13.3
Impact Factor
Theranostics 2023; 13(11):3582-3638. doi:10.7150/thno.82884 This issue Cite
Review
A systematic review of ultrasound-mediated drug delivery to the eye and critical insights to facilitate a timely path to the clinic
1. The University of Queensland, School of Pharmacy, Brisbane, Queensland, Australia.
2. The University of Queensland, Faculty of Medicine, Brisbane, Queensland, Australia.
3. The University of Queensland, School of Mechanical and Mining Engineering, Brisbane, Queensland, Australia.
4. Al-Asala University, Department of Industrial Engineering, Dammam, Saudi Arabia.
5. The University of Queensland, Queensland Brain Institute, Brisbane, Queensland, Australia.
Abstract
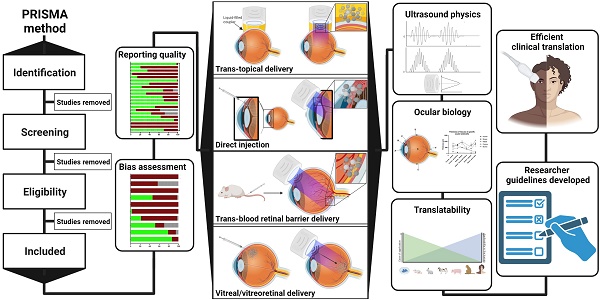
Ultrasound has long been identified as a promising, non-invasive modality for improving ocular drug delivery across a range of indications. Yet, with 20 years of learnings behind us, clinical translation remains limited. To help address this, and in accordance with PRISMA guidelines, the various mechanisms of ultrasound-mediated ocular drug delivery have been appraised, ranging from first principles to emergent applications spanning both ex vivo and in vivo models. The heterogeneity of study methods precluded meta-analysis, however an extensive characterisation of the included studies allowed for semi-quantitative and qualitative assessments.
Methods: In this review, we reflected on study quality of reporting, and risk of bias (RoB) using the latest Animal Research: Reporting of In Vivo Experiments (ARRIVE 2.0) guidelines, alongside the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) RoB tools. Literature studies from 2002 to 2022 were initially characterised according to methods of ultrasound application, ultrasound parameters applied, animal models employed, as well as safety and efficacy assessments. This exercise contributed to developing a comprehensive understanding of the current state of play within ultrasound-mediated ocular drug delivery. The results were then synthesised and processed into a guide to aid future study design, with the goal of improving the reliability of data, and to support efficient and timely translation to the clinic.
Results: Key attributes identified as hindering translation included: poor reporting quality and high RoB, skewed use of animals unrepresentative of the human eye, and the over reliance of reductionist safety assessments. Ex vivo modelling studies were often unable to have comprehensive safety assessments performed on them, which are imperative to determining treatment safety, and represent a pre-requisite for clinical translation.
Conclusion: With the use of our synthesised guide, and a thorough understanding of the underlying physicochemical interactions between ultrasound and ocular biology provided herein, this review offers a firm foundation on which future studies should ideally be built, such that ultrasound-mediated ocular drug delivery can be translated from concept to the coalface where it can provide immense clinical benefit.
Keywords: acoustofluidics, ultrasound, ocular drug delivery, clinical translation, guideline
 Global reach, higher impact
Global reach, higher impact