129. Luo D, Carter KA, Razi A, Geng J, Shao S, Giraldo D. et al. Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release. Biomaterials. 2016;75:193-202
130. Luo D, Li N, Carter KA, Lin C, Geng J, Shao S. et al. Rapid light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin-phospholipids. Small. 2016;12:3039-47
131. Khatun Z, Nurunnabi M, Nafiujjaman M, Reeck GR, Khan HA, Cho KJ. et al. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale. 2015;7:10680-9
132. Yang G, Sun X, Liu J, Feng L, Liu Z. Light-responsive, Singlet-oxygen-triggered on-demand drug release from photosensitizer-doped mesoporous silica nanorods for cancer combination therapy. Adv Funct Mater. 2016;26:4722-32
133. Li H, Yang X, Zhou Z, Wang K, Li C, Qiao H. et al. Near-infrared light-triggered drug release from a multiple lipid carrier complex using an all-in-one strategy. J Control Release. 2017;261:126-37
134. Zhang W, Wang F, Wang Y, Wang J, Yu Y, Guo S. et al. pH and near-infrared light dual-stimuli responsive drug delivery using DNA-conjugated gold nanorods for effective treatment of multidrug resistant cancer cells. J Control Release. 2016;232:9-19
135. Zhao YL, Stoddart JF. Azobenzene-based light-responsive hydrogel system. Langmuir. 2009;25:8442-6
136. Wang DQ, Ren YB, Shao YP, Yu DM, Meng LJ. Facile preparation of doxorubicin-loaded and folic acid-conjugated carbon nanotubes@poly(N-vinyl pyrrole) for targeted synergistic chemo photothermal cancer treatment. Bioconjugate Chem. 2017;28:2815-22
137. Wang DQ, Hou C, Meng LJ, Long JG, Jing JG, Dang DF. et al. Stepwise growth of gold coated cancer targeting carbon nanotubes for the precise delivery of doxorubicin combined with photothermal therapy. J Mater Chem B. 2017;5:1380-7
138. Zhang XK, Meng LJ, Lu QH, Fei ZF, Dyson PJ. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 2009;30:6041-7
139. Wang SG, Chen Y, Li X, Gao W, Zhang LL, Liu J. et al. Injectable 2D MoS2-integrated drug delivering implant for highly efficient NIR-triggered synergistic tumor hyperthermia. Adv Mater. 2015;27:7117-22
140. Yang GB, Gong H, Liu T, Sun XQ, Cheng L, Liu Z. Two-dimensional magnetic WS2@Fe3O4 nanocomposite with mesoporous silica coating for drug delivery and imaging-guided therapy of cancer. Biomaterials. 2015;60:62-71
141. Zhao Y. Light-responsive block copolymer micelles. Macromolecules. 2012;45:3647-57
142. Moon H, Kumar D, Kim H, Sim C, Chang JH, Kim JM. et al. Amplified photoacoustic performance and enhanced photothermal stability of reduced graphene oxide coated gold nanorods for sensitive photoacoustic imaging. ACS Nano. 2015;9:2711-9
143. Huang P, Rong P, Lin J, Li W, Yan X, Zhang MG. et al. Triphase interface synthesis of plasmonic gold bellflowers as near-infrared light mediated acoustic and thermal theranostics. J Am Chem Soc. 2014;136:8307-13
144. Huang P, Rong P, Jin A, Yan X, Zhang MG, Lin J. et al. Dye-loaded ferritin nanocages for multimodal imaging and photothermal therapy. Adv Mater. 2014;26:6401-8
145. Lin J, Wang M, Hu H, Yang X, Wen B, Wang Z. et al. Multimodal-imaging-guided cancer phototherapy by versatile biomimetic theranostics with UV and gamma-irradiation protection. Adv Mater. 2016;28:3273-9
146. Wang S, Lin J, Wang Z, Zhou Z, Bai R, Lu N. et al. Core-satellite polydopamine-gadolinium-metallofullerene nanotheranostics for multimodal imaging guided combination cancer therapy. Adv Mater. 2017;29:1701013
147. Fan W, Lu N, Xu C, Liu Y, Lin J, Wang S. et al. Enhanced afterglow performance of persistent luminescence implants for efficient repeatable photodynamic therapy. ACS Nano. 2017;11:5864-72
148. Li X, Gao M, Xin K, Zhang L, Ding D, Kong D. et al. Singlet oxygen-responsive micelles for enhanced photodynamic therapy. J Control Release. 2017;260:12-21
149. Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45:6597-626
150. Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L. et al. Biomineralization-inspired synthesis of copper sulfide-ferritin nanocages as cancer theranostics. ACS Nano. 2016;10:3453-60
151. Tong R, Chiang HH, Kohane DS. Photoswitchable nanoparticles for in vivo cancer chemotherapy. P Natl Acad Sci USA. 2013;110:19048-53
152. Jin H, Zheng Y, Liu Y, Cheng H, Zhou Y, Yan D. Reversible and large-scale cytomimetic vesicle aggregation: light-responsive host-guest interactions. Angew Chem Int Ed. 2011;50:10352-6
153. Timko BP, Arruebo M, Shankarappa SA, McAlvin JB, Okonkwo OS, Mizrahi B. et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc Natl Acad Sci U S A. 2014;111:1349-54
154. Wang S, Lin J, Wang T, Chen X, Huang P. Recent advances in photoacoustic imaging for deep-tissue biomedical applications. Theranostics. 2016;6:2394-413
155. Wang J, Liu Y, Ma Y, Sun C, Tao W, Wang Y. et al. NIR-activated supersensitive drug release using nanoparticles with a flow core. Adv Funct Mater. 2016;26:7516-25
156. Lv R, Yang P, He F, Gai S, Yang G, Dai Y. et al. An imaging-guided platform for synergistic photodynamic/photothermal/chemo-therapy with pH/temperature-responsive drug release. Biomaterials. 2015;63:115-27
157. Lin G, Zhang Y, Zhu C, Chu C, Shi Y, Pang X. et al. Photo-excitable hybrid nanocomposites for image-guided photo/TRAIL synergistic cancer therapy. Biomaterials. 2018;176:60-70
158. Bao Y, Hu X, Song Q, Wang D, Sun Y, Zhang Z. RGD targeting, pH-sensitive hybrid micelles to overcome drug resistance in cancer cells. Nanomedicine: NBM. 2016;12:548
159. Jin H, Zhu T, Huang X, Sun M, Li H, Zhu X. et al. ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: Light-triggered size-reducing and enhanced tumor penetration. Biomaterials. 2019;211:68-80
160. Sun XQ, Wang C, Gao M, Hu AY, Liu Z. Remotely controlled red blood cell carriers for cancer targeting and near-infrared light-triggered drug release in combined photothermal-chemotherapy. Adv Funct Mater. 2015;25:2386-94
161. Qian C, Chen Y, Zhu S, Yu J, Zhang L, Feng P. et al. ATP-responsive and near-infrared-emissive nanocarriers for anticancer drug delivery and real-time imaging. Theranostics. 2016;6:1053-64
162. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177-82
163. Dong Z, Feng L, Zhu W, Sun X, Gao M, Zhao H. et al. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60-70
164. Wang S, Ni D, Yue H, Luo N, Xi X, Wang Y. et al. Exploration of antigen induced CaCO3 nanoparticles for therapeutic vaccine. Small. 2018;14:e1704272
165. Mi P, Dewi N, Yanagie H, Kokuryo D, Suzuki M, Sakurai Y. et al. Hybrid calcium phosphate-polymeric micelles incorporating gadolinium chelates for imaging-guided gadolinium neutron capture tumor therapy. ACS Nano. 2015;9:5913-21
166. Huang D, He B, Mi P. Calcium phosphate nanocarriers for drug delivery to tumors: imaging, therapy and theranostics. Biomater Sci. 2019;7:3942-60
167. Nomoto T, Fukushima S, Kumagai M, Miyazaki K, Inoue A, Mi P. et al. Calcium phosphate-based organic-inorganic hybrid nanocarriers with pH-responsive on/off switch for photodynamic therapy. Biomater Sci. 2016;4:826-838
168. Liu J, Luo Z, Zhang J, Luo T, Zhou J, Zhao X. et al. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials. 2016;83:51-65
169. Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S. et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34:414-8
170. Wang F, Wen L, Liu J, Peng W, Meng Z, Chen Q. et al. Albumin nanocomposites with MnO2/Gd2O3 motifs for precise MR imaging of acute myocardial infarction in rabbit models. Biomaterials. 2019:119614
171. Zou J, Zhang F, Zhang S, Pollack SF, Elsabahy M, Fan J. et al. Poly(ethylene oxide)-block-polyphosphoester-graft-paclitaxel conjugates with acid-labile linkages as a pH-sensitive and functional nanoscopic platform for paclitaxel delivery. Adv Healthc Mater. 2014;3:441-8
172. Pang X, Jiang Y, Xiao Q, Leung AW, Hua H, Xu C. pH-responsive polymer-drug conjugates: Design and progress. J Control Release. 2016;222:116-29
173. Quader S, Cabral H, Mochida Y, Ishii T, Liu X, Toh K. et al. Selective intracellular delivery of proteasome inhibitors through pH-sensitive polymeric micelles directed to efficient antitumor therapy. J Control Release. 2014;188:67-77
174. Li P, Sun M, Xu Z, Liu X, Zhao W, Gao W. Site-selective in situ growth-induced self-assembly of protein-polymer conjugates into pH-responsive micelles for tumor microenvironment triggered fluorescence imaging. Biomacromolecules. 2018;19:4472-9
175. Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648-54
176. Vila-Caballer M, Codolo G, Munari F, Malfanti A, Fassan M, Rugge M. et al. A pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine)-decorated liposome system for protein delivery: An application for bladder cancer treatment. J Control Release. 2016;238:31-42
177. Chen W, Meng FH, Cheng R, Zhong ZY. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: A comparative study with micelles. J Control Release. 2010;142:40-6
178. Zhan FX, Chen W, Wang ZJ, Lu WT, Cheng R, Deng C. et al. Acid-activatable prodrug nanogels for efficient intracellular doxorubicin release. Biomacromolecules. 2011;12:3612-20
179. Madhusudana Rao K, Krishna Rao KS, Ramanjaneyulu G, Ha CS. Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery. Int J Pharm. 2015;478:788-95
180. Huang K, He YH, Zhu ZH, Guo JK, Wang GL, Deng C. et al. Small, traceable, endosome-disrupting, and bioresponsive click nanogels fabricated via microfluidics for CD44-targeted cytoplasmic delivery of therapeutic proteins. ACS Appl Mater Inter. 2019;11:22171-80
181. Mingming W, Yu W, Ke H, Naimin S, Yiyun C. Tumor extracellular acidity activated idquooff-onrdquo release of bortezomib from a biocompatible dendrimer. Biomater Sci. 2015;3:480-9
182. Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z. et al. pH-sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. WIRES: Nanomed Nanobi. 2016;8:696-716
183. Deirram N, Zhang C, Kermaniyan SS, Johnston APR, Such GK. pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Comm. 2019;40:1800917
184. Kocak G, Tuncer C, Butun V. pH-Responsive polymers. Polymer Chem. 2017;8:144-76
185. Chen PP, Qiu M, Deng C, Meng FH, Zhang J, Cheng R. et al. pH-responsive chimaeric pepsomes based on asymmetric poly(ethylene glycol)-b-poly(L-leucine)-b-poly(L-glutamic acid) triblock copolymer for efficient loading and active intracellular delivery of doxorubicin hydrochloride. Biomacromolecules. 2015;16:1322-30
186. Wu W, Luo L, Wang Y, Wu Q, Dai H-B, Li J-S. et al. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8:3038-58
187. Hou SL, Chen SS, Dong Y, Gao S, Zhu BS, Lu QH. Biodegradable cyclomatrix polyphosphazene nanoparticles: a novel pH-responsive drug self-framed delivery system. ACS Appl Mater Inter. 2018;10:25983-93
188. Lee Y, Kataoka K. Biosignal-sensitive polyion complex micelles for the delivery of biopharmaceuticals. Soft Matter. 2009;5:3810-7
189. Wang W, Wu S, Wang J, Li Z, Cui H, Lin S. et al. Superoxide dismutase transcellular shuttle constructed from dendritic MOF and charge reversible protein derivatives. Chem Sci. 2019;10:4476-85
190. Lee Y, Ishii T, Kim HJ, Nishiyama N, Hayakawa Y, Itaka K. et al. Efficient delivery of bioactive antibodies into the cytoplasm of living cells by charge-conversional polyion complex micelles. Angew Chem Int Ed. 2010;49:2552-5
191. Lee Y, Ishii T, Cabral H, Kim HJ, Seo JH, Nishiyama N. et al. Charge-conversional polyionic complex micelles-efficient nanocarriers for protein delivery into cytoplasm. Angew Chem Int Ed. 2009;48:5309-12
192. Tangsangasaksri M, Takemoto H, Naito M, Maeda Y, Sueyoshi D, Kim HJ. et al. siRNA-loaded polyion complex micelle decorated with charge-conversional polymer tuned to undergo stepwise response to intra-tumoral and intra-endosomal pHs for exerting enhanced RNAi efficacy. Biomacromolecules. 2016;17:246-55
193. Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H. et al. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J Control Release. 2014;178:18-24
194. Lee Y, Miyata K, Oba M, Ishii T, Fukushima S, Han M. et al. Charge-conversion ternary polyplex with endosome disruption moiety: a technique for efficient and safe gene delivery. Angew Chem Int Ed. 2008;47:5163-6
195. Ranneh AH, Takemoto H, Sakuma S, Awaad A, Nomoto T, Mochida Y. et al. An ethylenediamine-based switch to render the polyzwitterion cationic at tumorous pH for effective tumor accumulation of coated nanomaterials. Angew Chem Int Ed. 2018;57:5057-61
196. Zhang H, Liu J, Chen Q, Mi P. Ligand-installed anti-VEGF genomic nanocarriers for effective gene therapy of primary and metastatic tumors. J Control Release. 2020;320:314-27
197. Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew Chem Int Ed. 2011;50:6109-14
198. Zhou KJ, Liu HM, Zhang SR, Huang XN, Wang YG, Huang G. et al. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J Am Chem Soc. 2012;134:7803-11
199. Wang Y, Wang C, Li Y, Huang G, Zhao T, Ma X. et al. Digitization of endocytic pH by hybrid ultra-pH-sensitive nanoprobes at single-organelle resolution. Adv Mater. 2017;29:1603794
200. Guo YJ, Muharnmad F, Guo MY, Qi WX, Sun FX, Wang AF. et al. pH-triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J Am Chem Soc. 2011;133:8778-81
201. Choi KY, Silvestre OF, Huang XL, Min KH, Howard GP, Hida N. et al. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano. 2014;8:4559-70
202. Fan B, Kang L, Chen L, Sun P, Jin M, Wang Q. et al. Systemic siRNA delivery with a dual pH-responsive and tumor-targeted nanovector for inhibiting tumor growth and spontaneous metastasis in orthotopic murine model of breast carcinoma. Theranostics. 2017;7:357-76
203. Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed. 2003;42:4640-3
204. Quader S, Liu X, Chen Y, Peng M, Chida T, Ishii T. et al. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J Control Release. 2017;258:56-66
205. Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T. et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat Nanotechnol. 2016;11:724-30
206. Zhao T, Huang G, Li Y, Yang S, Ramezani S, Lin Z. et al. A Transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat Biomed Eng. 2016;1:0006
207. Gao GH, Im GH, Kim MS, Lee JW, Yang J, Jeon H. et al. Magnetite-nanoparticle-encapsulated pH-responsive polymeric micelle as an MRI probe for detecting acidic pathologic areas. Small. 2010;6:1201-4
208. Mi P, Kokuryo D, Cabral H, Kumagai M, Nomoto T, Aoki I. et al. Hydrothermally synthesized PEGylated calcium phosphate nanoparticles incorporating Gd-DTPA for contrast enhanced MRI diagnosis of solid tumors. J Control Release. 2014;174:63-71
209. Li HJ, Du JZ, Du XJ, Xu CF, Sun CY, Wang HX. et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Natl Acad Sci U S A. 2016;113:4164-9
210. Sun QH, Zhou ZX, Qiu NS, Shen YQ. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628
211. Li HJ, Du JZ, Liu J, Du XJ, Shen S, Zhu YH. et al. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano. 2016;10:6753-61
212. Zhang C, An T, Wang D, Wan G, Zhang M, Wang H. et al. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(beta-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J Control Release. 2016;226:193-204
213. Hung CC, Huang WC, Lin YW, Yu TW, Chen HH, Lin SC. et al. Active tumor permeation and uptake of surface charge-switchable theranostic nanoparticles for imaging-guided photothermal/chemo combinatorial therapy. Theranostics. 2016;6:302-17
214. Wang C, Cheng L, Liu YM, Wang XJ, Ma XX, Deng ZY. et al. Imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light. Adv Funct Mater. 2013;23:3077-86
215. Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325-9
216. Lee ES, Gao ZG, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J Control Release. 2008;129:228-36
217. Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465-72
218. Brown JM, William WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437-47
219. Liu Y, Jiang Y, Zhang M, Tang Z, He M, Bu W. Modulating hypoxia via nanomaterials chemistry for efficient treatment of solid tumors. Acc Chem Res. 2018;51:2502-11
220. Liu HM, Zhang YF, Xie YD, Cai YF, Li BY, Li W. et al. Hypoxia-responsive ionizable liposome delivery siRNA for glioma therapy. Int J Nanomedicine. 2017;12:1065-83
221. Im S, Lee J, Park D, Park A, Kim YM, Kim WJ. Hypoxia-triggered transforming immunomodulator for cancer immunotherapy via photodynamically enhanced antigen presentation of dendritic cell. ACS Nano. 2019;13:476-88
222. Poon Z, Chang D, Zhao XY, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5:4284-92
223. Zhen J, Tian S, Liu Q, Zheng C, Zhang Z, Ding Y. et al. Nanocarriers responsive to a hypoxia gradient facilitate enhanced tumor penetration and improved anti-tumor efficacy. Biomater Sci. 2019;7:2986-95
224. Kulkarni P, Haldar MK, Karandish F, Confeld M, Hossain R, Borowicz P. et al. Tissue-penetrating, hypoxia-responsive echogenic polymersomes for drug delivery to solid tumors. Chem Eur J. 2018;24:12490-4
225. Yang G, Phua SZF, Lim WQ, Zhang R, Feng L, Liu G. et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv Mater. 2019 e1901513
226. Li SY, Cheng H, Qiu WX, Zhang L, Wan SS, Zeng JY. et al. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials. 2017;142:149-61
227. Zhang G, Palmer GM, Dewhirst MW, Fraser CL. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat Mater. 2009;8:747-51
228. Zheng X, Tang H, Xie C, Zhang J, Wu W, Jiang X. Tracking cancer metastasis in vivo by using an iridium-based hypoxia-activated optical oxygen nanosensor. Angew Chem Int Ed. 2015;54:8094-9
229. Liu J, Liu Y, Bu W, Bu J, Sun Y, Du J. et al. Ultrasensitive nanosensors based on upconversion nanoparticles for selective hypoxia imaging in vivo upon near-infrared excitation. J Am Chem Soc. 2014;136:9701-9
230. Feng L, Cheng L, Dong Z, Tao D, Barnhart TE, Cai W. et al. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano. 2017;11:927-37
231. Yuan P, Zhang H, Qian L, Mao X, Du S, Yu C. et al. Intracellular delivery of functional native antibodies under hypoxic conditions by using a biodegradable silica nanoquencher. Angew Chem Int Ed. 2017;56:12481-5
232. Wang YZ, Xie Y, Li J, Peng ZH, Sheinin Y, Zhou JP. et al. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano. 2017;11:2227-38
233. Qian C, Yu J, Chen Y, Hu Q, Xiao X, Sun W. et al. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy. Adv Mater. 2016;28:3313-20
234. Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed. 2014;53:3362-6
235. Perche F, Biswas S, Patel NR, Torchilin VP. Hypoxia-responsive copolymer for siRNA delivery. Methods Mol Biol. 2016;1372:139-62
236. Yin W, Qiang M, Ke W, Han Y, Mukerabigwi JF, Ge Z. Hypoxia-responsive block copolymer radiosensitizers as anticancer drug nanocarriers for enhanced chemoradiotherapy of bulky solid tumors. Biomaterials. 2018;181:360-71
237. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891-9
238. Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S. et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735-43
239. Ahmad Z, Lv S, Tang Z, Shah A, Chen X. Methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for potential hypoxia-responsive delivery of doxorubicin. J Biomater Sci Polym. 2016;27:40-54
240. Tseng SJ, Kempson IM, Huang KY, Li HJ, Fa YC, Ho YC. et al. Targeting tumor microenvironment by bioreduction-activated nanoparticles for light-triggered virotherapy. ACS Nano. 2018;12:9894-902
241. He H, Zhu R, Sun W, Cai K, Chen Y, Yin L. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale. 2018;10:2856-65
242. Hua L, Wang Z, Zhao L, Mao H, Wang G, Zhang K. et al. Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy. Theranostics. 2018;8:5088-105
243. Li Y, Lu A, Long M, Cui L, Chen Z, Zhu L. Nitroimidazole derivative incorporated liposomes for hypoxia-triggered drug delivery and enhanced therapeutic efficacy in patient-derived tumor xenografts. Acta Biomater. 2019;83:334-48
244. Son S, Rao NV, Ko H, Shin S, Jeon J, Han HS. et al. Carboxymethyl dextran-based hypoxia-responsive nanoparticles for doxorubicin delivery. Int J Biol Macromol. 2018;110:399-405
245. Wang W, Lin L, Ma X, Wang B, Liu S, Yan X. et al. Light-induced hypoxia-triggered living nanocarriers for synergistic cancer therapy. ACS Appl Mater Inter. 2018;10:19398-407
246. Zhang X, Wu M, Li J, Lan S, Zeng Y, Liu X. et al. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemo-therapy. ACS Appl Mater Inter. 2018;10:21909-19
247. Zheng X, Mao H, Huo D, Wu W, Liu B, Jiang X. Successively activatable ultrasensitive probe for imaging tumour acidity and hypoxia. Nat Biomed Eng. 2017;1:0057
248. Zhang M, Ye JJ, Xia Y, Wang ZY, Li CX, Wang XS. et al. Platelet-mimicking biotaxis targeting vasculature-disrupted tumors for cascade amplification of hypoxia-sensitive therapy. ACS Nano. 2019;13:14230-40
249. Shen L, Huang Y, Chen D, Qiu F, Ma C, Jin X. et al. pH-responsive aerobic nanoparticles for effective photodynamic therapy. Theranostics. 2017;7:4537-50
250. Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N. et al. Facile, template-free synthesis of stimuli-responsive polymer nanocapsules for targeted drug delivery. Angew Chem Int Ed. 2010;49:4405-8
251. Luo Z, Cai KY, Hu Y, Zhao L, Liu P, Duan L. et al. Mesoporous silica nanoparticles end-capped with collagen: redox-responsive nanoreservoirs for targeted drug delivery. Angew Chem Int Ed. 2011;50:640-3
252. Mi P, Yanagie H, Dewi N, Yen HC, Liu X, Suzuki M. et al. Block copolymer-boron cluster conjugate for effective boron neutron capture therapy of solid tumors. J Control Release. 2017;254:1-9
253. Zou Y, Meng FH, Deng C, Zhong ZY. Robust, tumor-homing and redox-sensitive polymersomal doxorubicin: a superior alternative to Doxil and Caelyx? J Control Release. 2016;239:149-58
254. Chang Y, Yang K, Wei P, Huang S, Pei Y, Zhao W. et al. Cationic vesicles based on amphiphilic pillar [5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed. 2014;53:13126-30
255. Liu J, Ai X, Zhang H, Zhuo W, Mi P. Polymeric micelles with endosome escape and redox-responsive functions for enhanced intracellular drug delivery. J Biomed Nanotechnol. 2019;15:373-81
256. Sun H, Meng F, Cheng R, Deng C, Zhong Z. Reduction-responsive polymeric micelles and vesicles for triggered intracellular drug release. Antioxid Redox Signal. 2014;21:755-67
257. Liu H, Wang R, Wei J, Cheng C, Zheng Y, Pan Y. et al. Conformation-directed micelle-to-vesicle transition of cholesterol-decorated polypeptide triggered by oxidation. J Am Chem Soc. 2018;140:6604-10
258. Elkassih SA, Kos P, Xiong H, Siegwart DJ. Degradable redox-responsive disulfide-based nanogel drug carriers via dithiol oxidation polymerization. Biomater Sci. 2019;7:607-17
259. Kim HJ, Takemoto H, Yi Y, Zheng M, Maeda Y, Chaya H. et al. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano. 2014;8:8979-91
260. Qiu L, Zhao L, Xing C, Zhan Y. Redox-responsive polymer prodrug/AgNPs hybrid nanoparticles for drug delivery. Chinese Chemical Letters. 2018;29:301-4
261. Guo X, Cheng Y, Zhao X, Luo Y, Chen J, Yuan WE. Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnology. 2018;16:74
262. Xu HP, Cao W, Zhang X. Selenium-containing polymers: promising biomaterials for controlled release and enzyme mimics. Acc Chem Res. 2013;46:1647-58
263. Ma N, Li Y, Xu H, Wang Z, Zhang X. Dual redox responsive assemblies formed from diselenide block copolymers. J Am Chem Soc. 2010;132:442-3
264. Li J, Ke W, Wang L, Huang M, Yin W, Zhang P. et al. Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy. J Control Release. 2016;225:64-74
265. Lin LS, Huang T, Song J, Ou XY, Wang Z, Deng H. et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J Am Chem Soc. 2019;141:9937-45
266. Chen H, Li F, Yao Y, Wang Z, Zhang Z, Tan N. Redox dual-responsive and O2 evolving theranostic nanosystem for highly selective chemotherapy against hypoxic tumors. Theranostics. 2019;9:90-103
267. Chen H, Tian J, He W, Guo Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J Am Chem Soc. 2015;137:1539-47
268. Liang Y, Li S, Wang X, He B, He B, Dai W. et al. A nanosystem of amphiphilic oligopeptide-drug conjugate actualizing both alphavbeta3 targeting and reduction-triggered release for maytansinoid. Theranostics. 2017;7:3306-18
269. Chen J, Zou Y, Deng C, Meng FH, Zhang J, Zhong ZY. Multifunctional click hyaluronic acid nanogels for targeted protein delivery and effective cancer treatment in vivo. Chem Mater. 2016;28:8792-9
270. Zhong YN, Yang WJ, Sun HL, Cheng R, Meng FH, Deng C. et al. Ligand-directed reduction-sensitive shell-sheddable biodegradable micelles actively deliver doxorubicin into the nuclei of target cancer cells. Biomacromolecules. 2013;14:3723-30
271. Christie RJ, Matsumoto Y, Miyata K, Nomoto T, Fukushima S, Osada K. et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano. 2012;6:5174-89
272. Wu LL, Zou Y, Deng C, Cheng R, Meng FH, Zhong ZY. Intracellular release of doxorubicin from core-crosslinked polypeptide micelles triggered by both pH and reduction conditions. Biomaterials. 2013;34:5262-72
273. Oe Y, Christie RJ, Naito M, Low SA, Fukushima S, Toh K. et al. Actively-targeted polyion complex micelles stabilized by cholesterol and disulfide cross-linking for systemic delivery of siRNA to solid tumors. Biomaterials. 2014;35:7887-95
274. Hu X, Hu J, Tian J, Ge Z, Zhang G, Luo K. et al. Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J Am Chem Soc. 2013;135:17617-29
275. Li Z-Y, Hu J-J, Xu Q, Chen S, Jia H-Z, Sun Y-X. et al. A redox-responsive drug delivery system based on RGD containing peptide-capped mesoporous silica nanoparticles. J Mater Chem B. 2015;3:39-44
276. Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater. 2004;3:183-9
277. Li Y, Li Y, Zhang X, Xu X, Zhang Z, Hu C. et al. Supramolecular PEGylated dendritic systems as pH/redox dual-responsive theranostic nanoplatforms for platinum drug delivery and NIR imaging. Theranostics. 2016;6:1293-305
278. He X, Zhang J, Li C, Zhang Y, Lu Y, Zhang Y. et al. Enhanced bioreduction-responsive diselenide-based dimeric prodrug nanoparticles for triple negative breast cancer therapy. Theranostics. 2018;8:4884-97
279. Wang DQ, Ren YB, Shao YP, Meng LJ. Multifunctional polyphosphazene-coated multi-walled carbon nanotubes for the synergistic treatment of redox-responsive chemotherapy and effective photothermal therapy. Polymer Chem. 2017;8:6938-42
280. Hu JM, Zhang GQ, Liu SY. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev. 2012;41:5933-49
281. Chandrawati R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp Biol Med. 2016;241:972-9
282. Liu J, Zhang B, Luo Z, Ding X, Li J, Dai L. et al. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale. 2015;7:3614-26
283. Zhu Y, Meng W, Gao H, Hanagata N. Hollow mesoporous silica/Poly(l-lysine) particles for codelivery of drug and gene with enzyme-triggered release property. J Phys Chem C. 2011;115:13630-6
284. Zhang C, Pan D, Li J, Hu J, Bains A, Guys N. et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017;55:153-62
285. Gallo J, Kamaly N, Lavdas I, Stevens E, Nguyen QD, Wylezinska-Arridge M. et al. CXCR4-targeted and MMP-responsive iron oxide nanoparticles for enhanced magnetic resonance imaging. Angew Chem Int Ed. 2014;53:9550-4
286. Huang J, Shu Q, Wang L, Wu H, Wang AY, Mao H. Layer-by-layer assembled milk protein coated magnetic nanoparticle enabled oral drug delivery with high stability in stomach and enzyme-responsive release in small intestine. Biomaterials. 2015;39:105-13
287. Gu XL, Wei YH, Fan QY, Sun HL, Cheng R, Zhong ZY. et al. cRGD-decorated biodegradable polytyrosine nanoparticles for robust encapsulation and targeted delivery of doxorubicin to colorectal cancer in vivo. J Control Release. 2019;301:110-8
288. Hu Q, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014;6:12273-86
289. Fu H, Shi K, Hu G, Yang Y, Kuang Q, Lu L. et al. Tumor-targeted paclitaxel delivery and enhanced penetration using TAT-decorated liposomes comprising redox-responsive poly(ethylene glycol). J Pharm Sci. 2015;104:1160-73
290. Hu QY, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014;6:12273-86
291. Van den Mooter G, Samyn C, Kinget R. The relation between swelling properties and enzymatic degradation of azo polymers designed for colon-specific drug delivery. Pharm Res. 1994;11:1737-41
292. Callmann CE, Barback CV, Thompson MP, Hall DJ, Mattrey RF, Gianneschi NC. Therapeutic enzyme-responsive nanoparticles for targeted delivery and accumulation in tumors. Adv Mater. 2015;27:4611-5
293. Liu Y, Ding X, Li J, Luo Z, Hu Y, Liu J. et al. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology. 2015;26:145102
294. Nguyen MM, Carlini AS, Chien MP, Sonnenberg S, Luo C, Braden RL. et al. Enzym-responsive nanoparticles for targeted accumulation and prolonged retention in heart tissue after myocardial infarction. Adv Mater. 2015;27:5547-52
295. Xin X, Teng C, Du X, Lv Y, Xiao Q, Wu Y. et al. Drug-delivering-drug platform-mediated potent protein therapeutics via a non-endo-lysosomal route. Theranostics. 2018;8:3474-89
296. Nosrati H, Mojtahedi A, Danafar H, Kheiri Manjili H. Enzymatic stimuli-responsive methotrexate-conjugated magnetic nanoparticles for target delivery to breast cancer cells and release study in lysosomal condition. J Biomed Mater Res A. 2018;106:1646-54
297. Cai H, Wang X, Zhang H, Sun L, Pan D, Gong Q. et al. Enzyme-sensitive biodegradable and multifunctional polymeric conjugate as theranostic nanomedicine. Appl Mater Today. 2018;11:207-18
298. Zhang H, Fei J, Yan X, Wang A, Li J. Enzyme-responsive release of doxorubicin from monodisperse dipeptide-based nanocarriers for highly efficient cancer treatment in vitro. Adv Funct Mater. 2015;25:1193-204
299. Hou X-F, Chen Y, Liu Y. Enzyme-responsive protein/polysaccharide supramolecular nanoparticles. Soft Matter. 2015;11:2488-93
300. Jiang J, Shen N, Ci T, Tang Z, Gu Z, Li G. et al. Combretastatin A4 Nanodrug-Induced MMP9 Amplification Boosts Tumor-Selective Release of Doxorubicin Prodrug. Adv Mater. 2019;31:e1904278
301. Andresen TL, Davidsen J, Begtrup M, Mouritsen OG, Jorgensen K. Enzymatic release of antitumor ether lipids by specific phospholipase A2 activation of liposome-forming prodrugs. J Med Chem. 2004;47:1694-703
302. Hou Y, Zhou J, Gao Z, Sun X, Liu C, Shangguan D. et al. Protease-activated ratiometric fluorescent probe for pH mapping of malignant tumors. ACS Nano. 2015;9:3199-205
303. Rao J, Khan A. Enzyme sensitive synthetic polymer micelles based on the azobenzene motif. J Am Chem Soc. 2013;135:14056-9
304. Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W. et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108:2426-31
305. Huang P, Gao Y, Lin J, Hu H, Liao HS, Yan X. et al. Tumor-specific formation of enzyme-instructed supramolecular self-assemblies as cancer theranostics. ACS Nano. 2015;9:9517-27
306. Yingyuad P, Mevel M, Prata C, Furegati S, Kontogiorgis C, Thanou M. et al. Enzyme-triggered PEGylated pDNA-nanoparticles for controlled release of pDNA in tumors. Bioconjug Chem. 2013;24:343-62
307. Gu G, Xia H, Hu Q, Liu Z, Jiang M, Kang T. et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34:196-208
308. Gunawan ST, Kempe K, Bonnard T, Cui J, Alt K, Law LS. et al. Multifunctional thrombin-activatable polymer capsules for specific targeting to activated platelets. Adv Mater. 2015;27:5153-7
309. Byrne M, Thornton PD, Cryan S-A, Heise A. Star polypeptides by NCA polymerisation from dendritic initiators: synthesis and enzyme controlled payload release. Polymer Chem. 2012:3
310. Xing Y, Wang C, Han P, Wang Z, Zhang X. Acetylcholinesterase responsive polymeric supra-amphiphiles for controlled self-assembly and disassembly. Langmuir. 2012;28:6032-6
311. Datta LP, Chatterjee A, Acharya K, De P, Das M. Enzyme responsive nucleotide functionalized silver nanoparticles with effective antimicrobial and anticancer activity. New J Chem. 2017;41:1538-48
312. Li Y, Hu H, Zhou Q, Ao Y, Xiao C, Wan J. et al. alpha-Amylase- and Redox-Responsive Nanoparticles for Tumor-Targeted Drug Delivery. ACS Appl Mater Inter. 2017;9:19215-30
313. Renoux B, Raes F, Legigan T, Peraudeau E, Eddhif B, Poinot P. et al. Targeting the tumour microenvironment with an enzyme-responsive drug delivery system for the efficient therapy of breast and pancreatic cancers. Chem Sci. 2017;8:3427-33
314. Zhou M, Wei W, Chen X, Xu X, Zhang X, Zhang X. pH and redox dual responsive carrier-free anticancer drug nanoparticles for targeted delivery and synergistic therapy. Nanomedicine. 2019
315. Li J, Meng X, Deng J, Lu D, Zhang X, Chen Y. et al. Multifunctional micelles dually responsive to hypoxia and singlet oxygen: enhanced photodynamic therapy via interactively triggered photosensitizer delivery. ACS Appl Mater Inter. 2018;10:17117-28
316. Han H, Valdepérez D, Jin Q, Yang B, Li Z, Wu Y. et al. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano. 2017;11:1281-91
317. Zhu R, He H, Liu Y, Cao D, Yan J, Duan S. et al. Cancer-selective bioreductive chemotherapy mediated by dual hypoxia-responsive nanomedicine upon photodynamic therapy-induced hypoxia aggravation. Biomacromolecules. 2019;20:2649-56
318. Du JZ, Du XJ, Mao CQ, Wang J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc. 2011;133:17560-3
319. Lu J, Chen Q, Ding X, Wen J, Zhang Y, Li H. et al. BSA modified, disulfide-bridged mesoporous silica with low biotoxicity for dual-responsive drug delivery. Micropor Mesopor Mater. 2019;278:257-66
320. Zhao X, Yang CX, Chen LG, Yan XP. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat Commun. 2017;8:14998
321. Wu MX, Gao J, Wang F, Yang J, Song N, Jin XY. et al. Multistimuli responsive core-shell nanoplatform constructed from Fe3O4@MOF equipped with pillar [6]arene nanovalves. Small. 2018;14:1704440
322. Zhou Q, Shao S, Wang J, Xu C, Xiang J, Piao Y. et al. Enzyme-activatable polymer-drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019;14:799-809
323. Fang S, Lin J, Li C, Huang P, Hou W, Zhang C. et al. Dual-stimuli responsive nanotheranostics for multimodal imaging guided trimodal synergistic therapy. Small. 2017;13:1602580
324. Jing XN, Zhi Z, Jin LM, Wang F, Wu YS, Wang DQ. et al. pH/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale. 2019;11:9457-67
325. Meng LJ, Xu CQ, Liu TH, Li H, Lu QH, Long JG. One-pot synthesis of highly cross-linked fluorescent polyphosphazene nanoparticles for cell imaging. Polymer Chem. 2015;6:3155-63
326. Sun LJ, Liu TH, Li H, Yang L, Meng LJ, Lu QH. et al. Fluorescent and cross-linked organic-inorganic hybrid nanoshells for monitoring drug delivery. ACS Appl Mater Inter. 2015;7:4990-7
327. Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465-76
328. Takahashi A, Yamamoto Y, Yasunaga M, Koga Y, Kuroda J, Takigahira M. et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013;104:920-5
329. Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med. 2015;7:314ra183
330. Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F. et al. Nanomedicine and macroscale materials in immuno-oncology. Chem Soc Rev. 2019;48:351-81
331. Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52:1543-54
Author contact
![]() Corresponding author: Prof. P. Mi, E-mail: miedu.cn
Corresponding author: Prof. P. Mi, E-mail: miedu.cn
 Global reach, higher impact
Global reach, higher impact