13.3
Impact Factor
Theranostics 2023; 13(13):4636-4649. doi:10.7150/thno.86623 This issue Cite
Research Paper
Functional mass spectrometry imaging maps phospholipase-A2 enzyme activity during osteoarthritis progression
1. Centre for Biomedical Technologies, Queensland University of Technology, Brisbane, Australia.
2. School of Mechanical, Medical & Process Engineering, Queensland University of Technology, Brisbane, Australia.
3. Central Analytical Research Facility and School of Chemistry and Physics, Queensland University of Technology, Brisbane, Australia.
4. Molecular Horizons and School of Chemistry and Molecular Biosciences, University of Wollongong, Wollongong, Australia.
5. Centre for Microscopy and Microanalysis, University of Queensland, Brisbane, Australia.
6. The Prince Charles Hospital, Orthopedic department, Brisbane, Australia.
Abstract
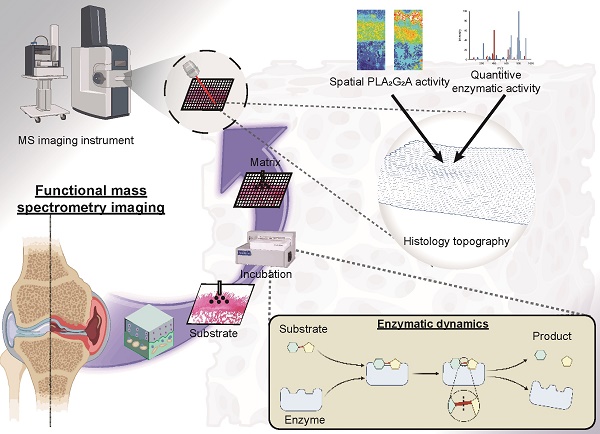
Background: Enzymes are central components of many physiological processes, and changes in enzyme activity are linked to numerous disease states, including osteoarthritis (OA). Assessing changes in enzyme function can be challenging because of difficulties in separating affected tissue areas that result in the homogenisation of healthy and diseased cells. Direct correlation between spatially-resolved enzyme distribution(s) and diseased cells/tissues can thus lead to advances in our understanding of OA pathophysiology. Herein, we present a method that uses mass spectrometry imaging (MSI) to visualise the distribution of lipase enzymes and their downstream lipid products in fresh bone and cartilage tissue sections. Immunohistostaining of adjacent tissue sections was then used to identify OA cells/tissues, which were then statistically correlated with molecular-level images.
Methods: MSI was used to image lipase enzymes, their substrates, and their metabolic products to validate enzymatic activity and correlate to OA regions determined by immunohistochemistry (IHC). Based on the modified Mankin score, six non-OA and OA patient-matched osteochondral samples were analysed by matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI). Due to the involvement of phospholipase A2 (PLA2) in inflammatory pathways, explant tissues were treated with IL-1β to mimic inflammation observed in OA. Bovine explant tissues were then subject to MSI methods to observe the spatial distribution of PLA2.
Results: Compared with non-OA samples, OA samples showed an elevated level of multiple arachidonic acid (AA)-containing phospholipids (P < 0.001), in which the elevation in the surface and deep layer cartilage of OA tissues is correlated to elevated PLA2 activity (P < 0.001). Bovine explant tissues treated with IL-1β to mimic OA pathophysiology validated these results and displayed elevated PLA2 levels in OA mimic samples relative to the controls (P < 0.001). It was established that the PLA2G2A isoform specifically was responsible for PLA2 enzyme activity changes in OA tissues (P < 0.001).
Conclusion: Our results present a reliable method for imaging enzyme dynamics in OA cartilage, which sets up the foundation for future spatial enzyme dynamics in the OA field. We demonstrated that OA patients exhibit increased expression of PLA2G2A at the superficial and deep cartilage zone that degrades cartilage differently at the spatial level. A tissue-specific PLA2G2A precision inhibition may be the potential target for OA.
Keywords: osteoarthritis, lipidomics, microenvironment, MALDI-MSI, enzyme dynamics
 Global reach, higher impact
Global reach, higher impact