13.3
Impact Factor
Theranostics 2020; 10(11):4839-4850. doi:10.7150/thno.43771 This issue Cite
Review
The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach
1. Department of Oral and Maxillofacial-Head & Neck Oncology, Shanghai Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine; National Clinical Research Center for Oral Diseases; Shanghai Key Laboratory of Stomatology & Shanghai Research Institute of Stomatology, Shanghai, China
2. Department of Dentistry, Government Medical College & Hospital, Chandigarh, India
*Contributed equally
Received 2020-1-8; Accepted 2020-3-12; Published 2020-3-26
Abstract
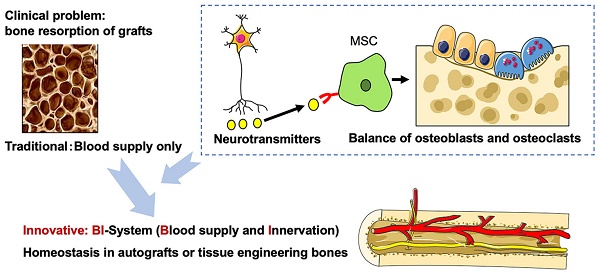
Large bone reconstruction is a major clinical issue associated with several challenges, and autograft is the main method for reconstructing large defects of maxillofacial bone. However, postoperative osteoporosis of the bone graft, even with sufficient vascularization, remains a primary problem. Therefore, better understanding of the mechanisms and clinical translation of bone homeostasis is required. Neuronal innervation of the bone is an emerging research topic, especially with regards to the role of peripheral nerves in regulating bone homeostasis. Moreover, sensory and autonomic nerves regulate this process via different types of neurotransmitters, but the specific mechanism is still elusive. In this review article, the current understanding of the interaction between the peripheral nerve and the skeleton system is summarized, with a particular focus on bone marrow mesenchymal stem cells (BMMSCs), except for osteoblasts and osteoclasts. The novel application of nerve-based bone regeneration via BMMSCs may provide a new strategy in tissue engineering and clinical treatment of osteoporosis and bone disorders.
Keywords: bone marrow mesenchymal stem cell, bone homeostasis, peripheral nerves, bone regeneration, bone graft
Introduction
Large defects of the maxillofacial bone caused by tumors, trauma and congenital malformation, etc., seriously affect the appearance and function of the patient. Moreover, functional reconstruction is clinically difficult, but is highly important. Tissue engineering is a promising technology, with previous cutting-edge studies showing the importance of advanced scaffold materials with controlled release or molecularly imprinted intelligence for tissue regeneration or cancer treatment [1-6], using biomaterial alignment and optimized mechanical stimulation driven by the differentiation of mesenchymal stem cells (MSCs) [7]. Autograft remains the main method for reconstructing large continuous defects of the maxillofacial bone, but severe postoperative osteoporosis can be found after non-vascularized bone grafts [8-11]. Although the effect of free vascularized bone grafts is higher compared with non-vascularized bone grafts, the postoperative spontaneous osteoporosis of the bone graft is severe and hinders the success of dental implants, even after microsurgical vascularization [8, 11, 12]. Currently, there is no effective preventive method and the underlying mechanism remains unknown. Hence, this has become a focus of the reparative and reconstructive surgery research field.
Based on the fact that postoperative osteoporosis, despite a sufficient blood supply, inevitably develops in autografted bones indicates that there may be systemic factors other than just blood supply that control the internal environment of the bone graft. As the blood supply of bone is accompanied by innervation, it has been hypothesized that nerves play an important role in regulating the bone homeostasis. Previous studies have revealed that sympathetic nerves and sensory nerves affect bone metabolism and bone remodeling via certain neurotransmitters [13-16]. Furthermore, it has been demonstrated that the peripheral nervous system is involved in bone metabolism, osteogenic differentiation, bone mineralization and bone remodeling. Our previous studies have demonstrated that the sympathetic nervous system (SNS) inhibits bone remodeling by inhibiting bone marrow mesenchymal stem cells (BMMSCs), as well as indicating that, Nerve Growth Factor (NGF) and Substance P (SP) can promote bone formation via sensory nerves [17-20]. Therefore, based on the results of animal experiments and necropsy, our research group has investigated in the development of a vascularized iliac bone grafting method with simultaneous innervation using neurorrhaphy between the nerves innervating the iliac bone and recipient site. Our clinical retrospective study suggested that this novel method significantly decreases postoperative osteoporosis and improves the success of dental implants [21]. Moreover, a previous tissue engineering study has found that in addition to vascularization, innervation may also play an important role in promoting tissue engineered bone formation [22].
Remodeling of innervation effectively prevents osteoporosis of the bone graft, suggesting that the mechanism via which innervation regulates bone homeostasis may involve BMMSCs. Both sympathetic and sensory nerves play a crucial role in regulating bone remodeling via specific neurotransmitters. In particular, sensory neurotransmitters, such as NGF, Calcitonin Gene-related Peptide (CGRP), SP, and Semaphorin 3A (Sema3A) positively regulate bone formation via BMMSCs. The present review examines how peripheral nerve regulates bone homeostasis via MSCs, which may provide a basis for translational research of systemically regulating bone remodeling, regeneration and prevention of osteoporosis.
Relationship between bone homeostasis and 'aging' BMMSCs in osteoporosis
Osteoporosis is a very common skeletal degenerative disease in the elderly and menopausal women. Moreover, it is characterized by decreased bone mineral density (BMD) and destruction of bone microarchitecture, which give rise to an increased risk of fragility fracture of by more than 40% [23, 24]. Therefore, there is thus an urgent need for novel therapies to not only reduce the risk of fracture, but also to prevent the active bone loss in early disease phases. Furthermore, it is essential to identify the mechanism of osteoporosis, which may be caused by an imbalance in bone formation and resorption [23].
Osteoporosis is related to 'bone homeostasis', which mainly refers to the relatively stable state of the intraosseous environment under the precise regulation of the system network, which should be the bone remodeling balance between osteogenic activity of osteoblasts and bone resorption activity of osteoclasts. In addition, osteoporosis occurs when the activity of osteoblasts decreases and that of osteoclasts increases, due to the disturbed balance in bone homeostasis.
Bone homeostasis is closely related to the 'aging' of BMMSCs. Friedenstein et al. [25] identified a group of cells with osteogenic potential that were derived from bone marrow, and had a morphotype similar to fibroblasts, displaying rapid adherence to tissue culture vessels. These cells are referred to as 'Mesenchymal stem cells (MSCs)' [26]. MSCs are defined as self-renewable, multipotent progenitor cells with the capacity to differentiate into several distinct mesenchymal lineages, and are uniformly positive for CD29, CD44, CD71, CD90, CD106, CD120a, CD124, amongst other surface proteins [27]. BMMSCs, the mesenchymal progenitors for both osteoblasts and adipocytes, have two features: self-renewal and a multipotent ability [28, 29]. Aging is characterized by common environmental changes, such as hormonal, immunological and metabolic disorders. Moreover, 'aged' BMMSCs refer to dysfunctional cells, with differentiations shifting toward adipogenesis rather than osteogenesis [30]. Furthermore, in an osteoporosis mouse model BMMSCs are shown to display an 'aging' phenomenon, resulting in decreased osteogenic differentiation ability, enhanced adipogenic capacity, decreased self-renewal ability and decreased ability of induced-apoptosis in osteoclasts and T cells, ultimately leading to unbalanced bone homeostasis [31-33]. Therefore, 'aging' BMMSCs may be an important factor involved in unbalanced bone graft homeostasis, and thus further examination of the promotion of osteogenesis differentiation should be considered. Moreover, the osteogenic activity of BMMSCs should be present in both autograft and tissue-engineered bones to avoid postoperative absorption.
BMMSCs, as key cells for bone regeneration and maintenance, have become a hot topic of research in recent years. Furthermore, there are ongoing clinical trials for treating non-union of the bone, osteonecrosis of the femoral head and other bone diseases by directly injecting autologous MSCs or implanting with carriers [34]. In addition, studies using stem-cell-based regeneration of bone with bone tissue engineering grafts and growth factors have been successful [34]. A previous study used this method with bone marrow aspirates and customized titanium cage culturing in latissimus dorsi muscle to regenerate mandibular bone, and the results provide a good example of ectopic bone formation [35]. Since additional surgery increases the risk of complications in the donor site, dental implants can be inserted into the new bone in in-situ bone formation with autologous adipose stem cells and β-tricalcium phosphate granules [36]. It has been demonstrated that BMMSCs promote cartilage regeneration to treat osteoarthritis in vitro [37]. Interestingly, MSC-based immunotherapy using systemic infusion has beneficial effects in patients with graft-versus-host disease [38]. Moreover, recipient glycemic micro-environments result in enhanced effects of BMMSCs therapy following BMMSCs infusion in an experimental osteopenia model [39]. In addition, systemic transplantation of stem cells from human exfoliated deciduous teeth has been used to treat systemic lupus erythematosus in mice [40]. MSCs therapies in different diseases, including heart failure and enterocutaneous perianal fistular disease have made progress, but standardizing MSC preparation, fitness and functionality remains difficult [41].
Microenvironmental regulation of BMMSCs
Stem cells are controlled by a microenvironment called the 'stem cell niche', rather than existing independently [42]. Furthermore, there has been increasing attention on how the systematic microenvironment regulates the stem cells. It has been shown that osteoblasts differentiate from BMMSCs, while osteoclasts differentiate from hematopoietic stem cells (HSCs), and both BMMSCs and HSCs coexist in the microenvironment of interacting stem cells [42]. Moreover, the interaction of BMMSCs with cells differentiated from HSCs, including T cells, natural killer cells and osteoclasts, influences bone remodeling [43]. The role of T cells in bone remodeling was first identified in 1983, where it was found that bone resorption occurred in T-cell-deficient nude mice [44]. Furthermore, in an osteoporosis model, T cells are demonstrated to induce apoptosis of BMMSCs and osteoblasts via the CD40/CD40L pathway [43]. It has also been shown that BMMSCs express Fas ligand (FASL) and can recruit activated T cells to promote apoptosis [42, 45, 46]. Moreover, cytotoxic T‑lymphocyte protein 4 (CTLA-4), which is secreted by regulatory T cells, can bind to CD80/CD86 on osteoclast precursors to promote apoptosis and reduce bone resorption [47]. Receptor activator of NF-κΒ ligand (RANKL) was first discovered to regulate T cell differentiation and is an important factor in osteoclast differentiation [48]. It has also been demonstrated that osteoprotegerin (OPG) is an osteoclast differentiation inhibitor, and serves as a decoy receptor of RANKL. Furthermore, B cells can secrete OPG to inhibit RANK-RANKL binding, which slows osteoclast differentiation and stabilize bone mass [49].
Previous studies have shown that immune cells can secrete pro-inflammatory factors that damage BMMSCs and affect tissue regeneration [50, 51]. For example, HSC-derived lymphocytes secrete interferon (IFN)-γ and tumor necrosis factor (TNF)-α to inhibit BMMSC differentiation [43, 47]. Using an ovariectomy-induced osteoporotic mice in vitro model, our previous study reported that inflammatory microenvironment can cause loss of differentiation potential of normal BMMSC and apoptosis but can also lead to a reduced ability of BMMSC to induce apoptosis of osteoclasts [32, 33, 46]. Moreover, TNF-α and IFN-γ negatively regulate the osteogenic capacity of BMMSC via the NFκB and Wnt pathways, and the alteration of these pathways affects runt-related transcription factor 2 (Runx2) and peroxisome proliferator-activated receptor γ (PPARγ), which are two key transcription factors regulating osteogenesis and adipogenesis, and thus causes stem cell aging [30, 32]. Bone marrow stromal cells (BMSCs), differentiated from BMMSCs, can either secrete RANKL, which binds RANK on osteoclast precursor cells, or secrete macrophage - colony stimulating factor (M-CSF), which binds to its receptors expressed by osteoclast precursors, thus promoting osteoclast differentiation [47].
As nerve fibers are involved in the formation of the bone marrow stem cell niche, the regulation of stem cells by the nervous system has become an increasing focus of research [42]. Furthermore, a previous study suggested a role of the sympathetic system in regulating mobilization of HSC to the stem cell niche [13]. Zhao et al. [52] first discovered the presence of a MSC niche around a neurovascular bundle in the mouse incisor model and demonstrated that the vascular nerve bundle regulates MSC homeostasis by secreting Sonic Hedgehog protein. Previous studies using tissue engineering have shown that innervation, in addition to vascularization, may also play a crucial role in promoting tissue engineered bone formation [22, 53]. Moreover, it has been revealed that adipocytes regulate various tissues by transmitting signals to local nerve fibers [54]. The study of neurotransmitters, such as leptin, CGRP, SP and Sema3A, on bone homeostasis has been increasing [55, 56], with a focus on examining, how various neurotransmitters regulate 'aging' stem cells and may reduce osteogenic capacity.
Innervation of bone
According to Hilton's rule [57], nerves innervating the muscles also innervate the attached bones. In addition, large nerve bundles accompanying blood vessels nourish bones at different locations. Therefore, the relationship between the innervation of sensory nerves and autonomic nerves and the regulation of bone homeostasis has attracted increasing attention. A large number of sensory nerve fibers, which are sensitive to mechanical stress and pain, innervate the trabecular bone and periosteum [58, 59]. Furthermore, it has been shown that these sensory nerve fibers promote osteogenesis by secreting sensory neurotransmitters, such as NGF, CGRP and SP [55, 60]; although detailed understanding of their function in different tissues remains elusive (Table 1). The autonomic nervous system is divided anatomically and functionally into two opposed arms, the SNS and the parasympathetic nervous system (PSNS). The SNS plays a crucial role in the connection between the central control and terminal effectors. Furthermore, norepinephrine (NE) is a major neurotransmitter of the SNS and inhibits bone formation by stimulating α- and β-receptors, while Neuropeptide Y (NPY) is regarded as an inhibitor of NE [55, 61]. Moreover, the parasympathetic neurotransmitter acetylcholine (ACh) plays a role in bone protection by activating muscarinic and nicotinic cholinergic receptors [61]. However, the precise distribution and density of the SNS and PSNS in bone are not fully understood. In general, preganglionic neurons are cholinergic, while postganglionic neurons are noradrenergic. In addition, sympathetic postganglionic neurons cover the majority of tissues in the body, along with major nerves that contain predominantly sensory and somatic motor nerve fibers.
Sensory and sympathetic neurotransmitters expressed in cells and tissues
| Neurotransmitters | Expressed cells | Localization | Identification | References |
|---|---|---|---|---|
| NGF | Keratinocytes Mast cells Macrophages Osteoblasts Adipocyte | Bone (osteoblasts) Periosteal sensory nerves Adipose tissue | RT-PCR; Immunocytochemistry | 54, 65, 66 |
| CGRP | Sensory afferents Peptidergic primary Sensory neurons (spinal cord) | Bone/facture callus (BMSCs, Osteoblasts, Osteoclasts) Bone periosteum Bone trabecular Adipose tissue (Sensory nerve, Adipose stem cells) Endothelium (endothelial cells) Serum | RT-PCR; Immunocytochemistry; Immunostaining | 14, 54, 56, 64, 71, 100 |
| SP | Peptidergic sensory neurons Type B cells in the DRG Osteocytes | Articular cartilage/fracture callus/OA cartilage (Chondrocytes) Bone/facture callus (BMMSCs, BMSCs, BMMs) Wound region of bone (CD29+ MSCs) Osteoblasts Mature osteoclasts Immune cells Bone (bone periosteum, bone trabecular, epiphyseal growth plate, subchondral bone, ligaments, synovium) Adipose tissue (endothelial cells, immune cells) Blood vessel Smooth muscle Immune system (T- or B-lymphocytes, monocytes, mast cells, macrophages) Gland | In situ hybridization; Immunofluorescence; Immunoenzyme; RT-PCR; Immunohistochemistry | 14, 18, 54, 56, 66, 76, 77, 101, 102 |
| Sema3A | Axon Peripheral nerves Spinal cord Activated T cells Dendritic cells Angiogenic endothelial cells Bone cell lineages (chondrocytes, osteoblasts, and osteoclasts) | Bone (bone periosteum, bone marrow) Hypertrophic chondrocytes in ossification centers Endothelial cells Serum Brain Heart Lung Liver Fat | RT-PCR; Immunocytochemistry; Cytoenzymology; Immunostaining | 84, 85, 86, 88, 103, 104 |
| NE | Noradrenergic fibers Postsynaptic sympathetic neurons | Adipose tissue (efferent nerves, adipose stem cells, T cells, Macrophages) Osteoblasts | RT-PCR; Immunocytochemistry | 54, 60 |
Abbreviations: DRG: dorsal root ganglion; NGF: nerve growth factor; CGRP, Calcitonin gene-related peptide; SP: substance P; Sema3A: Semaphorin 3A; NE: Norepinephrine; BMMSCs: bone marrow mesenchymal stem cells; BMSCs: bone marrow stromal cells; BMMs: bone marrow macrophages; RT-PCR: real-time polymerase chain reaction; OA: osteoarthritis.
Regulation of BMMSC by different nerve fibers via neurotransmitters
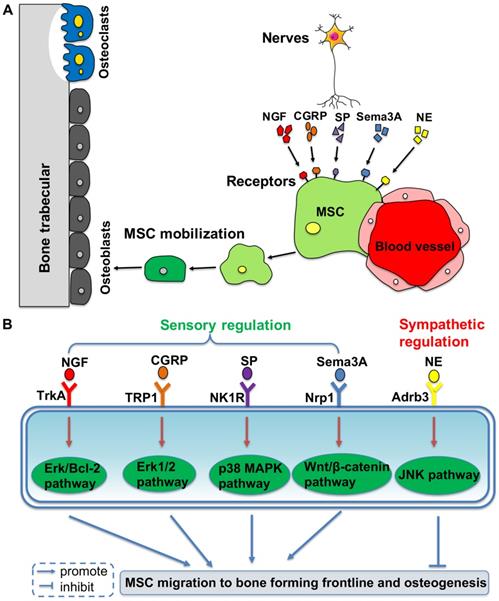
Based on the clinical discovery that patients who are paralyzed are prone to osteoporosis, the role of the nervous system in maintaining bone mass in bone metabolism has become an important research topic [62]. It has been revealed that there is a close relationship between the central nervous system and bone metabolism. Leptin from the hypothalamus is related to osteogenesis via stimulation from the sympathetic nerves and it can inhibit bone formation via the β2 receptor expressed on osteoblasts [15]. A recent study has demonstrated that leptin can also act as a physiologic signal to leptin receptor expressed by BMMSCs, thus inhibiting osteogenesis and inducing adipogenesis [63]. Moreover, sensory and sympathetic nerves promote the migration of BMMSCs to the osteogenesis front line by using different neurotransmitters, which maintains an active bone environment in osteogenesis (Figure 1).
Peripheral nerves regulate mobilization and differentiation of mesenchymal stem cells (MSCs) from their perivascular niche to frontlines of osteogenesis, via different molecular pathways. (A) Neurotransmitters binding to the membrane receptors trigger the intracellular pathways to promote or inhibit MSC migration to the bone forming sites. (B) It shows the detailed molecular pathways.
NGF
NGF is a member of the neurotrophic factor family and plays a critical role in the development of various types of nerve cells in the central and peripheral nervous systems [64]. It has been shown that NGF increases osteoblast differentiation, proliferation and activity, and is also involved in osteoclastogenesis [65, 66]. Our previous study reported that NGF can promote the recovery of mandibular sensory nerves in distraction osteogenesis (DO) and indirectly promote bone regeneration [19, 20]. In addition, our previous study found that, in the rabbit mandibular DO model, the local application of NGF during the consolidation period can accelerate wound healing to shorten this period [20]. Our previous results also suggested that using Collagen/nano-hydroxyapatite/kappacarrageenangels to inject NGF at the DO region can achieve improved bone-promoting effects [19]. Moreover, NGF promotes osteoblast differentiation and inhibits its apoptosis for bone remodeling, as well as accelerates the recovery of inferior alveolar nerve in DO [67, 68]. It has been revealed that NGF directly regulates wound repair during bone fracture healing by activating the NGF/TrkA signaling pathway, which causes load-induced nerve sprouting, particularly in the early stage [66]. A previous study also showed that NGF binding to TrkA enhances the survival and regenerative capacity of bone marrow stromal stem cells via the upregulation of the Erk/ Bcl-2 pathway [69]. Collectively, these findings suggest that NGF promotes bone regeneration and reconstruction.
CGRP
CGRP is a 37 amino acid peptide synthesized by the CGRP gene on the short arm of chromosome 11 and is widely found in the central and peripheral nervous systems [14]. In the maxillofacial region, CGRP is secreted from the trigeminal semilunar neuron and is transported to the bone and other effector tissues via the trigeminal axons, and can perform its biological effects by binding to the main receptor transient receptor potential vanilloid 1 (TRPV1). Moreover, the effect of CGRP in bone metabolism has been demonstrated in osteoporosis-phenotype CGRP-knockout mice [14]. In addition, our previous study revealed that numerous CGRP-based neuropeptides promote mobilization and osteogenic differentiation of BMMSCs in jaw regeneration [70].
CGRP can act on various intraosseous cells such as osteoblasts and osteoclasts. Furthermore, it has been shown that CGRP inhibits osteoclasts via RANKL/OPG pathways under the activation of TRPV1, resulting in reduced bone resorption in animal models with experimental periodontitis [71]. Previous studies have also revealed that CGRP receptors are expressed on osteoclasts and that CGRP can inhibit osteoclastogenesis in vivo and in vitro [72-74], especially inhibiting the differentiation of early osteoclasts [71]. The p38 signaling pathway is important for regulation of BMMSC osteogenesis, and our previous study demonstrated that jaw-derived BMMSCs mobilize to the osteogenesis front line and play an important role in osteogenesis under the effect of static strain activated p38-MAPK signaling [70, 75]. Therefore, these results provide experimental evidence for enhancing DO efficiency. Moreover, based on these findings it was speculated that the CGRP neuropeptide stops the 'aging' BMMSC microenvironment, but further studies are required to investigate the potential involvement of other pathways in which CGRP may participate.
SP
SP, a neuropeptide of the tachykinin family, is a highly conserved 11-amino acid neuropeptide involved in pain perception, and has identical protein sequences in mice, rabbits and humans [76]. SP mainly binds to the neurokinin 1 receptor (NK1R) on non-neuronal cells, including BMSCs, BMMSCs, osteoblasts and osteoclasts, and thus regulates both osteogenesis and osteoclastogenesis [77]. Furthermore, SP can stimulate osteoblast osteogenesis via NK1R in advanced bone formation. It has also been demonstrated that in skull osteoblast SP induces increased mineralization and expression levels of the bone-related proteins Runx2 and osteocalcin [78]. SP stimulation also promotes osteoclastogenesis of isolated bone marrow macrophages and bone resorption activity of mature osteoclasts [14, 78, 79]. It has been reported that RANKL-induced cytosolic free Ca2+ signaling accelerates NF-κB nuclear translocation in osteoclasts, and SP activates NF-κB in bone marrow macrophages (BMMs), which directly facilitates RANKL-induced macrophage osteoclastogenesis and bone resorption activity [77, 80]. Moreover, SP stimulates BMSCs to produce RANKL, but at concentrations that are too low to evoke osteoclastogenesis [77]. Furthermore, a lack of SP may lead to a decrease in bone resorption rate, as well as late bone formation and mineralization rate, resulting in a net bone loss due to a greater rate of bone resorption than bone formation [79, 81].
In addition, previous studies have shown that SP can stimulate the proliferation of BMMSCs and the mineralization of differentiated BMMSCs in vitro [77, 81]. It has been reported that SP facilitates the proliferation of BMMSC in a concentration-dependent manner: high concentrations of SP stimulate BMMSC proliferation and mineralization, while low concentrations of SP stimulate osteoblast differentiation at a later stage [77]. Our previous study also demonstrated that local injection of SP in a rat mandibular DO model can accelerate bone remodeling and bone maturity, and also increases MSC migration during osteogenesis [18]. Furthermore, systemic injection of SP increases the migration of CD29+ MSCs to the wound region, which accelerates bone remodeling and bone maturation by activating the Erk1/2 signaling pathway [18, 76]. Collectively, SP stimulates the migration of BMMSCs to the osteogenesis area and promotes differentiation to increase mineralization, thus leading to bone formation. However, the appropriate concentration and signaling pathways involved in the process requires further examinations. SP can also stimulate immune cells to secrete pro-inflammatory factors, and thus acts as a mediator of adipose tissue inflammation, leading to metabolic dysfunction [54].
Sema3A
Sema3A, an axon guidance molecule, belongs to the semaphorin family and is an important member of the vertebrate sensory neurotransmitter microenvironment. In addition, Sema3A is a membrane-associated secretory protein in the central nervous system, which is involved in guiding axonal and neuronal migration [82]. It has been shown that Sema3A is also involved in organogenesis and angiogenesis [83]. Fukuda et al. [84] were the first to discover the positive role of sensory nerves in bone remodeling, and reported a low bone mass phenotype in a neuron-specific Sema3A-deficient mouse model, but normal bone formation and bone mass in osteoblast-specific Sema3A-deficient mice [84]. Thus, high expression levels of Sema3A in bone may be derived from neurons, and a decrease in Sema3A expression in nerves innervating the intraosseous is a major factor in the reduction of bone formation and increase of bone resorption. Therefore, it was speculated that Sema3A may regulate bone metabolism via peripheral nerves.
Neuropilin 1 (Nrp1), the main receptor for Sema3A, is a protein encoded by the npr1 gene, which is located at 10p11.22 and plays a broad role in angiogenesis, axon guidance, cell survival, migration and invasion. Another receptor of Sema3A Plexin A1 (PlxnA1) forms a complex with Nrp1 to protect bone [85]. Furthermore, Sema3A binds to Nrp1 to mediate signal transduction, activate osteoblast differentiation, and inhibit adipocyte differentiation, osteoclast precursor migration and osteoclast differentiation [85, 86]. Nrp1 has two complement binding domains, two coagulation factor domains and one MAM domain. Moreover, Sema3A binding to the extracellular complement-binding domain causes conformational changes of PlxnA1 and activates intracellular signals to promote osteogenic differentiation [87]. It has been demonstrated that expression of Sema3A in bone participates in not only innervation but also regulation of vascular invasion, which may contribute to bone formation.
The majority of the factors regulate bone homeostasis from a single aspect, while Sema3A both inhibits bone resorption and increases bone formation to protect bone [85]. Competing with TREM2, Nrp1 binds to PlxnA1 to inhibit both osteoclast differentiation pathways ITAM and RhoA and promote the Wnt osteogenic differentiation pathway [85]. In addition, Sema3A stimulation upregulates Nrp1 expression, causing BMMSCs differentiation into osteoblasts [85]. It has also been shown that Sema3A binds to the Nrp1 receptor and specifically activates Rac1 mediated by FARP2, which leads to the accumulation of Wnt3a-activated β-catenin in the nucleus, ultimately resulting in increased osteogenic differentiation and reduced adipogenic differentiation [88]. Such increased osteogenic differentiation can be contributed by promoting osteogenesis genes such as RUNX2, whereas reduced adipogenic differentiation can be caused by inhibiting lipogenic genes such as PPAR-γ [87, 89]. Furthermore, the self-renewal and osteogenic differentiation potential of BMMSCs is determined by the expression level of Wnt3a [89]. Previous studies have reported that the expression of the osteogenesis-associated gene RUNX2 is increased in stem cells that have high expression of Sema3A [90]. Thus, Sema3A signaling is important for neuronal targeting in the peripheral nervous system. In addition, neuronal-derived Sema3A can act as an autocrine factor to promote the normal development of the nervous system [84, 91].
NE
The negative regulation of bone remodeling by SNS has also been previously reported [55, 56, 61]. NE, as a main neurotransmitter of SNS, participates in the regulation of bone homeostasis mainly via its β2 receptor, which stimulates osteoclast formation leading to bone resorption [92, 93]. With regards to its underlying mechanism, noradrenergic nerve terminals in bone release NE to stimulate β2-AR expressed on osteoblasts and osteocytes. This activation leads to an increase in RANKL expression, reduced bone formation and increased bone resorption [56]. In contrast, the expression levels of the β1 and β3 receptors on osteoblast cell lines are weak to nondetectable [56]. Furthermore, application of β-blockers can reduce fracture risk [55, 94]. Our previous studies reported that sympathetic nerves mainly play a negative regulatory role. In addition, the resection of sympathetic nerves down-regulates NE-β3 receptor expression and distraction stress can promote local BMMSCs, causing sympathetic nerve and endothelial stem cells to migrate to the osteogenesis front line via Stromal cell derived factor 1 (SDF-1), matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinase 3 (TIMP-3). Moreover, this process results in decreased bone mass via the NE/abrd3/JNK pathway [17, 95].
Clinical studies using nerve-supported bone homeostasis
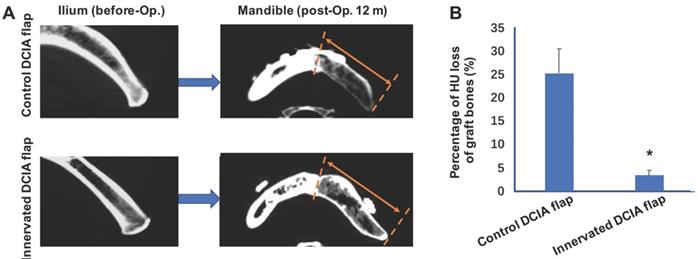
In the reconstruction of the jaw, the commonly used vascularized iliac bone, the DCIA flap, often undergoes severe postoperative absorption, which affects the success of dental implants. In the preparation of a conventional iliac bone flap, the ilioinguinal nerve adjacent to the vascular pedicle is usually severed or sacrificed. The ilioinguinal nerve travelling via the internal oblique muscle is attached to the ilium and innervates the internal oblique muscle, but also innervates the ilium periosteum and bone marrow. Therefore, the reconstruction of sensory nerves may play an important role in maintaining the homeostasis of the bone graft. Our previous studies developed a method of using neurorrhaphy between the ilioinguinal nerve and the inferior alveolar nerve or auricular nerve during reconstruction of the mandibular bone [21]. Moreover, this novel technique was applied in clinical setting with promising clinical results; it was found that 10/22 patients who underwent mandibular reconstruction with innervation, showed less bone resorption after 12 months at the CT scan and in histomorphometric analysis of the bone graft. Furthermore, it was identified that the bone quality around the dental implant was significantly higher compared with the non-innervated group. At 12 months after mandibular reconstruction, the Hounsfield unit (HU) loss of the grafted bone in the innervated group was significantly less compared with the non-innervated group (Figure 2) [21]. Therefore, it was speculated that simultaneous innervation of a vascularized iliac bone graft can significantly reduce the risk of bone resorption after bone graft surgery. In addition, a clinical trial (Clinicaltrials.gov ID: NCT03889587) is currently underway, which includes a larger sample size of patients and there are other clinical trials and studies on bone regeneration or reduction of bone graft resorption (Table 2).
Innervated deep circumflex iliac artery (DCIA) flap presented less bone resorption than control (non-innervated) DCIA flaps after mandibular reconstruction. (A) Computed tomographic (CT) scans of the ilium right before grafted to segmental mandibular defects and the mandibles including the graft regions 12 months after bone reconstruction surgeries. The area between red broken lines and red arrows shows regions of iliac bone grafts. (B) Evaluations of graft bone resorption determined by calculating the percentage loss in graft bone in Hounsfield units (HU) of the CT scans. Significant decreases of bone density were found in innervated DCIA flaps when compared with non-innervated ones (t test; n=10 for each group, *p < 0.05).
Clinical trials and researches on bone regeneration or reduction of bone graft resorption
| Authors | Research | Intervention | Methods | Outcome |
|---|---|---|---|---|
| Gjerde et al | Clinical trial (NCT02751125) | Cell therapy (BMMSC) induced regeneration of severely atrophied mandibular bone | 11 subjects (aged 52-79 years) with severe mandibular ridge resorption. Bone marrow cells were aspirated from the posterior iliac crest and plastic adherent cells were expanded in culture medium containing human platelet lysate. The MSCs and biphasic calcium phosphate granules as scaffolds were inserted subperiosteally onto the resorbed alveolar ridge. | The bone marrow cells were expanded in vitro. Significant new bone formation was induced. The regenerated bone volume was adequate for dental implant installation. Healing was uneventful, without adverse events. |
| Marrella et al [105] | Biomaterial research | Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration | Highlight the structure and osteogenic functions of the vascular and nervous systems in bone, in a coupled manner. Discuss important design criteria for engineering vascularized, innervated, and neurovascularized bone implant materials. | Emphasised that bone implant materials with neurovascularized networks can more accurately mimic native skeletal tissue and improve the regeneration of bone tissue. |
| Wang et al [21] | Clinical research | Preventing early-stage graft bone resorption by simultaneous innervation | Reported a new technique for simultaneous innervation of vascularized iliac flaps in mandibular reconstruction. 22 patients (aged 50 to 69 years) with postoncologic continuity defects of the mandible underwent mandibular reconstruction (10 innervated flaps and 12 control flaps). | Graft bone density loss in the control group was significantly higher than in the innervated group. Bone quality evaluation indicated a suitable condition for dental implantation in all patients in the innervated group. Histologic and histomorphometric analyses showed successful innervation in the innervated group but not in the control group. Osteoclast activity was significantly higher in the control group than in the innervated group. |
| Wang et al | Clinical trial (NCT03889587) | Innervation of vascularized iliac transplant avoids resorption in jaw bone reconstruction | Randomized controlled trial with 40 participants between the age of 17 to 65 years, irrespective of gender. Patients with post resection segmental defect of mandible between 5-9 cm long will be randomly assigned to 2 groups. Group 1 (Innervation)-There will be simultaneous innervation of vascularized iliac or fibular bone flaps through neurorrhaphy between the nerves innervating iliac or fibular bones and recipient site. Group 2 (Non-innervation)-This will be the traditional method of vascularized iliac or fibular bone flaps, and neurorrhaphy will not be performed. | The decreased ratio of the graft bone Hounsfield unit calculated by Spiral CT examination. It is used to reflect the degree of bone resorption. The index of successful innervated reconstruction. The innervation and sensation in the muscle island of innervated graft bone flap will be tested using neuroelectrophysiological and needling response examination. The graft bone samples taken by hollow drilling technique during the dental implant(s) procedure will be observed by silver staining. |
Abbreviations: BMMSC: bone marrow mesenchymal stem cell; MSCs: mesenchymal stem cells.
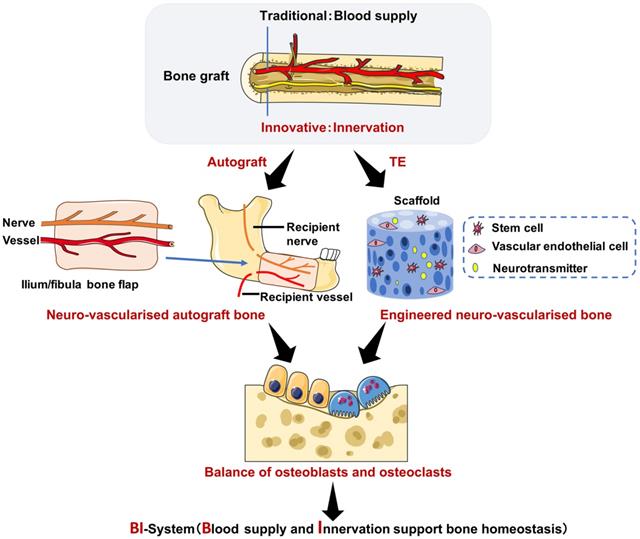
A perspective in translational research of nerve-supported bone homeostasis. On the one hand, a bony flap with both vascular anastomosis and neuroanastomosis to restore the mandibular defection leads to decreased post osteoporosis (the iliac/fibula bone graft is an example of autograft). On the other hand, stem cells derived from bone marrow, vascular endothelial cells and neurotransmitters all cultured in an artificial scaffold to reconstruct bone defection show balanced bone homeostasis. Abbreviations: TE: tissue engineering.
Tissue engineered bone research on nerve regulating bone remodeling
As autologous bone grafts can cause damage and complications to surrounding bone and tissue in the donor site, there has been increasing research into tissue engineering of bone. Moreover, tissue-engineered bone is becoming a promising novel tool and contains four key elements: a scaffold with osteoconductivity; growth factors that induce osteogenesis; seeded cells with osteogenic potential; and tissue engineered vascularization or adequate blood supply [4]. Previous studies have focused on advanced scaffold materials for bone regeneration [6], with cutting-edge research showing the importance of controlled release or molecularly imprinted intelligence for tissue regeneration or cancer treatment [1-5]. In addition, previous studies have shown the importance of vascularization for tissue-engineered bone, especially early vascularization to provide nutrition for the formation of bone tissue [4, 96, 97]. It has also been revealed that biomaterial alignment and optimized mechanical stimulation drive MSC differentiation, thus promoting osteogenesis via the stimulation of osteogenic cell recruitment to new bone formation areas [7, 98]. Furthermore, studies have focused on the role of nerves in tissue engineered bone [53, 98, 99], and therefore, further research is required to investigate mature tissue-engineered products for the use in patients.
Conclusions
The role of central and peripheral nervous systems in bone remodeling has been proposed in the present review, and the latter system is of particular interest. Sensory and autonomic nerves regulate the peripheral nervous system via different types of neurotransmitters, but the specific mechanism is still elusive. Sensory nerves positively regulate bone remodeling via different sensory neurotransmitters acting on BMMSCs, such as CGRP, SP and Sema3A. Furthermore, sensory neurotransmitters can inhibit BMMSC aging and promote osteogenesis, which is of great significance for osteoporosis after clinical bone transplantation. Preliminary clinical studies have reported a positive role of innervation in maintaining the bone homeostasis in bone grafts. However, the innervation of bone graft requires further investigation. It is emphasized that there may be an important connection between the nervous system and bone remodeling, which will facilitate the development of bone grafting and tissue engineering. Furthermore, the novel application of nerve-based bone regeneration using BMMSCs provides a new insight in tissue engineering and clinical treatment of osteoporosis and other bone disorders (Figure 3).
Abbreviations
BMMSC: Bone marrow mesenchymal stem cell; MSC: Mesenchymal stem cells; NGF: Nerve growth factor; SP: Substance P; CGRP: Calcitonin gene-related peptide; BMD: Bone mineral density; β-TCP: β-tricalcium phosphate; GVHD: Graft-versus-host disease; SLE: Systemic lupus erythematosus; HSCs: Hematopoietic stem cells; CD40L: CD40 ligand; FASL: Fas ligand; CTLA-4: Cytotoxic T‑lymphocyte protein 4; OPG: Osteoprotegerin; IFN-γ: Interferon γ; TNF-α: Tumor necrosis factor α; NFκB: Nuclear factor kappa B; PPARγ: Activated receptor gamma; Runx2: Runt-related transcription factor 2; M-CSF: Macrophage colony stimulating factor; SNS: Sympathetic nervous system; PSNS: Parasympathetic nervous system; NE: Norepinephrine; NPY: Neuropeptide Y; ACh: Acetylcholine; DO: Distraction osteogenesis; Col/nHA/Carr: Collagen/nano-hydroxyapatite/kappacarrageenan; TrkA: Tropomyosin receptor kinase A; Erk/Bcl-2: Extracellular signaling-regulated kinase/B-cell lymphoma-2; TRPV1: Transient receptor potential vanilloid 1; MAPK: Mitogen-activated protein kinase; NK1R: Neurokinin 1 receptor; BMMs: Bone marrow macrophages; Nrp: Neuropilin 1; Sema3A: Semaphorin 3A; PlxnA1: Plexin A1; TREM2: Triggering receptor expressed on myeloid cells 2; ITAM: Immune-receptor tyrosine-based activation motif; RhoA: Ras homolog family member A; Rac1: Rac family small GTPase 1; FARP2: FERM, ARH/RhoGEF and pleckstrin domain protein 2; β2-AR: Adrenoceptor beta 2; SDF-1: Stromal cell derived factor 1; MMP-2: Matrix metallopeptidase 2; TIMP-3: Tissue inhibitor of metalloproteinase 3; JNK: c-Jun N-terminal kinase; DCIA: Deep circumflex iliac artery; HU: Hounsfield unit; CT: Computed tomography.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (No. 81771046, No. 81970907 and No. 81270015), Clinical Plus Project of Shanghai 9th People's Hospital (No. JYLJ201817), Shanghai Talent Development (No. 2018042) and Science and Technology Commission Foundation of Shanghai (No. 10DZ1951300).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Neves MI, Wechsler ME, Gomes ME, Reis RL, Granja PL, Peppas NA. Molecularly imprinted intelligent scaffolds for tissue engineering applications. Tissue Eng Part B Rev. 2017;23:27-43
2. Clegg JR, Wechsler ME, Peppas NA. Vision for functionally decorated and molecularly imprinted polymers in regenerative engineering. Regen Eng Transl Med. 2017;3:166-75
3. Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14:89-97
4. Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240-50
5. Hu Q, Sun W, Wang J, Ruan H, Ye Y, Wang C. et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukeamia efficacy. Nat Biomed Eng. 2018;2:831-40
6. Ding X, Yang G, Zhang W, Li G, Lin S, Kaplan D. et al. Increased stem cells delivered using a silk gel/scaffold complex for enhanced bone regeneration. Sci Rep. 2017;7:2175
7. Subramony SD, Dargis BR, Castillo M, Azeloglu EU, Tracey MS, Su A. et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34:1942-53
8. Mertens C, Decker C, Seeberger R, Hoffmann J, Sander A, Freier K. Early bone resorption after vertical bone augmentation - a comparison of calvarial and iliac grafts. Clin Oral Implants Res. 2013;24:820-5
9. Johansson B, Grepe A, Wannfors K, Hirsch JM. A clinical study of changes in the volume of bone grafts in the atrophic maxilla. Dentomaxillofac Radiol. 2001;30:157-61
10. Li L, Blake F, Heiland M, Schmelzle R, Pohlenz P. Long-term evaluation after mandibular reconstruction with fibular grafts versus microsurgical fibular flaps. J Oral Maxillofac Surg. 2007;65:281-6
11. Mertens C, Decker C, Engel M, Sander A, Hoffmann J, Freier K. Early bone resorption of free microvascular reanastomized bone grafts for mandibular reconstruction - a comparison of iliac crest and fibula grafts. J CranioMaxillofac Surg. 2014;42:e217-23
12. Krimmel M, Hoffmann J, Zerfowski M, Reinert S. Central resorption in an iliac crest transplant with microvascular anastomosis - report of 2 cases. J CranioMaxillofac Surg. 2003;31:176-8
13. Larsson J, Scadden D. Nervous activity in a stem cell niche. Cell. 2006;124:253-5
14. Grässel SG. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 2014;16:485
15. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305-17
16. Jones KB, Mollano AV, Morcuende JA, Cooper RR, Saltzman CL. Bone and brain: a review of neural, hormonal, and musculoskeletal connections. Iowa Orthop J. 2004;24:123-32
17. Du Z, Wang L, Zhao Y, Cao J, Wang T, Liu P. et al. Sympathetic denervation-induced MSC mobilization in distraction osteogenesis associates with inhibition of MSC migration and osteogenesis by norepinephrine/adrb3. PLoS One. 2014;9:e105976
18. Zhang YB, Wang L, Jia S, Du ZJ, Zhao YH, Liu YP. et al. Local injection of substance P increases bony formation during mandibular distraction osteogenesis in rats. Br J Oral Maxillofac Surg. 2014;52:697-702
19. Wang L, Cao J, Lei DL, Cheng XB, Zhou HZ, Hou R. et al. Application of nerve growth factor by gel increases formation of bone in mandibular distraction osteogenesis in rabbits. Br J Oral Maxillofac Surg. 2010;48:515-9
20. Wang L, Zhou S, Liu B, Lei D, Zhao Y, Lu C. et al. Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J Orthop Res. 2006;24:2238-45
21. Wang L, Wei JH, Yang X, Yang ZH, Sun MY, Cheng XB. et al. Preventing early-stage graft bone resorption by simultaneous innervation: Innervated iliac bone flap for mandibular reconstruction. Plast Reconstr Surg. 2017;139:1152e-1161e
22. Fan JJ, Mu TW, Qin JJ, Bi L, Pei GX. Different effects of implanting sensory nerve or blood vessel on the vascularization, neurotization, and osteogenesis of tissue-engineered bone in vivo. Biomed Res Int. 2014;2014:412570
23. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010-8
24. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276-87
25. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403
26. Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-9
27. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7
28. Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42-60
29. Sui B, Hu C, Liao L, Chen Y, Zhang X, Fu X. et al. Mesenchymal progenitors in osteopenias of diverse pathologies: differential characteristics in the common shift from osteoblastogenesis to adipogenesis. Sci Rep. 2016;6:30186
30. Sui B, Hu C, Zheng C, Jin Y. Microenvironmental views on mesenchymal stem cell differentiation in aging. J Dent Res. 2016;95:1333-40
31. Jing H, Liao L, An Y, Su X, Liu S, Shuai Y. et al. Suppression of EZH2 prevents the shift of osteoporotic MSC fate to adipocyte and enhances bone formation during osteoporosis. Mol Ther. 2016;24:217-29
32. Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C. et al. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells. 2013;31:1383-95
33. Wang L, Liu S, Zhao Y, Liu D, Liu Y, Chen C. et al. Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ. 2015;22:1654-64
34. Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11:140-50
35. Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmöller M. et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766-70
36. Sándor GK, Tuovinen VJ, Wolff J, Patrikoski M, Jokinen J, Nieminen E. et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938-50
37. Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF. et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8:906-20
38. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-41
39. Sui BD, Hu CH, Zheng CX, Shuai Y, He XN, Gao PP, Zhao P. et al. Recipient glycemic micro-environments govern therapeutic effects of mesenchymal stem cell infusion on osteopenia. Theranostics. 2017;7:1225-44
40. Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S. et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1:5
41. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824-33
42. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829-34
43. Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012;91:1003-10
44. Gyarmati J, Mándi B, Fachet J, Varga S, Sikula J. Alterations of the connective tissue in nude mice. Thymus. 1983;5:383-92
45. Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY. et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2010;108:768-73
46. Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T. et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544-55
47. Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover - role of the immune system. Nat Rev Endocrinol. 2016;12:518-32
48. Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER. et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175-9
49. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050-5
50. Liu Y, Wang S, Shi S. The role of recipient T cells in mesenchymal stem cell-based tissue regeneration. Int J Biochem Cell Biol. 2012;44:2044-50
51. Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X. et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594-601
52. Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P. et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160-73
53. Ngan CGY, Quigley A, Kapsa RMI, Choong PFM. Engineering skeletal muscle - from two to three dimensions. J Tissue Eng Regen Med. 2018;12:e1-e6
54. Guilherme A, Henriques F, Bedard AH, Czech MP. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol. 2019;15:207-25
55. Corr A, Smith J, Baldock P. Neuronal control of bone remodeling. Toxicol Pathol. 2017;45:894-903
56. Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol Rev. 2018;98:1083-112
57. Hébert-Blouin MN, Tubbs RS, Carmichael SW, Spinner RJ. Hilton's law revisited. Clin Anat. 2014;27:548-55
58. Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD. et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155-66
59. Mahns DA, Ivanusic JJ, Sahai V, Rowe MJ. An intact peripheral nerve preparation for monitoring the activity of single, periosteal afferent nerve fibres. J Neurosci Methods. 2006;156:140-4
60. Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005;62:2339-49
61. Elefteriou F, Campbell P, Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int. 2014;94:140-51
62. Coupaud S, McLean AN, Purcell M, Fraser MH, Allan DB. Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone. 2015;74:69-75
63. Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154-68
64. Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154-62
65. Tomlinson RE, Li Z, Li Z, Minichiello L, Riddle RC, Venkatesan A. et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A. 2017;114:E3632-E3641
66. Sun S, Diggins NH, Gunderson ZJ, Fehrenbacher JC, White FA, Kacena MA. No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone. 2020;131:115109
67. Eppley BL, Snyders RV, Winkelmann TM, Roufa DG. Efficacy of nerve growth factor in regeneration of the mandibular nerve: a preliminary report. J Oral Maxillofac Surg. 1991;49:61-8
68. Wang L, Zhao Y, Cheng X, Yang Y, Liu G, Ma Q. et al. Effects of locally applied nerve growth factor to the inferior alveolar nerve histology in a rabbit model of mandibular distraction osteogenesis. Int J Oral Maxillofac Surg. 2009;38:64-9
69. Zheng MG, Sui WY, He ZD, Liu Y, Huang YL, Mu SH. et al. TrkA regulates the regenerative capacity of bone marrow stromal stem cells in nerve grafts. Neural Regen Res. 2019;14:1765-71
70. Yang Z, Wu B, Jia S, Zhao Y, Hou R, Liu X. et al. The mechanically activated p38/MMP-2 signaling pathway promotes bone marrow mesenchymal stem cell migration in rats. Arch Oral Biol. 2017;76:55-60
71. Takahashi N, Matsuda Y, Sato K, de Jong PR, Bertin S, Tabeta K. et al. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci Rep. 2016;6:29294
72. Lerner UH. Neuropeptidergic regulation of bone resorption and bone formation. J Musculoskelet Neuronal Interact. 2002;2:440-7
73. Valentijn K, Gutow AP, Troiano N, Gundberg C, Gilligan JP, Vignery A. Effects of calcitonin gene-related peptide on bone turnover in ovariectomized rats. Bone. 1997;21:269-74
74. Wang L, Shi X, Zhao R, Halloran BP, Clark DJ, Jacobs CR. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone. 2010;46:1369-79
75. Yang ZH, Wu BL, Ye C, Jia S, Yang XJ, Hou R. et al. Targeting P38 pathway regulates bony formation via MSC recruitment during mandibular distraction osteogenesis in rats. Int J Med Sci. 2016;13:783-9
76. Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W. et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat Med. 2009;15:425-35
77. Wang L, Zhao R, Shi X, Wei T, Halloran BP, Clark DJ. et al. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45:309-20
78. Goto T, Nakao K, Gunjigake KK, Kido MA, Kobayashi S, Tanaka T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides. 2007;41:25-31
79. Niedermair T, Schirner S, Seebröker R, Straub RH, Grässel S. Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci Rep. 2018;8:9199
80. Komarova SV, Pilkington MF, Weidema AF, Dixon SJ, Sims SM. RANK ligand-induced elevation of cytosolic Ca2+ accelerates nuclear translocation of nuclear factor kappa B in osteoclasts. J Biol Chem. 2003;278:8286-93
81. Adamus MA, Dabrowski ZJ. Effect of the neuropeptide substance P on the rat bone marrow-derived osteogenic cells in vitro. J Cell Biochem. 2001;81:499-506
82. Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789-800
83. Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525-8
84. Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T. et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490-3
85. Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69-74
86. Togari A, Mogi M, Arai M, Yamamoto S, Koshihara Y. Expression of mRNA for axon guidance molecules, such as semaphorin-III, netrins and neurotrophins, in human osteoblasts and osteoclasts. Brain Res. 2000;878:204-9
87. Xu R. Semaphorin 3A a new player in bone remodeling. Cell Adh Migr. 2014;8:5-10
88. Li Z, Hao J, Duan X, Wu N, Zhou Z, Yang F. et al. The role of semaphorin 3A in bone remodeling. Front Cell Neurosci. 2017;11:40
89. Jeoung JY, Nam HY, Kwak J, Jin HJ, Lee HJ, Lee BW. et al. A decline in Wnt3a signaling is necessary for mesenchymal stem cells to proceed to replicative senescence. Stem Cells Dev. 2015;24:973-82
90. Liu L, Wang J, Song X, Zhu Q, Shen S, Zhang W. Semaphorin 3A promotes osteogenic differentiation in human alveolar bone marrow mesenchymal stem cells. Exp Ther Med. 2018;15:3489-94
91. Janssen BJ, Robinson RA, Pérez-Brangulí F, Bell CH, Mitchell KJ, Siebold C. et al. Structural basis of semaphoring-plexin signalling. Nature. 2010;467:1118-22
92. Moore RE, Smith CK, Bailey CS, Voelkel EF, Tashjian AH Jr. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23:301-15
93. Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407-21
94. Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers and risk of fractures. JAMA. 2004;292:1326-32
95. Wu B, Wang L, Yang X, Mao M, Ye C, Liu P. et al. Norepinephrine inhibits mesenchymal stem cell chemotaxis migration by increasing stromal cell-derived factor-1 secretion by vascular endothelial cells via NE/abrd3/JNK pathway. Exp Cell Res. 2016;349:214-20
96. Mitrousis N, Fokina A, Shoichet MS. Biomaterials for cell transplantation. Nat Rev Mater. 2018;3:441-56
97. Saran U, Gemini Piperni S, Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109-17
98. Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today. 2013;99:170-91
99. Yu X, Liu S, Chen X, Du Y, Yin X, Du Y. et al. Calcitonin gene related peptide gene-modified rat bone mesenchymal stem cells are effective seed cells in tissue engineering to repair skull defects. Histol Histopathol. 2019;34:1229-41
100. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099-142
101. Liu D, Jiang LS, Dai LY. Substance P and its receptors in bone metabolism. Neuropeptides. 2007;41:271-83
102. Ytteborg E, Torgersen JS, Pedersen ME, Helland SJ, Grisdale-Helland B, Takle H. Exercise induced mechano-sensing and substance P mediated bone modeling in Atlantic salmon. Bone. 2013;53:259-68
103. Gomez C, Burt-Pichat B, Mallein-Gerin F, Merle B, Delmas PD, Skerry TM. et al. Expression of semaphorin-3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev Dyn. 2005;234:393-403
104. Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649-66
105. Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A. et al. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today (Kidlington). 2018;21:362-76
Author contact
![]() Corresponding authors: Lei Wang (MD, PhD) or Chen-ping Zhang (MD, PhD), Department of Oral & Maxillofacial-Head & Neck Oncology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, 639 Zhizaoju Road, Shanghai 200011, P.R. China. Email: wangleiorg or chenping.zhangcom. Tel: +8615921941601.
Corresponding authors: Lei Wang (MD, PhD) or Chen-ping Zhang (MD, PhD), Department of Oral & Maxillofacial-Head & Neck Oncology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, 639 Zhizaoju Road, Shanghai 200011, P.R. China. Email: wangleiorg or chenping.zhangcom. Tel: +8615921941601.
 Global reach, higher impact
Global reach, higher impact