13.3
Impact Factor
Theranostics 2023; 13(13):4316-4332. doi:10.7150/thno.82907 This issue Cite
Research Paper
Erythroid-transdifferentiated myeloid cells promote portal vein tumor thrombus in hepatocellular carcinoma
1. Department of Medical Oncology, the Third Affiliated Hospital of Sun Yat-sen University, 600 Tianhe Road, Guangzhou 510630, China.
2. Guangdong Key laboratory of Liver Disease Research, the Third Affiliated Hospital of Sun Yat-sen University, 600 Tianhe Road, Guangzhou 510630, China.
3. Department of Obstetrics, the Third Affiliated Hospital of Sun Yat-sen University, 600 Tianhe Road, Guangzhou 510630, China.
4. Department of Infectious Diseases, the Third Affiliated Hospital of Sun Yat-sen University, 600 Tianhe Road, Guangzhou 510630, China.
5. Department of Laboratory Medicine, the Third Affiliated Hospital of Sun Yat-sen University, 600 Tianhe Road, Guangzhou, 510630, China.
6. Department of Hepatic Surgery and Liver Transplantation Center & Guangdong Provincial Key Laboratory of Liver Disease Research, the Third Affiliated Hospital, Sun Yat-sen University, 600 Tianhe Road, Guangzhou, 510630, China.
#These authors contributed equally to this work.
Abstract
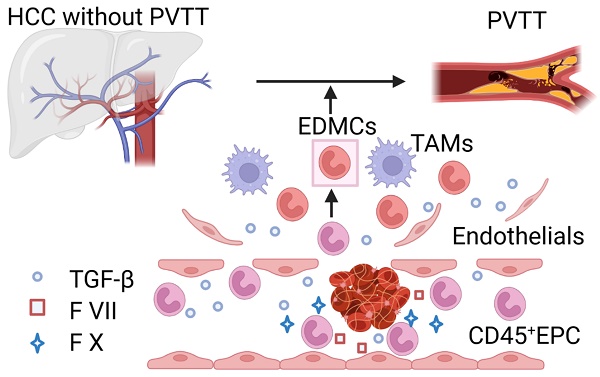
Rationale: Hepatocellular carcinoma (HCC) is primarily characterized by a high incidence of vascular invasion. However, the specific mechanism underlying portal vein tumor thrombus (PVTT) in HCC remains unclear. As a consequence of myeloid cell developmental arrest, CD71+ erythroid progenitor cells (EPCs) and myeloid-derived suppressor cells play important roles in HCC; however, their roles in PVTT remain unclear.
Methods: The role of CD71+ EPCs in the HCC tumor microenvironment (TME) was evaluated via morphological, RNA-sequencing, enzyme-linked immunosorbent assay, and flow cytometric analyses. Co-culture techniques were employed to assess the CD45+ EPCs and their vascular compromising effect. Additionally, the PVTT-promoting function of CD45+ EPCs was explored in vivo in a murine model.
Results: The CD45+EPCs in HCC tissues exhibited increased myeloid cell features, including morphology, surface markers, transforming growth factor (TGF)-β generation, and gene expression, compared with those in circulation. Hence, a large proportion of CD45+EPCs, particularly those in TMEs, comprise erythroid-transdifferentiated myeloid cells (EDMCs). Additionally, the expression of C-C chemokine receptor type 2 (CCR2) mRNA was upregulated in CD45+EPCs within the TME. Tumor macrophages from HCC tissues induced substantial migration of CD45+EPCs in a dose-dependent manner. Meanwhile, results from immunofluorescence analyses revealed that these two cell types are positively associated in the TME and circulation. That is, EDMCs are chemoattracted by HCC macrophages mainly via CCR2 from CD45+ EPCs in the circulation. Additionally, the expressions of FX, FVII, FGB, C4b, CFB, and CFH were elevated in CD45+EPCs within the TME compared with those in the spleen. The CD45+EPCs from the HCC TME promoted vessel endothelial cell migration and compromised tube formation through TGF-β and FGB, respectively. Additionally, CD45+EPCs from the TME induced HCC cell migration. HCC macrophage-induced CD45+EPCs to exhibit higher levels of FX, FVII, FGB, and TGF-β. Meanwhile, upregulation of CCAAT/enhancer binding protein beta expression induced FGB and TGF-β generation in CD45+EPCs in the TME. WTAP, a major RNA m6A writer, stabilized FX and FVII mRNA and enhanced their nuclear export in CD45+EPCs from the TME. CD45+EPCs from the TME were positively associated with PVTT and poor prognosis. Splenectomy reduced the level of CD45+EPCs in the circulation and TME, as well as the incidence of microvascular invasion. The incidence of microvascular invasion increased following the transfer of HCC tissue CD45+EPCs to splenectomized HCC-bearing mice.
Conclusions: The CD45+EPCs enriched in the HCC microenvironment are EDMCs, which are induced by HCC macrophages to migrate from the circulation to the TME. Subsequently, EDMCs promote PVTT by compromising the blood vessel endothelium, aggravating coagulation, and promoting HCC cell migration.
Keywords: Portal vein tumor thrombus, Coagulation, CD45+ EPC, Hepatocellular carcinoma, Vascular endothelial cells
 Global reach, higher impact
Global reach, higher impact