13.3
Impact Factor
Theranostics 2023; 13(13):4667-4693. doi:10.7150/thno.87316 This issue Cite
Review
Targeting materials and strategies for RNA delivery
1. College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China.
2. Department of Biomedical Engineering, College of Future Technology, Peking University, Beijing 100871, China.
3. Liangzhu Laboratory, Zhejiang University, Hangzhou 311121, China.
4. Eye Center, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang, China.
5. National Key Laboratory of Advanced Drug Delivery and Release Systems, Zhejiang University, Hangzhou 310058, China.
#These authors contributed equally to this work: Lixin Lin, Kexin Su.
Received 2023-6-19; Accepted 2023-8-9; Published 2023-8-21
Abstract
RNA-based therapeutics have shown great promise in various medical applications, including cancers, infectious diseases, and metabolic diseases. The recent success of mRNA vaccines for combating the COVID-19 pandemic has highlighted the medical value of RNA drugs. However, one of the major challenges in realizing the full potential of RNA drugs is to deliver RNA into specific organs and tissues in a targeted manner, which is crucial for achieving therapeutic efficacy, reducing side effects, and enhancing overall treatment efficacy. Numerous attempts have been made to pursue targeting, nonetheless, the lack of clear guideline and commonality elucidation has hindered the clinical translation of RNA drugs. In this review, we outline the mechanisms of action for targeted RNA delivery systems and summarize four key factors that influence the targeting delivery of RNA drugs. These factors include the category of vector materials, chemical structures of vectors, administration routes, and physicochemical properties of RNA vectors, and they all notably contribute to specific organ/tissue tropism. Furthermore, we provide an overview of the main RNA-based drugs that are currently in clinical trials, highlighting their design strategies and tissue tropism applications. This review will aid to understand the principles and mechanisms of targeted delivery systems, accelerating the development of future RNA drugs for different diseases.
Keywords: Targeting materials, Targeting strategies, RNA-based therapeutics, mRNA delivery, Specific organ/tissue tropism
Introduction
The world has recently witnessed the remarkable impact of messenger RNA (mRNA) vaccines in effectively combating the COVID-19 pandemic, opening a new era of RNA drugs for therapeutic applications. mRNA therapeutics have demonstrated immense potential in the fields of protein replacement therapies, vaccines, and gene editing, as it can regulate gene expression and produce specialized proteins [1-3]. In addition to mRNA, other RNA molecules such as small interfering RNA (siRNA), microRNA (miRNA), and antisense oligonucleotide (ASO) have also been used to treat diverse diseases, including cancer, rheumatoid arthritis, infection, ischemic stroke, and others [4-7]. However, RNA drugs all encounter the critical obstacle of precise delivery to targeted sites of interest.
To achieve the intended therapeutic effects, RNA drugs should be precisely delivered to specific organs, tissues, or even cells. Targeted delivery endows several acknowledged advantages. Firstly, delivering RNA cargoes to diseased cells is the prerequisite to realize treatment. Secondly, targeting enables us to address the issue of off-target effects, which often limits RNA therapy by causing toxicity and affecting drug safety as well as efficacy. Precise delivery aims to enhance the bioavailability of RNA drugs at the target site while minimizing their distribution to non-target tissues. Lastly, targeted delivery can lower the required dosage of RNA for administration, improve the biosafety of drugs, and increase the clinical tolerance [8,9].
Despite the enthusiastic potential of RNA-based drugs for targeted treatment of various diseases, multiple obstacles remain to be addressed to fully realize their clinical applications [4,5]. One of the major challenges is the instability of RNA molecules. Unlike traditional drugs, RNA molecules are highly susceptible to degradation by endonucleases and hydrolases in blood or physiological fluids, resulting in a short half-life [6]. Additionally, the physicochemical properties of RNA, such as its negative charge, hydrophilicity, and high molecular weight, make it difficult for RNA to cross the cell membranes [10-14]. Therefore, functional delivery vectors are required to transport RNA to the desired sites. Numerous work on systemic administration of unmodified RNA molecules have indicated that the pharmacokinetic characteristics are usually unsatisfied [7,8]. Although chemically modified RNA molecules are significantly more stable, overcoming intracellular barriers for cell entry and endosomal escape remains challenging [15]. Hence, to tackle the limitations of RNA-based drugs, rationally designed vectors are needed for targeted delivery of RNA drugs. The targeting vector material design should satisfy the following characteristics: 1) protecting RNAs from degradation in serum; 2) remaining stable in the blood or body fluids; 3) showing excellent biocompatibility and biodegradability; 4) improving the cellular uptake and endosomal escape; 5) providing RNA drugs with a suitable half-life and low toxicity; 6) preventing non-specific interaction with non-target tissues, achieving selective accumulation at the targeted sites, and mediating precise gene regulation [10,16,17].
To address the limitations of RNA-based drugs in practical use, a variety of non-viral vectors have been employed for therapeutics delivery. Until now, to develop targeting materials and strategies remains the critical challenge for RNA drug clinical translation. Although several targeted delivery systems have arisen, the guideline behind organ/tissue tropism remains unclear [18]. In this review, we provide an overview of various RNA drugs and their mechanisms of action in exerting therapeutic effects. Following this, RNA targeting materials and strategies are summarized from four factors: the category of vector materials, chemical structures of vectors, administration routes, and physicochemical properties of RNA vectors. Ultimately, we highlight the recent advancements in the clinical applications of RNA-based drugs, particularly mRNA drugs, with a focus on the utility of targeted delivery approaches.
Mechanisms and therapeutic applications of representative RNA drugs
Different RNA drugs demonstrate distinct characteristics and therapeutic mechanisms [19], and these understandings facilitate to achieve optimal therapeutic effects and appropriate clinical applications. Herein, we summarize the therapeutic mechanisms of several representative RNA drugs and their commonly applied therapeutic fields.
Messenger RNA (mRNA) drugs
mRNA is a single-stranded ribonucleotide transcribed from a template strand of DNA, which carries the genetic code and directs the synthesis of proteins. Natural mRNA is composed of the following parts: 5' and 3' untranslated regions (UTRs), 3' poly (A) tail, 5' cap. Among these, UTRs do not encode proteins, but can regulate locations of mRNA and translation efficiency [9,20]. The 5'cap, also known as m7GpppN, can bind to the multi-subunit initiation factor eIF4F to promote mRNA binding with ribosomes and translation of mRNA [21]. Simultaneously, this cap structure can protect mRNA from degradation by exonuclease [10]. The length of poly A is generally 30-70 nucleotides, which is known to influence the half-life and translation efficiency of mRNA, and it is reported that 120 nucleotides can enhance the stability of mRNA [11,12].
Compared to DNA drugs, mRNA does not require entry into the nucleus and can function directly in the cytoplasm. This advantage avoids the risk of gene insertion. Additionally, mRNA has transient expression and will be degraded by enzymes in a limited time after entering the body. This property endows mRNA high safety for therapeutic use [13]. After in vitro transcribed (IVT) mRNA is delivered into the desired cells by a suitable vector, such as lipid nanoparticle (LNP), the mRNA is released from the vector. Then the mRNA directs protein synthesis with the help of ribosomes and transfer RNA (tRNA), which can be used for protein replacement to treat protein deficiency (e.g. haemophilia B) or protein malfunction (e.g. muscular dystrophy) [14,22].
To date, one of the most significant applications of mRNA is to be used as the vaccines in the field of cancer immunotherapy and viral infections. The immune system plays a crucial role in fighting cancer and protecting the body from bacteria invasion. However, tumor-associated cells can secrete a variety of cytokines and chemokines through different mechanisms to inhibit immune system activities [23]. Individuals with weakened or inactive immune systems are more vulnerable to viruses and bacteria. mRNA can be utilized as vaccines by encoding the antigens of tumors or viruses and incorporating them into suitable vectors [24]. Upon appropriate administration, mRNA is translated into proteins, which are then degraded by the protease into small fragments, known as antigenic peptide epitopes. Afterwards, the fragments will be taken up by MHC molecules and immune response will be enhanced to activate CD4+ and CD8+ T cells and B cells, killing and eliminating viruses or tumor cells [15]. For instance, Oberli et al. used LNPs to deliver mRNA encoding melanoma-related antigens, which induced a strong immune response and prolonged the survival time of mice bearing melanoma [16]. However, no RNA-based drugs for treating cancer have been approved by FDA thus far. In this case, the marketing of two mRNA-based COVID-19 vaccines is encouraging. Compared to the inactivated viral vaccine, the mRNA vaccines have shown the ability to elicit a stronger immune response, resulting in higher antibody level and higher protection rate of over 90% [25,26]. To cope with the virus's tendency to mutate, it is crucial to design vaccines that are effective against of viruses in different periods. One notable advantage of mRNA vaccines is that they can be rapidly designed and mass-produced, tailoring the characteristics of different virus strains. For instance, Moderna took only 63 days to inoculate the first dose of mRNA vaccine after completing of gene sequencing [19], which was also benefited from the advancement of LNP delivery technology. The success of mRNA vaccine has drawn increased attention to the potential of RNA-based drugs, simultaneously emphasizing the significance of delivery vector design.
Gene editing is another important therapeutic field of mRNA-based drugs, leveraging the development of Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)) technology [1]. Charpentier and Doudna have been awarded the 2020 Nobel Prize in Chemistry because of their pioneering work on CRISPR-associated protein 9 (Cas9) gene editing technology, proving the significant status of this technology in the field of gene therapy [27,28]. The CRISPR-Cas9 system contains Cas9 protein to cleave the genomic DNA and single-guide RNA (sgRNA) to decide the specific location for cutting [29]. Considering that to deliver plasmid DNA with Cas9 gene and sgRNA may result in off-target effects with sustaining expression of Cas9 protein and requiring nuclear entry of DNA, in contrast, using IVT mRNA encoding Cas9 protein with the characteristics of transient expression and cytoplasmic functioning can overcome these challenges [1,13,30]. Several studies have demonstrated that co-delivering Cas9 mRNA and sgRNA can achieve effective gene editing [17,31-33]. Overall, mRNA-based drugs hold tremendous potential in various therapeutic fields and warrant further exploration.
Small interfering RNA (siRNA) drugs
siRNA, also known as short interfering RNA or silencing RNA, is a type of short double-stranded RNA with the length of 21-23 nucleotides containing 2 nucleotides overhang [34]. Once exogenous siRNA enters the cell, it can combine with ribonucleoprotein to form an RNA-induced silencing complex (RISC) and two RNA strands are separated. The remaining antisense (guided) strand is complementary to the targeted mRNA sequence, allowing to guide the Argonaute protein to cut the targeted mRNA, thereby inhibiting the translational process of protein [35]. Although siRNA is usually double-stranded, only the antisense strand remains associated with RISC, as the sense strand is rapidly cleared after unwinding [36]. The discovery of siRNA earned Fire and Mello the Nobel Prize in Physiology or Medicine in 2006 [37], highlighting the significant value and potential of siRNA in medical field. In 2018, the first siRNA drug, Onpattro (Patisiran), was approved by FDA for the treatment of hereditary transthyretin (TTR)-mediated amyloidosis [38]. Subsequently, in November 2019, another siRNA drug, Givosiran (Givlaar), was approved by the FDA to treat acute hepatic porphyrias (AHP) [39]. Until now, five siRNA drugs have been approved by the FDA, including Patisiran, Givosiran, Lumasiran, Inclisiran, and Vutrisiran, all of which are developed by Alnylam, a pioneering company in the field of RNA therapeutics.
MicroRNA (miRNA) drugs
miRNA is a single-stranded non-coding RNA molecule that plays a crucial role in the regulation of gene expression and is involved in a series of essential cellular processes, such as cell proliferation, differentiation and apoptosis [40]. Similar to siRNA, miRNA can also mediate gene silencing by forming RISC to bind mRNA and inhibit the expression of targeted protein [41]. Nowadays, some miRNAs are also discovered to activate the transcription of targeted mRNAs to increase gene expression [44]. And this discovery has opened new possibilities for miRNA-based therapeutic strategies. However, no miRNA-based drug has been approved by FDA yet, possibly due to the fact that each miRNA can have hundreds of targets, This phenomenon is known as “too many targets for miRNA effect” (TMTME) [43], making it challenging to achieve specific gene regulation with miRNA, which could lead to unpredictable side effects. Currently, most clinical trials on miRNA drugs have been terminated due to safety issues, with only a few ongoing. Despite these problems, laboratory research continues to uncover the potential of miRNA for the treatment of various diseases such as ovarian cancer, polyomaviruses, and breast cancers [44-46].
Antisense oligonucleotides (ASOs) drugs
In 1978, Zamecnik and Stephenson reported the use of synthetic oligodeoxynucleotides to inhibit the replication of virus in vitro, demonstrating the antisense function of synthetic oligodeoxynucleotides for the first time [47]. ASO is a kind of synthesized oligonucleotide with 12-30 nucleotides in length which can bind to targeted RNA sequences through complementary base pairing. This binding can result in RNA cleavage or degradation by RNase H or inhibition of translation through occupancy-only mechanisms [48,49]. ASOs have evolved over three generations with different modifications, such as phosphorothioate, alkyl moieties or nucleobase, which can improve stability, prolong circulation time, increase affinity to the target, and reduce off-target effects [49,50]. Notably, some specific modifications might confer unique functions. For instance, unlike other RNA-based drugs, most of ASOs do not require a delivery vector, instead, the delivery can be achieved by particular chemical modifications, such as gapmers [51]. Encouragingly, numerous candidates have entered clinical trials for the treatment of various diseases, ranging from cancer, infectious disease, neurological disease to metabolic disease, making them the largest class of FDA approved RNA drugs with promising therapeutic potential [50,52,53].
The abundance of RNA species and functions endows huge potential in biological and medical field. However, the efficacy of RNA-based drugs can be affected by the challenge of delivering them to specific organs and sites in vivo. To fully realize the potential of RNA-based drugs for disease treatment, further research and strategies are in urgent demand to overcome the problems associated with targeted delivery.
Materials and strategies for targeted RNA delivery. The vector category, vector chemical structure, administration route, and physicochemical property all affect the targeting tropism of nanoparticles.
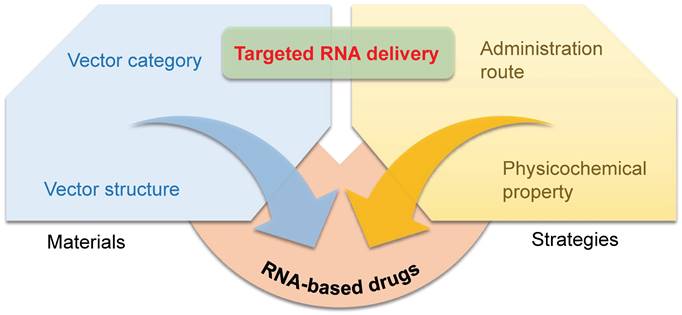
Materials and strategies to achieve targeting
RNA drugs open an era for treating chronic diseases, nonetheless, their full realization is seriously hindered by lack of targeted delivery technology. Despite the fact that RNA delivery systems have been studied for decades, the precise gene regulation in specific tissues or cells remains a huge challenging. Furthermore, understanding the guideline of organ/tissue tropism of nanoparticles will further the advance of next-generation gene delivery technology, facilitating future RNA-based drug development. Herein, we summarize current progress in targeted RNA delivery, from the point of four key factors: the category of vector materials, chemical structures of vectors, administration routes, and physicochemical properties of RNA vectors (Figure 1).
The category of vector materials
Lipid Nanoparticles (LNPs)
LNPs are one of the widely utilized non-viral vectors for delivering RNA-based drugs. Typically, LNPs are composed of four components with distinct functions, including permanently charged cationic or ionizable cationic lipids, phospholipids, cholesterol and polyethylene glycol (PEG)-lipids [1]. Initial LNPs employ permanent cationic lipids, which enable efficient encapsulation of negatively charged RNAs through electrostatic interaction. However, these permanently charged cationic lipids can be toxic to cells and make stimulus after entering the blood, with positive charges, leading to elimination from the body by the immune system [54]. Alternatively, ionizable cationic lipids remain neutral in physiological environment, ensuring higher safety, and become positively charged in the acidic endosomal environment of lower pH to facilitate endosomal escape of encapsulated drugs [55]. And Lee et al. found that ionizable cationic lipids with unsaturated tails could improve the delivery efficiency of LNPs due to the increased lipid fusion ability [56]. Cholesterol shows a rigid hydrophobic structure and can be inserted into the gap of the liposome membrane to enhance the stability of the LNP vector. Common helper lipids, such as DOPE, have a relatively small head group to form a tapered shape that facilitates the formation of hexagonal phase II, a transitional form during membrane fusion or bilayer rupture, finally promoting endosomal escape [57,58]. PEG-lipids play a significant role in reducing the opsonization effect of serum proteins and non-specific uptake of LNPs, extending the circulation time and half-life of LNPs in vivo [59]. Recently, studies demonstrated that some LNPs with dual-component enabled mRNA delivery as well [60]. Additionally, stability is an important issue to be taken into consideration during storage and transportation of LNP formulations. And changing formulation to lyophilized form or adding cryoprotectant, such as mannitol and sucrose, are feasible methods to improve the stability of LNP formulations [61].
Despite some studies show that lipid materials may cause liver or lung injuries and the cytotoxicity of LNPs is related to the dosage of administration and the properties of lipids used, LNPs offer several advantages compared to viral vectors, including ease of production, relatively low immunogenicity, high RNA loading capacity, and flexible design options [61,62]. Most LNPs are prone to target liver and usually be utilized to treat liver diseases. For instance, Onpattro with DLin-MC3-DMA LNP as the vector, is administrated intravenously and enables the inhibition of hepatic production of transthyretin [63]. Yang et al. demonstrated that LNPs encapsulating HNF4A mRNA could attenuate liver fibrosis in preclinical mouse model [64]. Finn et al. used a degradable lipid to formulate LNPs to deliver Cas9 mRNA/sgRNA for gene editing in vivo. The results showed that the editing efficiency was highly considerable for transthyretin gene editing in the liver, reducing serum protein levels by over 97% with a single dose [65]. Recently, some studies have revealed the rules of LNP targeting different liver cell subsets. It is reported that altering surface charge on the LNP in Onpattro formulation from neutral to anionic, the vector was prone to deliver mRNA to the hepatic reticuloendothelial system [66]. Additionally, researchers discovered that altering the size, adjusting PEG-lipid content, or incorporating ligands in LNPs affected the distribution of loaded cargoes in hepatocytes and liver sinusoidal endothelial cells (LSECs), or increased the delivery efficiency in extrahepatic organ. For instance, LNPs with 100 nm diameter in size are the most effective for targeting LSECs, while LNPs with smaller diameters, such as 60 nm, showed tropism for hepatocytes [67]. Another study showed that incorporating mannose PEG into LNPs led to increased delivery efficiency of siRNA to lungs in mouse model with pulmonary fibrosis after intratracheal injection [68].
Polymers
Despite the great potential in clinical translation, extrahepatic RNA delivery by LNPs remains challenging. Alternatively, polymers provide an option for delivery out of the liver. Polymers are divided into different classifications, such as cationic polymers, zwitterionic polymers, polymeric micelles, and dendrimers [1,2,69,70]. Polyethyleneimine (PEI) was the “golden standard” for gene delivery previously. The amine-containing PEI can not only improve the membrane affinity of polymeric nanoparticles, for enhanced cellular uptake, but also arise endosomatic effect. The polymers with amine groups show the ability for protonation and leading to osmotic pressure alternation in an acidic endosomal environment, leading to endosome rupture and drug release into the cytoplasm, known as 'proton sponge effect' [71]. However, cationic polymers bearing high positive charges would bind to serum proteins and red blood cells, resulting in disruption of plasma membrane [72]. Therefore, their applications are severely limited by safety concerns. It was reported that PEI polymers with low molecular weight was less cytotoxic compared to high molecular weight PEI, but the transfection efficacy was also decreased [73,74]. Researchers continue to develop next-generation cationic polymers, such as poly(β-amino ester) (PBAE) polymers, which are proved to be less cytotoxic and more efficient than PEI [75-78]. Zwitterionic biomaterials with oppositely charged groups have drawn great attention in many fields, like drug delivery, diagnosis, biosensors, and coating. For drug delivery, zwitterionic polymers enable protection of RNA drugs from degradation by inhibiting protein adsorption to extend circulation time. And researches revealed that zwitterionic polymers showed the ability to resist mucus tracking and increase the penetration of mucus to achieve drug delivery [79]. In regard to polymeric micelles, Langridge and Gemeinhart demonstrated that polymeric micelles composed of poly(ethylene glycol-block-caprolactone) (mPEG-CL) and poly(ethylene glycol-block-lactide) (mPEG-LA) were stable in cerebrospinal fluid [80], reminding us the potential utility of micelle vectors in targeted cerebrospinal fluid administration. And the variable sizes of polymeric micelles provide the suitable property to penetrate tumor tissue for delivery aim and many laboratory studies verified polymeric micelles could be used to deliver RNA drugs for multiple types of tumors, such as resistant ovarian tumor and solid tumors [81-83]. Natural polymers, like chitosan and protamine may overcome the disadvantage of cytotoxicity of synthetic polymers mentioned above, exhibiting good biocompatibility. However, production control between different batches is significant for clinical translation and little difference in molecular weight might alter the delivery efficiency of vectors, which is one main limitation for polymer application in delivering RNA drugs [84].
To further improve the functionalities of vectors, numerous hybrid copolymers, such as lipid-polymer hybrid nanoparticles (LPNs), graphene oxide (GO)-cationic PEI polymers (GO-PEI complexes), polymer-dendrimer hybrids, were developed [1,85]. The hybridized polymer-dendrimers exhibited enhanced permeability and retention (EPR) effect, outstanding permeability, and high drug loading capacity. However, several drawbacks still remained: the steric effects of multiple branches in dendrimers might hinder them from degradation, and the excess positive charges could lead lysis of cells and cause cytotoxicity [2,70,85]. Conjugation of ligands to improve the targeting ability, masking or reducing charges, and modification of ester bonds to improve degradability might be beneficial for solving these problems.
Numerous novel polymers have been reported to deliver therapeutic cargoes to special organs, particularly the lungs. For instance, hyperbranched PBAEs were synthesized to enable mRNA delivery to the lung epithelium through inhalation, resulting in consistent and controlled protein production without causing pulmonary or systemic toxicity [86]. Haque et al. utilized chitosan-coated PLGA nanoparticles to encapsulate chemically-modified mRNA encoding human cystic fibrosis transmembrane conductance regulator (CFTR), which was efficiently delivered to lungs of CFTR deficient mice following intravenous and intratracheal administrations [87]. Recently, some studies have revealed the potential of polymers to target organs other than the lungs. Liu et al. modified cationic polymers by zwitterionic phospholipidation to selectively deliver mRNA to the spleen and lymph nodes after intravenous administration in vivo, showing great potential in immunotherapy application [88]. McKinlay et al. designed charge-altering releasable transporters (CARTs) via organocatalytic ring-opening polymerization of various lipids and results demonstrated mixed-lipid CARTs could achieve effective mRNA delivery to B cells and T cells of spleen in vivo, and the delivery efficiency was higher than single-lipid CART [89].
Exosomes
Exosomes are extracellular vesicles (EVs) with diameters between 30 and 100 nm, and are naturally secreted by cells [90]. They play a crucial role in communications between cells via ligands, intercellular adhesion molecules on membranes or encapsulated cargo inside the exosomes [91]. Compared to other synthesized drug vectors, exosomes show a variety of advantages as natural vectors. For instance, the endogenous origin and membrane proteins of exosomes give them a long half-life in the body [92]. Studies have shown that the exosomes derived from foreskin fibroblast of normal human can reduce the phagocytosis of monocytes and macrophages, while enhancing the uptake of cancer cells by micropinocytosis [93]. Cancer cells are known to produce a large number of exosomes, and these exosomes show tropism to their source cells, which makes them a potential tool to target cancer cells. For instance, researchers discovered that compared to epithelial cell-derived exosomes, exosomes derived from an ovarian cancer cell line SKOV3 selectively targeted SKOV3 xenograft mice and achieved higher accumulation in tumor site, allowing for CRISPR/Cas9 gene editing to induce the apoptosis of ovarian cancer cells [94]. In addition, it was reported that exogenous miRNA-155 mimics or inhibitors were delivered via B cell-derived exosomes to hepatocytes or macrophages, with higher delivery efficiency and lower cytotoxicity in contrast to regular transfection methods [95]. Specifically, studies showed that the exosomes could cross the brain-blood barrier (BBB), bearing the innate character for brain-targeting [96,97]. Perets et al. developed a technique to track the exosomes from mesenchymal stem cells of bone marrow (MSC-exo), which could accumulate in mouse brains in different pathological models, including Alzheimer's disease, autism, stroke, and Parkinson's disease. To be more specific, the homing mechanism was driven by inflammation in pathological brains and MSC-exo could be selectively taken up by neuronal cells [98]. These findings highlight the potential of exosomes as vector to achieve targeted delivery of RNA-based drugs in various brain pathologic therapies.
Inorganic nanoparticles
Inorganic nanoparticles (INPs) are synthesized from inorganic particles, biodegradable polycations, and typical inorganic materials such as gold, silica, metallic oxide, and others. Among INPs, gold nanoparticles (AuNPs) have been extensively studied in inorganic chemistry and nanomaterials [99]. AuNPs are chemically inert, showing monodisperse nanostructures without toxicity, making them ideal for functionalization with a variety of ligands [100]. One notable application of AuNPs is their interaction with B lymphocytes, enabling targeted immune cell delivery and enhanced immune response. For instance, polymer-coated AuNPs loaded with antigen were utilized to target B lymphocytes and activate CD4 T cell responses [101], which might be used to improve efficacy of vaccines in the future. However, due to the inert property, it is difficult to metabolize AuNPs in time, leading to the long half-life that limits the clinical application of AuNPs. Wang et al. utilized copper sulfide, which was metabolizable in liver to improve the excretion of Au. And the results showed that the conjugation of copper sulfide and Au could accelerate the excretion of AuNPs in hepatocytes [102]. Other inorganic nanoparticles, such as silica nanoparticles, iron oxide nanoparticles, are also being investigated for targeted applications [99].
Chemical Structures of vectors
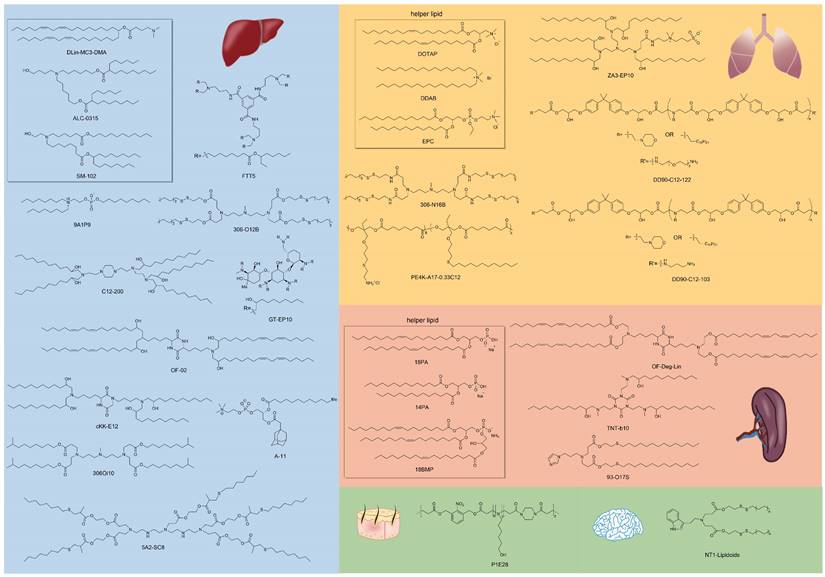
Similar classes of RNA vectors probably show the same tropism in vivo, for instance, most LNPs tend to deliver mRNA into the liver. Nonetheless, minimal alteration of chemical structures of vectors in the same class might also achieve the goal of targeting, and the research on exploring targeted material structures is in full swing. Beyond Dlin-MC3-DMA, ALC-0315, and SM-102 approved by FDA for clinical use in LNP formulations, numerous advancements have been made in the development of various lipids, lipidoids, and polymers for delivering RNA to targeted organs. Here, we outline the relationship between the chemical structures of these materials and their specific targeting capabilities for different organs (Figure 2).
Representative chemical structures of RNA vectors that mediate targeted delivery. RNA vectors for liver, lung, spleen, skin, and brain-targeting are shown.
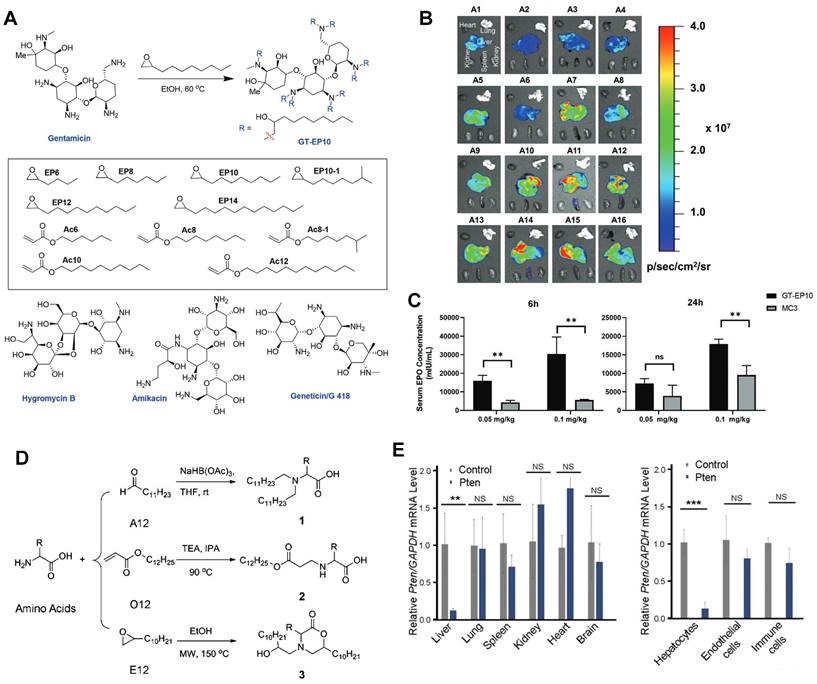
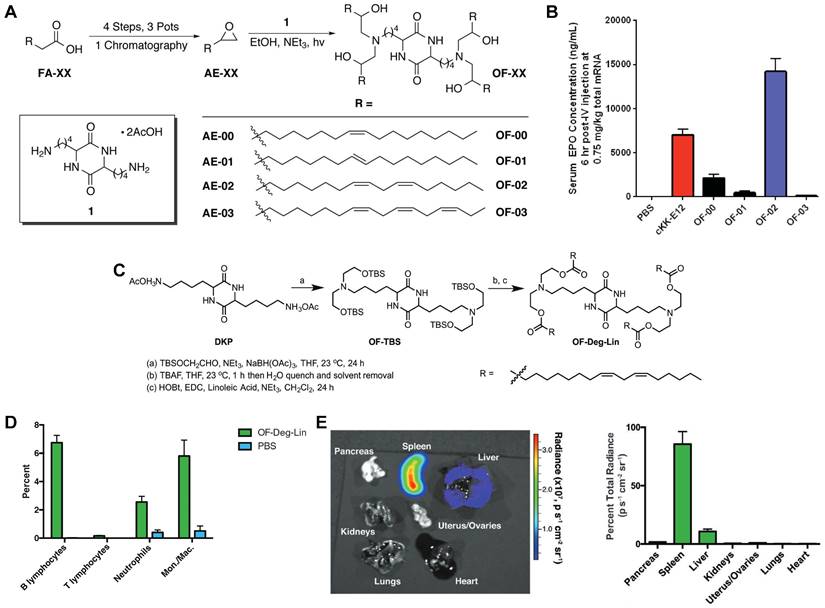
Lipids derived from ring-opening reaction or addition reaction of amines, acrylates, and epoxides for liver-targeting. (A) Synthesis route of cationic lipid-modified aminoglycosides according to ring-opening reaction and structures of aminoglycosides. (B) Luciferase expression in vivo of C57BL/6 mice with the delivery of CLA-based LNPs at 6h after intravenous injection. (C) Human erythropoietin expression after 6 h and 24 h with GT-EP10 LNP and MC3 LNP delivery. Adapted with permission from [106], copyright 2020, Wiley-VCH. (D) Synthesis routes of amino acid derivatives by addition reactions. (E) Expression level of Pten, in different organs and subtypes of liver cells. Adapted with permission from [107], copyright 2014, National Academy of Sciences.
Targeting of the liver
Most LNPs tend to target liver, and modifying the LNP vector can enhance their targeting ability or achieve targeting of specific subtypes of liver cells [103-105]. For instance, Yu et al. developed a class of LNPs with cationic lipid-modified aminoglycosides as shown in Figure 3A, and the results demonstrated that the top-performing GT-EP10 LNPs could deliver FLuc mRNA and human erythropoietin mRNA to liver at a higher delivery efficiency compared to DLin-MC3-DMA LNPs (Figure 3B-C) [106]. Gan et al. designed a series of LNPs with adamantyl-phospholipids to encapsulate Cre mRNA and DNA barcodes, and intravenous injection allowed the occurrence of tdTomato fluorescence in the liver of Ai14 mice. Further quantification demonstrated that A-11 without specific ligands tended to deliver inclusions to the liver immune cells, instead of common hepatocytes [104]. For siRNA delivery, Dong et al. designed a library of lipids according to the chemical reaction of amino acids and epoxide or acrylate esters presented in Figure 3D, and identified the top-performing lipid, cKK-E12, via iterative screening and structure-activity relationships (SAR) study. As a result, cKK-E12 LNPs delivered siRNA to liver and silenced Phosphatase and tensin homolog (Pten) in hepatocytes with high selectivity (Figure 3E) [107]. Furthermore, cKK-E12 LNPs were also utilized to co-deliver Cas9 mRNA and sgRNA for editing PCSK9 in hepatocytes of mice in a subsequent study [103]. Similarly, Whitehead and colleagues synthesized a series of lipidoids by Michael Addition for siRNA delivery. And subsequent investigation revealed that the lead 306Oi10 LNPs resulted in much higher siRNA accumulation in liver than naked siRNA [108].
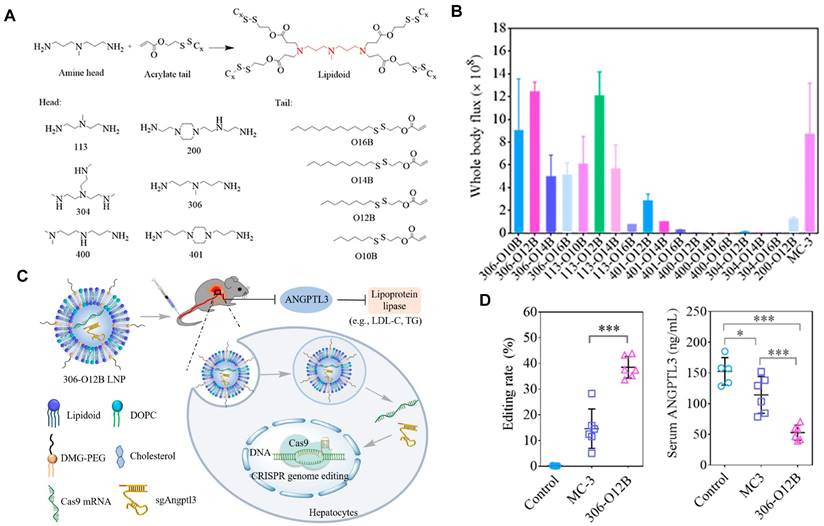
Currently, LNPs have been increasingly employed for loading mRNA to achieve liver targeting, and applications such as gene editing and protein replacement are explored [109,110]. The mechanism underlying the liver-targeting of LNPs has been extensively studied and several findings are publicized. Upon entry into the body, LNPs can interact with proteins in the biological environment and adsorb proteins on the membrane surface to form the protein corona [55,111]. Thus far, it has been demonstrated that the liver-targeting of LNPs is mediated by the absorbed corona proteins, particularly apolipoprotein E (ApoE), which can bind to low-density lipoprotein receptor (LDLR) expressed on hepatocytes and facilitate the uptake of LNP by liver cells [112,113]. In a recent study, Qiu et al. designed a library of O-series LNPs that contained ester bonds in their tails (Figure 4A). They discovered that 306-O12B LNPs tended to deliver Cas9 mRNA and sgRNA to the liver and led to higher Angptl3 gene knockdown compared to DLin-MC3-DMA LNPs (Figure 4B-D). Further studies revealed that the differentiation of tissue targeting might be determined by the corona proteins on the nanoparticle surface [114,115].
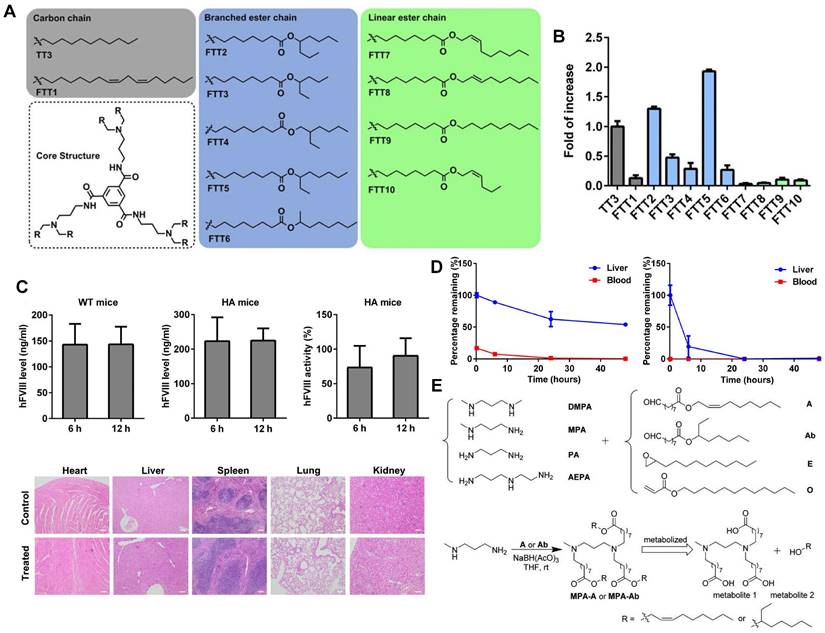
Lipid-like nanoparticles (LLNs) represent one of the predominant LNP delivery systems for RNA-based drugs. Dong and colleagues designed a series of LLNs with TT3 as the core structure as depicted in Figure 5A. FTT5 LLNs were formulated to deliver FLuc mRNA in vivo, mediating the highest luminescence signal in the liver (Figure 5B). Subsequently, FTT5 LLNs were utilized to package hFVIII mRNA and adenine base editor mRNA, achieving effective hFVIII protein expression and base editing in vivo of hemophilia A (HA) mice (Figure 5C). Further study showed that FTT5 LLNs with branched ester side chains exhibited greater resistance to degradation compared to FTT9 LLNs with linear chains (Figure 5D) [116]. In an effort to develop biodegradable materials, Zhang et al. rationally designed a series of LLNs with different amines and esters, bearing varied carbon chains as shown in Figure 5E. MPA-A and MPA-Ab showed higher Cas9 mRNA delivery efficiency compared to epoxide or acrylate series LLNs and C12-200 LNPs [117]. In a subsequent research, Luo et al. verified that the optimized MPA-Ab LLN formulations post orthogonal design mediated enhanced mRNA delivery compared to TT3 LLNs [118].
O-series LNPs for liver-targeting. (A) Synthesis of O-series lipidoids. (B) Whole body luminescence intensity of O-series LNPs compared to MC3 LNP in Balb/c mice at 6h after intravenous injection. (C) Schematic illustration of LNP-mediated gene editing in hepatocytes and reduction of Angptl3 protein resulting disinhibition of lipoprotein lipase. (D) Comparison of Angptl3 gene editing efficiency with the delivery of 306-O12B LNP and DLin-MC3-DMA LNP. Adapted with permission from [114], copyright 2021, National Academy of Sciences.
Novel LLNs for liver-targeting. (A) Chemical structures of FTT derivatives with TT3 as core. (B) mRNA delivery efficiency of FTT LLNs was represented by the fold of increase of luminescence intensity in vivo and FTT5 showed the highest delivery efficiency. (C) Expression level of hFVIII protein and hFVIII activity in wild-type mice and HA mice after intravenous injection of FTT5-hFVIII mRNA LLNs. And histopathological images of HA mice with injection of FTT5-hFVIII mRNA LLNs and untreated HA mice were shown. (D) FTT5 LLNs with branched ester side chains (left) were less likely to degrade than FTT9 LLNs with linear chains (right) in liver. Adapted with permission from [116], copyright 2020, American Association for the Advancement of Science. (E) The synthesized routes of amino-ester-derived LLNs. Adapted with permission from [117], copyright 2017, American Chemical Society.
In addition to LNPs mentioned above, several other vectors with different chemical structures can also realize liver-targeting. Rui et al. used linear diacrylate as the backbone and monomer E63 with two secondary amines as the end-cap to synthesize PBAE polymers, achieving preferential expression of mRNA in liver [119]. In another study, poly(acrylic acid) (PAA8k) was used as the core structure to synthesize polymers by conjugating with oligoalkylamine. The results demonstrated that PAA8k-(2-3-2) encapsulating FLuc mRNA could achieve higher delivery efficiency in the liver compared to PAA8k-(2-2-2) and PAA8k-(3-3-3). Similar results were observed when coupling the above oligoalkylamines with C12 [120]. The specific targeting mechanism is not mentioned in the above articles, but we infer that the liver-targeting may relate to the first pass effect of liver with intravenous injection or the protein corona formed on the vectors' surfaces. Beyond liver, researchers are exploring delivery systems for other organs of interest to fully realize the potential of RNA-based drugs.
Targeting of the lung and spleen
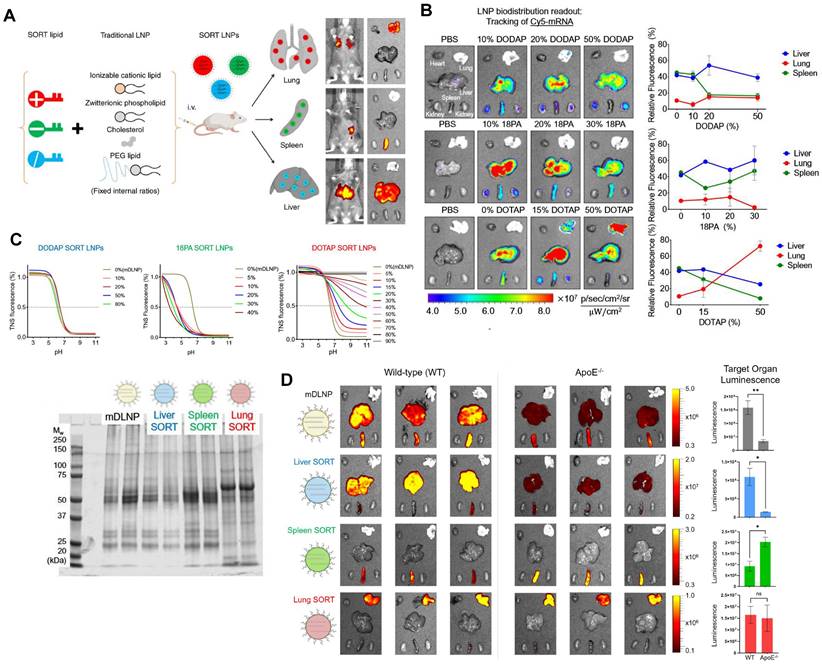
Typical LNPs are composed of four compositions and exert the characteristics of liver-targeting. Recently, Cheng et al. discovered that the presence of the fifth component, called 'Selective Organ Targeting (SORT)' molecule, could alter the organ-targeting effect of LNPs in vivo. The organ selectivity depended on the type and amount of SORT lipid added (Figure 6A-B). Notably, LNPs with four components primarily target the liver, but the luminescence activity would transfer from the liver to the spleen, and finally to the lung tissues with the increase of the fifth component-permanent cationic lipids, such as DOTAP, DDAB, and EPC. Encouragingly, the addition of negatively charged lipid, 18PA, led to spleen targeting. 14PA and 18BMP addition results demonstrated that the targeting ability was independent of the structure of negatively charged lipids used. In addition, ionizable cationic SORT lipids, such as DODAP and C12-200, enabled enhanced liver delivery without altering the tissue tropism [17,121]. Further mechanism was investigated for this organ-specific targeting. SORT lipid molecules were shown to affect the apparent pKa of LNP and the interaction between LNP and serum proteins (Figure 6C). Furthermore, targeting of LNPs to the spleen and lungs is apolipoprotein independent (Figure 6D) [121]. It is worth mentioning that this targeting strategy has been verified to deliver Cas9/sgRNA ribonucleoprotein complexes (RNPs) and achieved gene editing successively in liver and lung of the mice model [122]. These findings demonstrate tremendous potential, particularly in the field of gene therapy. Thus far, the LNP-SORT platform has been established and has the potential to be used in treating primary ciliary dyskinesia (PCD) and cystic fibrosis (CF) in the future [17].
SORT molecules allowed LNPs to achieve targeted delivery of mRNA for different organs. (A) Addition of different SORT molecules mediated the tissue-specific targeting delivery of LNPs. Adapted with permission from [17], copyright 2020, Springer Nature. (B) Increase of percentage of different SORT molecules altered the bio-distribution of fluorescence of Cy5-mRNA. (C) The addition of different percentages of SORT molecules affected the pKa of LNPs determined by TNS assay and plasma proteins absorbed in surface of LNPs visualized by SDS-PAGE. (D) The bioluminescence of functional proteins showed that the targeting of SORT LNPs in lung and spleen was ApoE-independent. Adapted with permission from [121], copyright 2021, National Academy of Sciences.
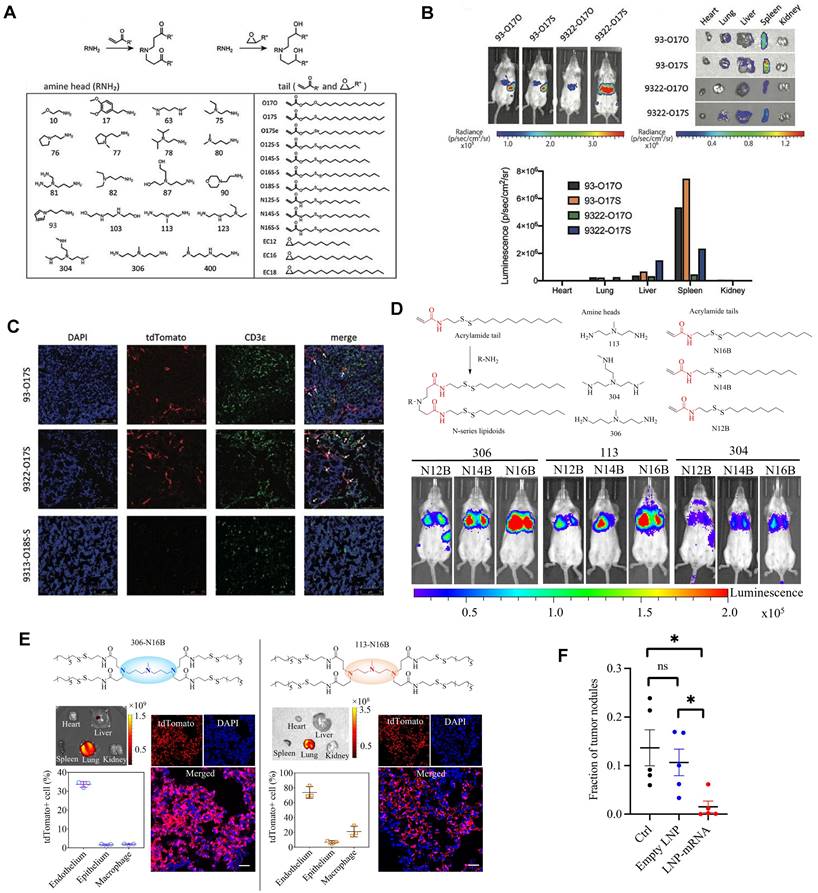
Cationic lipid has been regarded as the critical component in LNPs, and its chemical tailoring provides another option for mediating organ-targeting. For instance, Xu and colleagues developed imidazole derived cationic lipids that could preferentially deliver mRNA to the primary T lymphocytes of the spleen, achieving cellular level targeting (Figure 7A-C). Subsequently, N-series LNPs with amide bonds in the lipidoid tails, particularly 306-N16B LNPs, were identified as the ideal candidates for lung targeting (Figure 7D). More importantly, altering the head group of N-series cationic lipid targeted different subcellular populations of lung as shown in Figure 7E [114,115,123]. Following these, the lung targeting capacity of N-series LNPs was validated by delivering Tsc2 mRNA for the treatment of pulmonary lymphatic leiomyoma in a preclinical model (Figure 7F) [115]. Therefore, tailoring the chemical structures of lipids, such as the amine head and linker, might provide a way forward for organ tropisms.
Imidazole-based lipidoids for spleen-targeting and N-series LNPs for lung-targeting. (A) Synthesis of imidazole-based lipidoids. (B) Screening LNPs according to bioluminescence images of whole body and each organ with IVIS. (C) Detection of tdTomato expression in spleen by confocal microscopy and T cells were marked by CDε antibody. Adapted with permission from [123], copyright 2020, Wiley-VCH. (D) Synthesis and screening of N-series LNPs by whole body bioluminescence images with IVIS imaging system. (E) Changing the head structure of N-series LNPs could target different pulmonary cell types represented by immunofluorescence images of lung tissue and quantification of tdTomato+ cell percentages in different pulmonary cell types of 306-N16B and 113-N16B. (F) Tsc2 mRNA-loaded LNP could exert therapeutic effect of inhibiting growth of tumor in lung. Adapted with permission from [115], copyright 2022, National Academy of Sciences.
The spleen, which is rich in lymphocytes and macrophages, is an important immune organ. A library of ionizable amino-polyesters were synthesized via ring-opening polymerization of lactones and tertiary amino-alcohols. These polymers were incorporated in LNPs (APE-LNPs), which achieved mRNA expression in hepatocytes, pulmonary endothelial cells, and antigen presenting cells of the spleen [124]. In another study, Anderson and colleagues synthesized a series of alkenyl amino alcohols (AAA) lipids through ring-opening reaction of alkenyl epoxides and a polyamine core as shown in Figure 8A. OF-02 LNP was identified as the top vector to achieve liver-targeting in vivo, showing superior delivery efficiency compared to cKK-E12 LNPs (Figure 8B) [125]. Afterwards, based on OF-02 lipid, OF-Deg-Lin lipid was designed with a diketopiperazine core and four esterifiable unsaturated tails (Figure 8C). Further investigation showed that OF-Deg-Lin LNPs could target B lymphocytes of the spleen, inducing luciferase expression in the spleen after FLuc mRNA delivery (Figure 8D-E) [126]. Additionally, OF-Deg-Lin was further modified via changing the carbon linker length from two to four to obtain OF-C4-Deg-Lin lipid, showing higher efficacy for mRNA delivery [127]. Gomi et al. prepared a series of LNPs with alcohol-soluble phosphatidylserine (PS) molecules, which targeted spleen for mRNA delivery and translation, demonstrating application potential in immunotherapy and vaccines [128]. Based on a siRNA vector TNT-a10, Dong and colleagues optimized the position of functional groups to obtain the TNT-b10. Further optimizing the formulation of TNT-b10 LLNs resulted in a high signal of mRNA expression in the spleen, which was 10 times higher than that in the liver with intravenous injection [129]. Based on LNPs, Cao et al. combined helper-PBAEs and DOTAP to develop five-element nanoparticles (FNPs) for lung-targeted delivery of mRNA with high stability after lyophilization [130]. Tombácz et al. combined the CD4 antibody with LNPs, achieving much higher accumulation of radiolabeled mRNA in the spleen compared to non-modified LNPs after systemic administration. Further expression of a reporter gene showed CD4-targeted LNPs could specifically deliver Cre mRNA to the spleen and lymph nodes [131]. Notably, lymph nodes are place where various immune cells gather and immune responses occur and this research reminds that by conjugating antibodies or ligands for specific immune cells subsets, it is possible to achieve lymph node targeting for activating immunoreaction in some tumor or infection models.
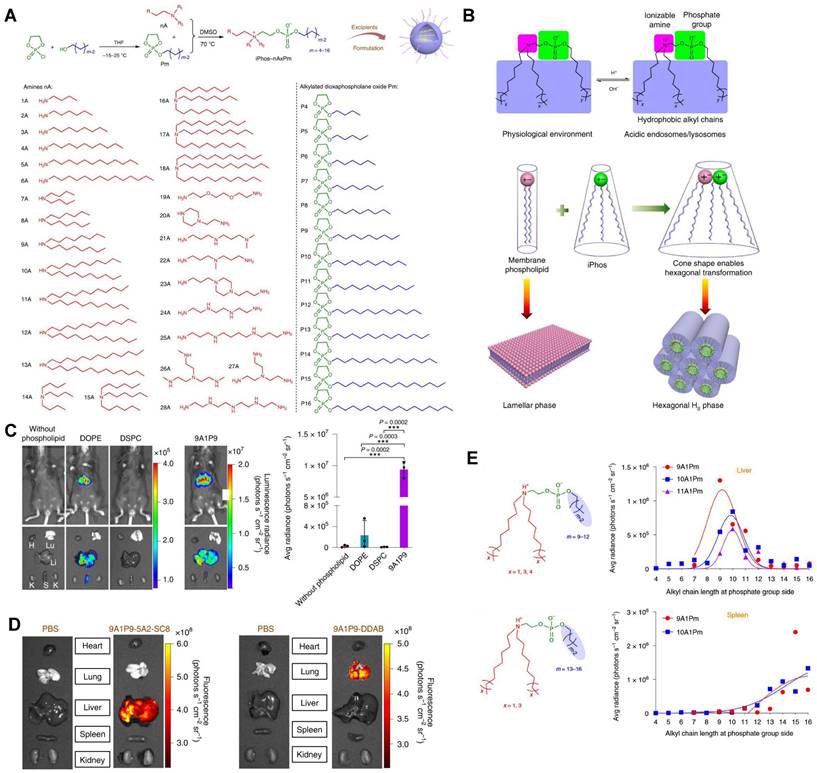
CRISPR/Cas9 gene editing technology has shown revolutionary impact on the gene therapy field. However, delivering the editing tool safely, effectively, and accurately to the target sites remains a significant challenge [27,28]. To address this issue, it is necessary to improve the endosomal escape of the drug-loading nanoparticles and achieve extrahepatic targeting. A library of novel ionizable phospholipids (iPhos) with strong endosomal escape properties were designed and synthesized by Liu et al. (Figure 9A) [31]. Most biofilm phospholipids adopted a bilayer architecture, but when iPhos entered the acidic endosomes, the tertiary amines of iPhos lipids would protonate to form a zwitterionic head and insert into endosomal membranes to produce a cone shape. This process would generate hexagonal transition and cause rupture of endosomes to release the contents (Figure 9B). iPhos based LNPs (called iPLNPs) were utilized to deliver mRNA or Cas9 mRNA/sgRNA in follow-up studies and this new delivery system achieved extremely high mRNA delivery superior to benchmark phospholipids, DOPE and DSPC (Figure 9C). Also, efficient CRISPR/Cas9 gene editing in liver and extrahepatic organs were achieved (Figure 9D). Moreover, the relationship between iPhos structures and organ selectivity were revealed: 1) The alkyl length next to the phosphate group could determine the organ selectivity: a shorter length of 9 to 12 carbons would deliver mRNA to the liver for protein expression, while a longer length (13 to 16 carbons) benefited spleen delivery (Figure 9E). 2) Selective mRNA expression or CRISPR/Cas9 gene editing could be achieved in the spleen, liver, and lungs with the delivery of 9A1P9 iPLNPs containing zwitterionic, ionizable cationic, and permanent cationic helper lipids, respectively [31]. In another study, Miller et al. reported that zwitterionic amino lipids (ZALs) could co-deliver Cas9 mRNA and sgRNAs with a high efficacy in vitro and in vivo. Specifically, ZA3-Ep10 LNPs enabled gene editing in the liver, kidney, and lungs [32]. High-throughput screening technique, as one of the effective methods to screen nanoparticles with extrahepatic tropism, is capable of quantifying numerous delivery nanoparticles and protein expression in vivo in a short time [132,133]. Dahlman and colleagues developed a high-throughput screening technique, called FIND, which could significantly improve the screening efficiency of LNPs. Using this technique, they verified two LNPs, 7C2 and 7C3, which were synthesized based on the 7C1 and composed of different four compositions and ratios, could deliver Cas9 mRNA and sgRNA into endothelial cells of the spleen [133].
OF-XX series lipids for spleen-targeting. (A) Synthesis route of OF-XX lipids by ring opening reaction between alkenyl epoxides and polyamine core. (B) EPO expression following mRNA delivery with OF-XX LNPs and cKK-E12 LNP in vivo. Adapted with permission from [125], copyright 2016, Wiley-VCH. (C) Synthesis route of OF-Deg-Lin. (D) Quantification of cell populations labeled by Cy5 mRNA delivered with OF-Deg-Lin showed that B lymphocytes were the main targeted cell population. (E) Luciferase expression showed that OF-Deg-Lin LNPs could deliver FLuc mRNA to the spleen of mice. Adapted with permission from [126], copyright 2017, Wiley-VCH.
Researchers have also investigated the relationship between other vector structures and their targeting abilities, beyond LNPs. Kaczmarek et al. reported that PBAE DD90-C12-122 could deliver mRNA to the lungs after intravenous administration, and PEGylation increased the delivery efficacy. Further optimizations, such as varying the carbon chain lengths of alkylamine and the molar ratios of diacrylate and amines, would lead to polymeric nanoparticles with greater delivery potential targeting different subtypes of lung. Analysis of the lung cell types after administrating the optimized formulation of polymers showed the transfected cells were mainly among pulmonary endothelial cells and few immune cells [69,134]. Recently, Kaczmarek et al. synthesized a library of PBAEs and identified two polymers, D90-C12-103 and DD90-C12-103, which used diacrylate-amine as the backbone, could deliver pDNA and mRNA to the lung of mice post systemic administration. Results showed that the fluorescence peak of mRNA was much higher than that of pDNA in vivo after delivery and area analysis of radiance flux and luminescent images at different time points demonstrated that DD90-C12-103 vector was superior to D90-C12-103 for mRNA delivery [135]. A degradable polyester library modified with amino thiols and alkyl thiols was built and screened in vitro and in vivo. A top polymeric vector, PE4K-A17-0.33C12, formulated with 5% F127 and FLuc mRNA was verified to show high luminescence activity in the lung after intravenous administration [136]. Recently, a polymersome library consisting of cationic and helper polymers was designed and synthesized by modifying poly(ethylene glycol) block poly(lactide-co-glycolide) (PEG-PLGA) with various oligopeptides and charged groups. PA9-ZP3 and PH9-Aln were identified which could achieve outstanding liver- and spleen-targeting respectively. Further investigation demonstrated the bisphosphonate group and oligo-histidine played an important role in spleen-targeting and the organ-selective delivery was relevant to the sizes, charges, protein corona formed on the surface of vectors [137]. In summary, organ-targeting is largely associated with vector structures. Cationic materials with excess positive charges (as the main or helper component) trend to deliver RNA cargoes to the lung, while negatively charged component incorporation shows spleen tropism.
Targeting of other organs or tissues
To date, the liver, lung, and spleen are commonly targeted organs in current research, however, targeting other organs such as the eyes, skin, heart, and brain holds great potential but remains more challenging in clinical applications.
A series of novel iPhos for mRNA targeted delivery. (A) Synthesis routes of iPhos lipids by conjugating amines and alkylated dioxaphospholane oxide molecules. (B) Schematic illustration of hexagonal transition of biofilm phospholipids with the addition of iPhos lipids, which contained one zwitterionic head and three hydrophobic alkyl tails, in acidic environment. (C) Bioluminescence images and quantification of luciferase expression showed that the delivery efficiency of mRNA by iPhos 9A1P9 were superior to that of commonly used phospholipids, DOPE and DSPC. (D) Cas9 mRNA delivery and gene editing were achieved in liver and lung with 9A1P9-5A2-SC8 iPLNPs and 9A1P9-DDAB iPLNPs respectively. (E) iPhos with alkyl group length of 9 to 12 carbons resulted in highest mRNA expression in liver, 13 to 16 carbons resulted in highest mRNA expression in spleen. Adapted with permission from [31], copyright 2021, Springer Nature.
Despite the translation potential, most LNPs tend to accumulate in the liver, and overcoming the natural liver targeting characteristics of LNPs has become a key challenge in nano-vector research. Recently, Sahay group developed LNPs decorated with an oligomer peptide to deliver mRNA to neural retina in rodents and non-human primates after intravitreal administration. This breakthrough demonstrates the potential of LNP-mRNA in the treatment of inherited retinal diseases and represents significant progress in LNP penetrating biological barriers to achieve extrahepatic targeting [138]. Xue et al. synthesized a series of LNPs with bisphosphonate (BP)-lipid, which showed higher affinity for bone-related fragments in vitro and higher bone-targeting in vivo compared to LNPs without BP-lipid [139]. Another example of extrahepatic targeting involves decorating LNPs with CD5 antibody to produce CAR T cells transiently by delivering mRNA to target T cells. In a mouse model of heart disease, the targeted-LNPs encapsulating modified-mRNA could reduce the fibrosis degree of heart and restore heart function [140].
The skin is the largest organ in the human body and skin aging is related closely to the fibroblast in dermis. Recently, Francisco and Ferreira designed hundreds of polymers and selected six polymers with similar chemical structures composed of diacrylate, special amine, and bisacrylamide, which could transfect mouse fibroblasts with high efficiency in vitro. Among them, P1E28 containing alkyl alcohol side chain and piperazine rings with two tertiary amines showed the highest transfected efficiency. In vivo results showed that P1E28 polymeric nanoparticles delivered Cre mRNA to skin dermal fibroblasts with a higher delivery efficiency than endothelial cells, keratinocytes and macrophages. Further mechanism analysis indicated the ability of P1E28 to target skin fibroblasts might be mediated by CD26 and FAP overexpressed in fibroblasts [141].
Brain represents another hard-to-target organ, and the presence of BBB limits the therapeutic effect of brain diseases [97,142]. One innovative solution is the use of neurotransmitter-derived lipidoids (NT-lipidoids), which can efficiently deliver drugs to the brain via intravenous injection. By lipidating the neurotransmitter, the formulated lipid nanoparticles could pass through the BBB by mediating receptors and enter the central nervous system (CNS) to eventually release encapsulated small molecule drugs, macromolecules and gene editing proteins in neuronal cells. It has been demonstrated that the addition of NT1-lipidoids to facilitate vectors penetrating the BBB is suitable for various types of LNPs [143]. Above study reminds us that delivering the drugs through receptors in the brain is an effective strategy, and transferrin receptor (TfR) has been regarded as one of the most promising targets [142]. Rodrigues et al. modified liposomes with cell-penetrating peptides and transferrin ligands, achieving the targeting of brain capillary endothelial cells expressing TfR [144]. TfR has also been identified as a promising target for glioblastoma, a lethal brain cancer, as its expression is up to 100 times higher in cancer cells than that in healthy cells [145,146]. Exosomes functionalized by T7 peptide with binding ability to TfR could deliver antisense miRNA oligonucleotides against overexpressed miR-21 in glioblastoma to reduce the tumor size in vivo [147]. Additionally, nanoparticles coated with polysorbate 80 (PS 80) were developed to combine with apolipoprotein for interacting with lipoprotein receptors. By adjusting coating density, siRNA delivery across BBB was achieved for the treatment of brain diseases via inhibiting the expression of tau protein in a traumatic brain injury mouse model [148]. Overall, these studies demonstrate that targeting BBB relevant receptors can be an effective strategy for delivering drugs into the brain.
Targeting of the tumor cells and immune cells
Cancer is one of the leading causes of death in humans. Targeting drugs provide a precise way to target tumor cells without affecting normal cells, reducing side effects and improving overall survival rates compared to traditional chemotherapeutic drugs [149]. Currently, one of the main methods to achieve tumor targeting is by attaching antibodies or ligands (such as peptide ligands mentioned above [147]) to vectors that bind to surface receptors of tumor cells [150]. Monoclonal antibodies (mAbs) are considered to be the most promising candidates against cancer [151]. Generally, mAbs have the function of both targeting and anti-tumor effect. Great progress has been made for siRNA delivery with the help of mAbs, but there is no successful clinical translation until now, which is limited by the acquirement of LNP optimization according to different mAbs to some extent [151]. To address this limitation, Peer et al. developed a modular targeting platform called anchored secondary scFv enabling targeting (ASSET), which was a membrane-anchored lipoprotein integrated with LNP and could interact with Fc constant domain of the antibody. The results showed that siRNA-loaded LNPs formed by adding ASSET and RIg could be taken up by targeted cells in vitro and produce desired gene knockdown in vivo. Further research found that altering RIg could achieve targeting of various leukocyte subsets specifically, demonstrating the potential applications of this platform in different disease models [152]. In another study, Peer et al. utilized the ASSET strategy to connect anti-Ly6c+ mAbs to LNPs, achieving targeted delivery of therapeutic mRNA to Ly6c+ inflammatory leukocytes of mice with inflammatory bowel disease. These results highlight the potential of ASSET in the field of cancers, inflammatory diseases, and rare genetic disorders [153].
Cancer immunotherapy is a highly effective approach to fight against cancer, including using cancer vaccines. However, a major obstacle lies in how to target dendritic cells (DCs) to activate the immune response. In a recent study, RNA-lipoplexes (RNA-LPX) without ligand decoration were designed to target DCs for the delivery of cancer antigens and activation of immunoreaction post intravenous administration. Through adjusting the charge ratio of formulation, the positively charged RNA-LPX primarily targeted the lungs and the fluorescence expression shifted from lung to spleen with a reduction of the cationic lipid content. Notably, in a phase I clinical trial (NCT02410733), three melanoma patients were treated with RNA-LPX encoding the tumor antigens, and the produced IFNα and T-cell responses demonstrated the broad applicability of the RNA-LPX considering that antigens of any tumor cells could be encoded by RNA [153]. RNA-based immunotherapy is making rapid progress in cancer treatment, with the help of advanced delivery technology.
Administration routes
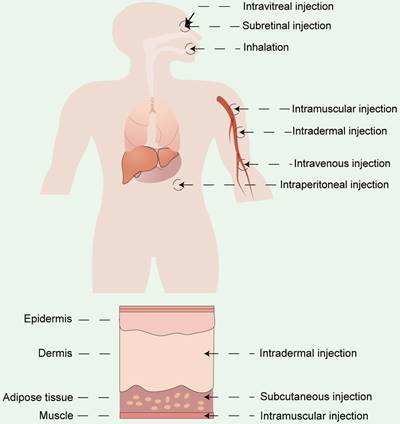
Apart from vector materials, the routes and sites of administration significantly influence the therapeutic outcomes and biodistribution of RNA drugs [154]. Currently, the two primary modes of drug delivery in clinical practice include systemic and local administration. RNA drugs are usually administrated systemically via intravenous (i.v.) injection or locally administrated via subcutaneous (s.c.) injection, intramuscular (i.m.) injection, intradermal (i.d.) injections, and so on [155,156]. Drugs can also be delivered by inhalation for pulmonary disease treatment [86,157] or by site-specific administration (e.g. heart [158], eyes [159,160], and brain [161]) (Figure 10). The selection of the appropriate administration routes depends on the physicochemical properties and the desired therapeutic effects.
Systemic administration
Intravenous injection is commonly used for LNP-mediated delivery of mRNA or siRNA drugs. This route of administration allows the drugs to reach the target site through circulation. Notably, most LNPs administrated via the intravenous injection are prone to concentrate selectively in the liver because of its abundance of ApoE, exerting an essential influence on LNP-mediated RNA drugs [162]. So far, systemic administration of non-viral vectors often results in significant hepatic specificity. Encouragingly, Melamed et al. reported that intraperitoneal administration of LNPs enabled a shift in specific mRNA expression from the liver to the pancreas [163]. However, non-liver targeting through systemic administration to broaden the application prospect of RNA drugs still remains challenging [18].
Different administration routes for RNA drugs, including systemic administration and local administration.
Numerous efforts have been made to systemically deliver RNA to the lung for therapeutic use in pulmonary diseases. For instance, by rationally designing the structure of cationic lipids, Qiu et al. converted LNPs from liver-targeting to lung-targeting [115]. In addition, suitable vectors enabled mRNA to be expressed in different cell subtypes of the spleen, including endothelial cells, macrophages, and antigen-presenting cells, enhancing the immune response [154,164,165]. To date, the majority of RNA-based vaccines have depended on draining lymph nodes to reach specific sites primarily through intramuscular or subcutaneous injections [166]. However, intravenous delivery of RNA might become another alternative approach, since there are plenty of non-immune cells at the injection sites. Luozhong et al. developed phosphatidylserine LNPs that could mediate effective mRNA expression in both lymph nodes and spleen after intravenous injection [167]. In a transformative study, Cheng et al. reported a SORT strategy, which achieved the redirection of LNPs to extrahepatic organs by supplementing SORT molecules. Specifically, LNPs were shown to target the spleen by adding anionic lipids and modulating their formulations [17]. Beyond LNPs, Liu et al. showed that zwitterionic phospholipidated polymers efficiently delivered mRNA to the spleen, and these polymers with specific amphiphilic and zwitterionic structures exhibited lymph node transfection capability [88]. These findings demonstrate that designing appropriate delivery materials can enable RNA drugs to be delivered into immune organs through systemic administration, opening new opportunities for immunotherapy.
Local administration
As systemic delivery targets specific organs with intricate difficulties, local administration can be a preferable alternative for certain clinical applications. RNA-based drugs administered by topical delivery are expected to show a therapeutic effect at the specific site. In the case of treating pulmonary diseases, RNA drugs can be administrated to the lung via intravenous administration, inhalation or intratracheal injection [68,168,169]. mRNA delivery by nebulization is shown to deliver more therapeutics to the lungs compared to systemic route [170]. Efficient delivery of therapeutic RNAs is also an appealing strategy for ophthalmic applications requiring pro-chromatic expression of proteins in the retina. However, due to the complicated biological barriers of the ocular surface and the unstable physicochemical characteristics of RNAs, topical administration becomes the preferred administration method for ophthalmic treatment regimens. Devoldere and colleagues demonstrated that higher therapeutic effect of chemically modified mRNAs could be achieved by administrating on the photoreceptor side rather than through the vitreous side [171]. Notably, a recent study reported an intradermal delivery of mRNA-loaded extracellular vesicles via a microneedle-based system and indicated that this technique could be used as a collagen replacement therapy for the treatment of skin diseases and aging [172]. Interestingly, local administration may also produce systemic therapeutic effects. For instance, therapeutics delivered subcutaneously are capable of entering the systemic circulation as well as lymphatic circulation at a slow release rate, providing a longer period of time for the receptors that are closely linked to mediated cellular uptake [173]. Specifically, RNA-based vaccines used in clinical practice are usually administered intradermally or intramuscularly, which are associated with the site of presence of antigen-presenting cells. Nowadays, many mRNA vaccines are administrated via intramuscular injection, enabling the drugs to be concentrated in the draining lymph nodes and trigger a strong immune response from T cells [61].
Physicochemical properties
After numerous studies presenting the challenges faced by vectors in delivering RNA, it is evident that the barriers preventing the selective accumulation of drugs may be closely associated with the physicochemical properties of designed vectors [4,81]. For the purpose of achieving site-specific accumulation of RNA drugs, researchers have scrutinized various physical parameters of non-viral vectors, including protein corona, particle size, surface and vector charge, and particle shape, with the aim of optimizing the potential for targeted delivery of nanoparticles.
Protein corona
The formation of protein coronas is believed to be closely associated with the biodistribution of nanoparticles in the body. Upon intravenous injection, the nanoparticles come into contact with the serum, leading proteins to adsorb on their surface and form a distinctive protein corona layer [121]. The presence of the protein corona may alter the properties of the nanoparticles, with profound influences for their tissue specificity. For instance, most LNPs exhibit hepatic specificity via intravenous administration, owing to the adsorption of ApoE by binding to the LDLR that are over-expressed on the hepatocytes [121]. Additionally, it has been validated that the knockdown of ApoE lowered the liver targeting of nanomaterials by delivering mRNA into a mouse model lacking ApoE expression [121]. Although the mechanism of endogenous targeting is still not fully explored, researchers have striven to investigate the rules. It is discovered that when the PEG-lipids desorb from LNPs, specific protein binding to LNPs is promoted. The desorption rate of PEG-lipids with longer hydrophobic chains is slower, which might not conducive to formulating appropriate protein coronas and achieving organ targeted delivery [121]. In parallel, nano-vectors with different structures and properties may adsorb different proteins, resulting in protein coronas with distinct components and properties. The formation of protein corona at the interface will alter the physicochemical characteristics of nanoparticles, exhibiting a crucial impact on their biodistribution and endocytosis [162]. It has been demonstrated that LNPs with different pKa values can influence their organ targeting. More specifically, the preferred pKa range for liver, spleen, and lung targeting is 6.2~6.5, 2~6, and 7~9, respectively [121,174,175]. These theories have implications for guiding the design of other delivery systems as well.
Particle size
The particle size and the dispersion of delivery vectors play a crucial role in constructing target delivery systems that meet different clinical needs. In order to overcome multiple intracellular and extracellular delivery barriers, attention should be paid to how the vector size affects the bio-interface interactions. Vectors of different sizes present different organ or tissue tropism in vivo. For instance, previous studies have revealed that nanoparticles with a diameter of approximately 5 nm are rapidly cleared by the renal filtration process [176]. Most delivery vectors with particle sizes of 50~150 nm are prone to exhibit selective accumulation in the liver, such as LNPs, owing to the structure of endothelial cells [81]. Furthermore, particles with diameters over 200 nm may possess splenic targeting capability due to the size of inter-endothelial cells. The pulmonary epithelium offers a large area (over 100 m2), making it an appealing target for RNA therapeutics upon systemic drug delivery. To maximize the drugs' efficacy in the lungs, particles with diameters of less than 3 μm are generally preferred, as they optimize deposition in the alveoli [169]. Also, previous studies have reported that the transport capacity of polymer vectors in the blood can be significantly altered by varying their sizes [177]. Specifically, the authors compared minor size differences to the effect of PAMAM dendrimers and results showed that the higher molecular weight polymers were able to accumulate more intensively in the brain [178]. Since brain-targeted delivery usually requires receptor-mediated or vector-mediated transport passing through the blood-brain barrier, it is assumed that nanocomplexes with ultra-small dimension are more likely to cross the blood-brain barrier [177]. Thus, small polyplexes functionalized with ligands, such as synthetic peptides, can be designed for targeting. Size is a crucial factor in determining how the particles are taken up by the cells, and it can significantly influence therapeutic sites of drugs.
Surface and vector charges
Surface and vector charges of nanoparticles are significant physicochemical properties that can be engineered to enhance the site-specific accumulation of RNA vectors and affect their delivery profile [179]. Surface charge plays an important role in cellular internalization of the nanoparticles and contributes to overcoming the biological barriers during the drug delivery process [162]. When the vectors show neutral or negative charges, they may have less adsorption of serum proteins, leading to a prolonged circulation time. In contrast, positively charged vectors tend to present a higher cellular uptake rate. Since polymers with neutral charges are prone to aggregation and precipitation, researchers usually use positively charged polyplexes to enhance cellular uptake via the interaction with negatively charged cell membranes. However, high-density surface positive charges can be correlated with corresponding toxicity, thus limiting their application to some extent. In accordance with the desired therapeutic effect, researchers have developed amphiphilic polymers or ionizable lipid nanoparticles that exhibit outstanding endosomal escape efficiency than conventional permanent positive vectors. These new materials are generally neutral under the physiological condition so as to mitigate the toxicity [177,180]. In addition, Cheng et al. adjusted the lipid charges in LNPs, mediating controlled pKa and specific organ tropism of nano-systems [17]. It was shown that spleen-targeted delivery was more likely to be achieved when negatively charged lipids were introduced into the vectors, whereas the introduction of positively charged lipids might facilitate the delivery of RNA to the lungs. Vectors with different pKa may likewise exhibit a propensity to accumulate in different organ tissues, which is one of the pivotal factors in the design of vectors. Therefore, the rational design of non-viral vectors with desired charges can lead to satisfactory therapeutic effects for RNA-based drugs.
Other properties
Other characteristics, such as particle shape and biodegradability, should also be taken into consideration when designing and evaluating the delivery performance of vectors. Studies have demonstrated that the shape of the nano-vectors plays a crucial role in its delivery journey. For instance, disc-shaped nanoparticles interact more effectively with the vessel wall and exhibit higher specificity for endothelial cells compared to spherical ones [181,182]. Moreover, the stiffness of nanoparticles has been implicated to affect their clearance in the blood. For instance, the increased clearance of cholesterol-modified liposomes in the spleen might be due to their increased stiffness [183]. Moreover, DeSimone and colleagues emphasized the significance of the elastic properties of the vectors on the circulation time and biodistribution of the therapeutics [184]. In summary, physicochemical properties are underestimated in designing delivery systems, and their exploration in depth would help development of next-generation RNA vectors.
Clinical advances in targeted delivery of RNAs
Over 20 years have passed since the first RNA-based drug was approved by FDA, and now several RNA drugs have been approved for therapeutic applications in different diseases, including TTR-mediated amyloidosis, duchenne muscular dystrophy, hypercholesterolemia, etc. Additionally, hundreds of candidate drugs are currently under clinical studies. Here, we present a summary RNA-based drugs that have been applied in clinics (Table 1), including information on the types of vectors used, administration routes, the diseases treated and the related organs.
Clinical advances of mRNA drugs
mRNA therapy has gained immense popularity since the outbreak of COVID-19. Although no mRNA drug for cancer treatment has been approved by the FDA, there are several mRNA-based therapies for cancer in various stages of clinical trials, which can be divided into two major categories roughly based on the encoded antigens [196]. The first category is cancer vaccines that encode tumor-associated antigens, representing the major type of mRNA-based cancer therapy. mRNA-4157 from Moderna & Merck has made a great breakthrough for the treatment of melanoma in phase II clinical trial (NCT03897881) and solid tumor in phase I clinical trial (NCT03313778) [197]. And recently, the FDA has granted a breakthrough therapy designation to mRNA-4157/V940 in combination with PD-1 antibody for adjuvant therapy for high-risk melanoma to prevent postoperative recurrence, which inspired the progress of mRNA therapy in the field of vaccine. In addition, the companies plan to conduct phase III studies on melanoma and expand the treatment range to other tumor types, including non-small cell lung cancer. The second category is immuno-oncological treatments that use mRNA drugs. For instance, mRNA-2752 is encapsulated in LNPs to encode OX40L, IL-23, and IL-36γ, which can promote cytokine release and activate T cells to kill tumor cells. It has shown the effect in slowing tumor growth of patients with intratumoral injection [187]. Currently, mRNA-2752 is in phase I clinical trial for the treatment of solid tumors and lymphoma (NCT03739931). The first inhaled mRNA drug MRT5005 is also in I/II clinical trial (NCT03375047) and aims to treat CF by delivering mRNA encoding CFTR to pulmonary epithelial cells through nebulization. Additionally, in the field of gene editing covering gene knockout and gene insertion, mRNA showed good clinical results. NTLA-2001, utilizing LNP encapsulating Cas9 mRNA and a sgRNA to target TTR, achieved knockout of TTR gene and decreased the concentration of TTR protein in serum by 52% with a dose of 0.1 mg/kg and 87% with a dose of 0.3 mg/kg in clinical phase I (NCT04601051). Additionally, NTLA-2002, targeting KLKB1 gene, could block production of kallikrein and further reduce bradykinin to treat hereditary angioedema. In clinical I/II trial (NCT05120830), the NTLA-2002 encapsulating Cas9 mRNA and KLKB1-specific sgRNA delivered by LNP resulted durable reductions of kallikrein protein in plasma with well tolerability.
All the mRNA drugs mentioned above utilize LNPs as the delivery vectors, highlighting their significance in the field of RNA-based drugs. However, one exception is AZD-8601 mRNA, which has entered clinical trial (NCT03370887) for heart failure. AZD-8601 mRNA is formulated in citrate buffered saline with biocompatibility and is delivered by epicardial injections to express vascular endothelial growth factor A protein directly. Developed by AstraZeneca and Moderna, this drug increased left ventricular ejection fraction and enhanced heart function compared to placebo group [188]. Despite the limited mRNA drug research in the past, recent laboratory experiments and clinical trials have demonstrated the potential of mRNA drugs in various fields, including protein replacement therapy, cancer immunotherapy, and gene editing, covering the therapy of liver, lung, heart-related diseases, and tumors. Table 2 provides some examples of mRNA drugs in clinical trials for diseases in different organs and tissue sites.
The ribonucleic acid therapeutics approved by FDA.
| Products | Category of ribonucleic acids | Approval year | Delivery vectors | Administration routes | Diseases | Targeted organs |
|---|---|---|---|---|---|---|
| Fomivirsen | ASO | 1998 (withdrawn) | - | Intravitreal | Cytomegalovirus infection | Eye |
| Pegaptanib | Aptamer | 2004 (withdrawn) | - | Intravitreal | Wet Macular Degeneration | Eye |
| Mipomersen | ASO | 2013 (withdrawn) | - | Subcutaneous | Hypercholesterolemia | Liver |
| Eteplirsen | ASO | 2016 | - | Intrathecal | Duchenne muscular dystrophy | Skeletal muscle |
| Nusinersen | ASO | 2016 | - | Intrathecal | Spinal muscular atrophy | Spinal nerves |
| Inotersen | ASO | 2018 | - | Subcutaneous | TTR-mediated amyloidosis | Liver |
| Patisiran | siRNA | 2018 | LNP | Intravenous | TTR-mediated amyloidosis | Liver |
| Volanesoren | ASO | 2019 | - | Subcutaneous | Familial chylomicronemia syndrome | Liver |
| Golodirsen | ASO | 2019 (confirmatory trial required) | - | Subcutaneous | Duchenne muscular dystrophy | Skeletal muscle |
| Givosiran | siRNA | 2019 | GalNAc | Subcutaneous | Acute hepatic porphyrias | Liver |
| Viltolarsen | ASO | 2020 | - | Intravenous | Duchenne muscular dystrophy | Skeletal muscle |
| Inclisiran | siRNA | 2020 | GalNAc | Subcutaneous | Hypercholesterolemia | Liver |
| Lumasiran | siRNA | 2020 | GalNAc | Subcutaneous | Primary hyperoxaluria type 1 | Liver |
| Casimersen | ASO | 2021 | - | Subcutaneous | Duchenne muscular dystrophy | Skeletal muscle |
| BNT162b2 | mRNA | 2021 | LNP | Intramuscular | COVID-19 (emergency use) | - |
| mRNA-1273 | mRNA | 2021 | LNP | Intramuscular | COVID-19 (emergency use) | - |
| Vutrisiran | siRNA | 2022 | GalNAc | subcutaneous | TTR-mediated amyloidosis | Liver |
The clinical trials of mRNA targeting different tissues or organs.
| Name | Delivery vectors | Administration routes | Diseases | Clinicaltrials.gov identifier | Phase |
|---|---|---|---|---|---|
| Liver | |||||
| ARCT-810 | LNP | Intravenous | Ornithine transcarbamylase deficiency | NCT05526066 | II |
| NTLA-2001 | LNP | Intravenous | TTR-mediated amyloidosis with polyneuropathy | NCT04601051 | I |
| mRNA-3745 | LNP | Intravenous | Glycogen storage disease | NCT05095727 | I |
| mRNA-3705 | LNP | Intravenous | Isolated methylmalonic acidemia | NCT04899310 | I/II |
| mRNA-3927 | LNP | Intravenous | Propionic acidemia | NCT05130437 | I/II |
| Lung | |||||
| ARCT-032 | LNP | Inhalation | Cystic fibrosis | NCT05712538 | I |
| MRT5005 | LNP | Inhalation | Cystic fibrosis | NCT03375047 | I/II |
| VX-522 | LNP | Inhalation | Cystic fibrosis | NCT05668741 | I |
| Heart | |||||
| AZD8601 | No vector | Epicardial Injection | Heart failure | NCT03370887 | II |
| mRNA-0184 | Unknown | Intravenous | Heart failure | NCT05659264 | I |
| Tumor | |||||
| mRNA-4157 | LNP | Intramuscular | Melanoma | NCT03897881 | II |
| mRNA-4157 | LNP | Intramuscular | Solid tumor | NCT03313778 | I |
| mRNA-2752 | LNP | Intratumoral | Solid tumor Lymphoma | NCT03739931 | I |
| BNT111 | Liposome | Intravenous | Melanoma | NCT04526899 | II |
| BNT122 | Liposome | Intravenous | Colorectal cancer | NCT04486378 | II |
| BNT151 | Liposome | Intravenous | Solid tumor | NCT04455620 | I/II |
| CV9201 | Protamine | Intradermal | Non-small cell lung cancer | NCT00923312 | I/II |
| CV-9202 | Protamine | Intradermal | Non-small cell lung cancer | NCT03164772 | I/II |
Clinical advances of other RNA drugs
Most of these drugs approved by FDA target the liver, particularly siRNA drugs. In addition to the five siRNA drugs receiving market authorization, there are also some siRNA drugs in clinical trials and most of them are designed to treat liver-related diseases, such as Nedosiran. Primary hyperoxaluria (PH) is a metabolic disease caused by abnormal liver metabolism, resulting in excessive oxalate production from glyoxylate via lactate dehydrogenase (LDH) and excretion of oxalate in urine, which can cause severe kidney injury or kidney failure [189-191]. LDHA and LDHB are two subunits of LDH encoded by their respective mRNA. Researches reveal that conjugating RNA with N-acetylgalactosamine (GalNAc) can achieve liver-targeting and the mechanism is that the GalNAc can combine with asialoglycoprotein receptors (ASGPRs), which are overexpressed by hepatocytes specifically [189]. Nedosiran is a double-stranded siRNA conjugated with GalNAc and can combine with mRNA encoding LDHA, reducing the generation of LDH proteins and thus inhibiting the production of oxalate in the liver [189]. Nedosiran is currently in phase II clinical trial (NCT05001269), and previous laboratory studies and clinical trials showed that this siRNA drug could target liver specifically to knock out LDHA without affecting other organs like muscle in animal models. It can also significantly reduce the oxalic acid content in urine after a subcutaneous injection in patients with high safety and tolerability [189,192,193]. Cemdisiran is another promising siRNA drug conjugated with GalNAc that targets the liver to treat immunoglobulin A nephropathy via inhibiting activation of complement component 5. Alnylam declared that in phase II clinical trials (NCT03841448), Cemdisiran reduced the ratio of urinary protein and creatinine (UPCR) by an average of 37% at 24 hour without adverse effects after subcutaneous administration compared to placebo group [194]. In summary, the therapeutic applications of siRNA are wide, covering the fields of metabolic diseases, ocular diseases, infectious diseases, tumors, and so on [194].
Similar to siRNA, ASO drugs on the market are primarily used to treat patients with rare diseases, but they have a wider range of target organs, including liver, muscle, eyes, and so on. The diseases they address include nervous system diseases, muscle diseases, cardiovascular diseases, and metabolic diseases. In addition to rare diseases, an increasing number of ASO drugs are being developed for other common diseases and are currently in middle- or late-stage clinical trials [195]. For instance, Pelacarsen, an ASO drug that targets mRNA expressing LPA in hepatocytes, reduced the level of lipoprotein(a) by 35-80% in phase II clinical trial (NCT03070782). This reduction is promising for patients with chronic kidney disease since elevated lipoprotein(a) levels are associated with kidney damage [196]. CDR132L is a synthetic ASO that function as the inhibitor to target miR-132 in heart failure to normalize cardiomyocytes. Thum et al. have demonstrated the powerful effect of CDR132L in large pig animals and the results showed that this drug significantly improved the systolic and diastolic function of the heart and reversed cardiac remodeling after repeated monthly administration [197]. Phase 1b clinical trial (NCT04045405) demonstrated the effectiveness in patients with heart failure after intravenous administration with safety and tolerability in the dose range of 0.32~10 mg/kg [198]. The development of delivery strategies targeting other tissues instead of the liver is the key to expand the applied range of small RNA drugs, and pulmonary administration is a reliable way to achieve lung targeting. IONIS-ENAC-2.5Rx and QR-010 that have completed phase I or II clinical trials (NCT03647228, NCT02564354/NCT02532764) are two ASO drugs for the treatment of CF via aerosol administration [195]. And results from clinical trials demonstrated the effectiveness of these ASO drugs in treating CF [194,195].
It is noteworthy that over half of the clinic trials on RNA-based drugs are focused on mRNA, highlighting the huge potential of mRNA therapeutics in RNA-based therapy at present. However, the majority of active clinic trials involving mRNA are derived from COVID-19 vaccines and for the indication of infections. If we exclude these vaccine-related trials, most remaining trials are still in the early-stage [185]. This reminds us that more progress should be made to expand the field of mRNA therapy and diversify its applications in other diseases.
Conclusion and Outlook
Since the discovery of the therapeutic potential of RNA drugs half a century ago, numerous delivery platforms have emerged to enable the safe and efficient delivery of RNA. Through the diligent efforts of researchers in library screening and rational design of delivery systems, several RNA-based drugs have received marketing approval or are currently undergoing clinical trials. As the focus has gradually shifted towards better adapting to clinical needs, efforts are being made to target specific sites for the treatment and reduce the side effects of RNA therapy. With a deeper understanding of the targeting mechanism, researchers are striving to achieve targeted therapies for RNA drugs by using different categories of vectors for delivery, tailoring vector chemical structures, adapting various administration routes, and altering physicochemical properties of vectors. These major advances in delivery systems provide great potential for RNA therapy.
Although the first siRNA drug utilizes the LNP vector, the following four siRNA approved drugs instead employ the GalNAc technology, which has become the primary strategy for siRNA-targeted delivery to hepatocytes. While in the field of mRNA-based therapy, advanced LNPs have been developed in two widely inoculated vaccines for COVID-19, known as BNT162b2 and mRNA-1273. This breakthrough has facilitated the exploitation of mRNA-target delivery systems, which have enabled selectivity for organs or tissues by modifying the lipid chemical structures and their formulations. Despite the progress, much work remains to be undertaken to achieve better clinical translation. 1) A deeper understanding of the targeting mechanism is required to develop universal guidelines for achieving targeting to each organ or even different cell subtypes, thus facilitating the design of next-generation delivery systems. 2) Additional investigations on the physicochemical properties, morphology, stability, and other characteristics of the vectors are required to facilitate further targeting and druggability. One can design vectors with specific sizes or charges to achieve targeting delivery of RNA according to the characteristics of different organs and tissues. 3) More attention can be paid to the inherent targeting ability of some natural vectors and figuring out the mechanism of their native tropism, which can guide the future specific targeting. Identifying the exact mechanism of targeting delivery will fundamentally help to design the appropriate vectors to achieve precise delivery. 4) Biocompatibility of vector materials should be considered to reduce toxicity and avoid undesired immunogenicity. 5) Inspired by protein corona-guided and GalNAc-conjugated targeting delivery system, more efforts should be made to screen specific receptors possessed by cell subsets for cellular level targeting. 6) Stepwise targeted drug delivery strategy from organ, tissue, cell to organelle may be utilized to overcome obstacles in delivery process and improve delivery precision.
Overall, the targeted RNA-based therapies have already demonstrated tremendous therapeutic potential in numerous clinical applications. As an integral part of RNA therapeutics, the development of a more precise delivery system is of undeniable significance. The prospects are promising that targeted RNA drugs have the potential of utility in diverse disease fields. Although some challenges remain for targeted RNA delivery, the enormous and continuous advances in RNA therapy will undoubtedly improve our health and lives.
Abbreviations
mRNA: messenger RNA; siRNA: small interfering RNA; miRNA: microRNA; ASO: antisense oligonucleotide; UTRs: untranslated regions; IVT: in vitro transcribed; LNP: lipid nanoparticle; tRNA: transfer RNA; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeat; Cas9: CRISPR-associated protein 9; sgRNA: single-guide RNA; RISC: RNA-induced silencing complex; TTR: transthyretin; AHP: acute hepatic porphyrias; TMTME: too many targets for miRNA effect; PEG: polyethylene glycol; LSECs: liver sinusoidal endothelial cells; PEI: polyethyleneimine; PBAE: poly(β-amino ester); mPEG-CL: poly(ethylene glycol-block-caprolactone); mPEG-LA: poly(ethylene glycol-block-lactide); LPNs: lipid-polymer hybrid nanoparticles; GO: graphene oxide; EPR: enhanced permeability and retention; CFTR: cystic fibrosis transmembrane conductance regulator; CARTs: charge-altering releasable transporters; EVs: extracellular vesicles; BBB: brain-blood barrier; MSC: mesenchymal stem cell; INPs: inorganic nanoparticles; AuNPs: gold nanoparticles; SAR: structure-activity relationships; Pten: Phosphatase and tensin homolog; ApoE: apolipoprotein E; LDLR: low-density lipoprotein receptor; LLNs: lipid-like nanoparticles; HA: hemophilia A; SORT: Selective Organ Targeting; RNPs: ribonucleoprotein complexes; PCD: primary ciliary dyskinesia; CF: cystic fibrosis; AAA: alkenyl amino alcohols; PS: phosphatidylserine; FNPs: five-element nanoparticles; iPhos: ionizable phospholipids; ZALs: zwitterionic amino lipids; PEG-PLGA: poly(ethylene glycol) block poly(lactide-co-glycolide); BP: bisphosphonate; NT: neurotransmitter; CNS: central nervous system; TfR: transferrin receptor; PS 80: polysorbate 80; mAbs: monoclonal antibodies; ASSET: anchored secondary scFv enabling targeting; DCs: dendritic cells; RNA-LPX: RNA-lipoplexes; PH: primary hyperoxaluria; LDH: lactate dehydrogenase; GalNAc: N-acetylgalactosamine; asialoglycoprotein receptors: ASGPRs; UPCR: urinary protein and creatinine.
Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ23H180003), the National Natural Science Foundation of China (Grant No. 22205201), and the Startup Package of Zhejiang University.
Author Contributions
L.X. Lin, K.X. Su and S. Liu conceived and wrote this review. L.X. Lin, K.X. Su, Q. Cheng and S. Liu searched and analyzed the literature. S. Liu and Q. Cheng reviewed and edited this review. All authors contributed to this manuscript for important intellectual content and approved the submission.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ibba ML, Ciccone G, Esposito CL, Catuogno S, Giangrande PH. Advances in mRNA non-viral delivery approaches. Adv Drug Deliv Rev. 2021;177:113930
2. Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol Ther. 2019;27:710-28
3. Swingle KL, Safford HC, Geisler HC, Hamilton AG, Thatte AS, Billingsley MM. et al. Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy. J Am Chem Soc. 2023;145:4691-706
4. Gupta A, Andresen JL, Manan RS, Langer R. Nucleic acid delivery for therapeutic applications. Adv Drug Deliv Rev. 2021;178:113834
5. Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35:222-9
6. Yan Y, Liu X-Y, Lu A, Wang X-Y, Jiang L-X, Wang J-C. Non-viral vectors for RNA delivery. J Control Release. 2022;342:241-79
7. Singh A, Trivedi P, Jain NK. Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol. 2018;46:274-83
8. Park J, Park J, Pei Y, Xu J, Yeo Y. Pharmacokinetics and biodistribution of recently-developed siRNA nanomedicines. Adv Drug Deliv Rev. 2016;104:93-109
9. Mayr C. What Are 3' UTRs Doing? Cold Spring Harb Perspect Biol. 2019;11:a034728
10. Evdokimova V, Ruzanov P, Imataka H, Raught B, Svitkin Y, Ovchinnikov LP. et al. The major mRNA-associated protein YB-1 is a potent 5' cap-dependent mRNA stabilizer. EMBO J. 2001;20:5491-502
11. Chang H, Lim J, Ha M, Kim VN. TAIL-seq: genome-wide determination of poly(A) tail length and 3' end modifications. Mol Cell. 2014;53:1044-52
12. Holtkamp S, Kreiter S, Selmi A, Simon P, Koslowski M, Huber C. et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009-17
13. Zhang H-X, Zhang Y, Yin H. Genome Editing with mRNA Encoding ZFN, TALEN, and Cas9. Mol Ther. 2019;27:735-46
14. Sahu I, Haque AKMA, Weidensee B, Weinmann P, Kormann MSD. Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases. Mol Ther J Am Soc Gene Ther. 2019;27:803-23
15. Iavarone C, O'hagan DT, Yu D, Delahaye NF, Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017;16:871-81
16. Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A. et al. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017;17:1326-35
17. Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313-20
18. Wei T, Cheng Q, Farbiak L, Anderson DG, Langer R, Siegwart DJ. Delivery of Tissue-Targeted Scalpels: Opportunities and Challenges for In Vivo CRISPR/Cas-Based Genome Editing. ACS Nano. 2020;14:9243-62
19. Liu L, Gao H, Guo C, Liu T, Li N, Qian Q. Therapeutic Mechanism of Nucleic Acid Drugs. ChemistrySelect. 2021;6:903-16
20. Jia L, Mao Y, Ji Q, Dersh D, Yewdell JW, Qian S-B. Decoding mRNA translatability and stability from the 5' UTR. Nat Struct Mol Biol. 2020;27:814-21
21. Haghighat A, Sonenberg N. eIF4G dramatically enhances the binding of eIF4E to the mRNA 5'-cap structure. J Biol Chem. 1997;272:21677-80
22. Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2:1-17
23. Xiang X, Niu Y-R, Wang Z-H, Ye L-L, Peng W-B, Zhou Q. Cancer-associated fibroblasts: Vital suppressors of the immune response in the tumor microenvironment. Cytokine Growth Factor Rev. 2022;67:35-48
24. Zhang Y, Yan J, Hou X, Wang C, Kang DD, Xue Y. et al. STING Agonist-Derived LNP-mRNA Vaccine Enhances Protective Immunity Against SARS-CoV-2. Nano Lett. 2023;23:2593-600
25. Lim WW, Mak L, Leung GM, Cowling BJ, Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2:e423
26. Fathizadeh H. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: A review. Int J Biol Macromol. 2021;188:740-50
27. Memi F, Ntokou A, Papangeli I. CRISPR/Cas9 gene-editing: Research technologies, clinical applications and ethical considerations. Semin Perinatol. 2018;42:487-500
28. Marsh S, Hanson B, Wood MJA, Varela MA, Roberts TC. Application of CRISPR-Cas9-Mediated Genome Editing for the Treatment of Myotonic Dystrophy Type 1. Mol Ther. 2020;28:2527-39
29. Liu Q, Yang J, Xing Y, Zhao Y, Liu Y. Development of delivery strategies for CRISPR-Cas9 genome editing. BMEMat. 2023 e12025
30. Vaughan HJ, Green JJ, Tzeng SY. Cancer-Targeting Nanoparticles for Combinatorial Nucleic Acid Delivery. Adv Mater. 2020;32:e1901081
31. Liu S, Cheng Q, Wei T, Yu X, Johnson LT, Farbiak L. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20:701-10
32. Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS. et al. Non-Viral CRISPR/Cas Gene Editing In Vitro and In Vivo Enabled by Synthetic Nanoparticle Co-Delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 2017;56:1059-63
33. Zhang D, Wang G, Yu X, Wei T, Farbiak L, Johnson LT. et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat Nanotechnol. 2022;17:777-87
34. Gaynor JW, Campbell BJ, Cosstick R. RNA interference: a chemist's perspective. Chem Soc Rev. 2010;39:4169-84
35. Alshaer W, Zureigat H, Al Karaki A, Al-Kadash A, Gharaibeh L, Hatmal MM. et al. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021;905:174178
36. Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008;13:842-55
37. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-11
38. Garber K. Alnylam launches era of RNAi drugs. Nat Biotechnol. 2018;36:777-8
39. Scott LJ. Givosiran: First Approval. Drugs. 2020;80:335-9
40. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-97
41. Bortolin-Cavaillé M-L, Dance M, Weber M, Cavaillé J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009;37:3464-73
42. Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931-4
43. Zhang S, Cheng Z, Wang Y, Han T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des Devel Ther. 2021;15:721-33
44. Patel M, Wang Y, Bartom ET, Dhir R, Nephew KP, Matei D. et al. The Ratio of Toxic-to-Nontoxic miRNAs Predicts Platinum Sensitivity in Ovarian Cancer. Cancer Res. 2021;81:3985-4000
45. Zou W, Imperiale MJ. Biology of Polyomavirus miRNA. Front Microbiol. 2021;12:662892
46. Lee E, Ito K, Zhao Y, Schadt EE, Irie HY, Zhu J. Inferred miRNA activity identifies miRNA-mediated regulatory networks underlying multiple cancers. Bioinformatics. 2016;32:96-105
47. Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978;75:280-4
48. Bennett CF. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu Rev Med. 2019;70:307-21
49. Tarn W-Y, Cheng Y, Ko S-H, Huang L-M. Antisense Oligonucleotide-Based Therapy of Viral Infections. Pharmaceutics. 2021;13:2015
50. Ramasamy T, Ruttala HB, Munusamy S, Chakraborty N, Kim JO. Nano drug delivery systems for antisense oligonucleotides (ASO) therapeutics. J Control Release. 2022;352:861-78
51. Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19:673-94
52. Aimo A, Castiglione V, Rapezzi C, Franzini M, Panichella G, Vergaro G. et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat Rev Cardiol. 2022;19:655-67
53. Boros BD, Schoch KM, Kreple CJ, Miller TM. Antisense Oligonucleotides for the Study and Treatment of ALS. Neurotherapeutics. 2022;19:1145-58
54. Samaridou E, Heyes J, Lutwyche P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv Drug Deliv Rev. 2020;154-155:37-63
55. Dilliard SA, Siegwart DJ. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat Rev Mater. 2023:1-19
56. Lee SM, Cheng Q, Yu X, Liu S, Johnson LT, Siegwart DJ. A Systematic Study of Unsaturation in Lipid Nanoparticles Leads to Improved mRNA Transfection In Vivo. Angew Chem Int Ed Engl. 2021;60:5848-53
57. Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36-48
58. Hattori Y, Suzuki S, Kawakami S, Yamashita F, Hashida M. The role of dioleoylphosphatidylethanolamine (DOPE) in targeted gene delivery with mannosylated cationic liposomes via intravenous route. J Control Release. 2005;108:484-95
59. Li S-D, Huang L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178-81
60. Huang Y, Yang M, Wang N, Li S, Liu Z, Li Z. et al. Intracellular delivery of messenger RNA to macrophages with surfactant-derived lipid nanoparticles. Mater Today Adv. 2022;16:100295
61. Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078-94
62. Cullis PR, Hope MJ. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol Ther. 2017;25:1467-75
63. Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang C-C, Ueda M, Kristen AV. et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:11-21
64. Yang T, Poenisch M, Khanal R, Hu Q, Dai Z, Li R. et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J Hepatol. 2021;75:1420-33
65. Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J. et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018;22:2227-35
66. Pattipeiluhu R, Arias-Alpizar G, Basha G, Chan KYT, Bussmann J, Sharp TH. et al. Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System. Adv Mater. 2022;34:2201095
67. Kim M, Jeong M, Hur S, Cho Y, Park J, Jung H. et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci Adv. 2021;7:eabf4398
68. Jin H, Jeong M, Lee G, Kim M, Yoo Y, Kim HJ. et al. Engineered Lipid Nanoparticles for the Treatment of Pulmonary Fibrosis by Regulating Epithelial-Mesenchymal Transition in the Lungs. Adv Funct Mater. 2023;33:2209432
69. Kaczmarek JC, Patel AK, Kauffman KJ, Fenton OS, Webber MJ, Heartlein MW. et al. Polymer-Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew Chem Int Ed Engl. 2016;55:13808-12
70. Palmerston Mendes L, Pan J, Torchilin V. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules. 2017;22:1401
71. Chou LYT, Ming K, Chan WCW. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233-45
72. Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100-9
73. Liu S, Zhou D, Yang J, Zhou H, Chen J, Guo T. Bioreducible Zinc(II)-Coordinative Polyethylenimine with Low Molecular Weight for Robust Gene Delivery of Primary and Stem Cells. J Am Chem Soc. 2017;139:5102-9
74. Liu S, Jia H, Yang J, Pan J, Liang H, Zeng L. et al. Zinc Coordinated Cationic Polymers Break Up the Paradox between Low Molecular Weight and High Transfection Efficacy. Biomacromolecules. 2018;19:4270-6
75. Wahane A, Waghmode A, Kapphahn A, Dhuri K, Gupta A, Bahal R. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy. Molecules. 2020;25:2866
76. Liu S, Gao Y, Zhou D, Zeng M, Alshehri F, Newland B. et al. Highly branched poly(β-amino ester) delivery of minicircle DNA for transfection of neurodegenerative disease related cells. Nat Commun. 2019;10:3307
77. Liu S, Gao Y, A S, Zhou D, Greiser U, Guo T. et al. Biodegradable Highly Branched Poly(β-Amino Ester)s for Targeted Cancer Cell Gene Transfection. ACS Biomater Sci Eng. 2017;3:1283-6
78. Liu S, Sun Z, Zhou D, Guo T. Alkylated branched poly(β-amino esters) demonstrate strong DNA encapsulation, high nanoparticle stability and robust gene transfection efficacy. J Mater Chem B. 2017;5:5307-10
79. Li Q, Wen C, Yang J, Zhou X, Zhu Y, Zheng J. et al. Zwitterionic Biomaterials. Chem Rev. 2022;122:17073-154
80. Langridge TD, Gemeinhart RA. Toward understanding polymer micelle stability: Density ultracentrifugation offers insight into polymer micelle stability in human fluids. J Control Release. 2020;319:157-67
81. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941-51
82. Salzano G, Navarro G, Trivedi MS, De Rosa G, Torchilin VP. Multifunctional Polymeric Micelles Co-loaded with Anti-Survivin siRNA and Paclitaxel Overcome Drug Resistance in an Animal Model of Ovarian Cancer. Mol Cancer Ther. 2015;14:1075-84
83. Salzano G, Costa DF, Sarisozen C, Luther E, Mattheolabakis G, Dhargalkar PP. et al. Mixed Nanosized Polymeric Micelles as Promoter of Doxorubicin and miRNA-34a Co-Delivery Triggered by Dual Stimuli in Tumor Tissue. Small. 2016;12:4837-48
84. Yang W, Mixich L, Boonstra E, Cabral H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv Healthc Mater. 2023;12:e2202688
85. Rodríguez-Acosta GL, Hernández-Montalbán C, Vega-Razo MFS, Castillo-Rodríguez IO, Martínez-García M. Polymer-dendrimer Hybrids as Carriers of Anticancer Agents. Curr Drug Targets. 2022;23:373-92
86. Patel AK, Kaczmarek JC, Bose S, Kauffman KJ, Mir F, Heartlein MW. et al. Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv Mater. 2019;31:e1805116
87. Haque AKMA, Dewerth A, Antony JS, Riethmüller J, Schweizer GR, Weinmann P. et al. Chemically modified hCFTR mRNAs recuperate lung function in a mouse model of cystic fibrosis. Sci Rep. 2018;8:16776
88. Liu S, Wang X, Yu X, Cheng Q, Johnson LT, Chatterjee S. et al. Zwitterionic Phospholipidation of Cationic Polymers Facilitates Systemic mRNA Delivery to Spleen and Lymph Nodes. J Am Chem Soc. 2021;143:21321-30
89. McKinlay CJ, Benner NL, Haabeth OA, Waymouth RM, Wender PA. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc Natl Acad Sci U S A. 2018;115:E5859-66
90. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-79
91. Belhadj Z, Qie Y, Carney RP, Li Y, Nie G. Current advances in non-viral gene delivery systems: Liposomes versus extracellular vesicles. BMEMat. 2023 e12018
92. Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles — Endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846:75-87
93. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
94. Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release. 2017;266:8-16
95. Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10:1517-27
96. Lakhal S, Wood MJA. Exosome nanotechnology: An emerging paradigm shift in drug delivery. Bioessays. 2011;33:737-41
97. Bashyal S, Thapa C, Lee S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J Control Release. 2022;348:723-44
98. Perets N, Betzer O, Shapira R, Brenstein S, Angel A, Sadan T. et al. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019;19:3422-31
99. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101-24
100. Han G, Ghosh P, De M, Rotello VM. Drug and gene delivery using gold nanoparticles. NanoBiotechnology. 2007;3:40-5
101. Hočevar S, Milošević A, Rodriguez-Lorenzo L, Ackermann-Hirschi L, Mottas I, Petri-Fink A. et al. Polymer-Coated Gold Nanospheres Do Not Impair the Innate Immune Function of Human B Lymphocytes in Vitro. ACS Nano. 2019;13:6790-800
102. Wang X, Guo L, Zhang S, Chen Y, Chen Y-T, Zheng B. et al. Copper Sulfide Facilitates Hepatobiliary Clearance of Gold Nanoparticles through the Copper-Transporting ATPase ATP7B. ACS Nano. 2019;13:5720-30
103. Yin H, Song C-Q, Suresh S, Wu Q, Walsh S, Rhym LH. et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat Biotechnol. 2017;35:1179-87
104. Gan Z, Lokugamage MP, Hatit MZC, Loughrey D, Paunovska K, Sato M. et al. Nanoparticles containing constrained phospholipids deliver mRNA to liver immune cells in vivo without targeting ligands. Bioeng Transl Med. 2020;5:e10161
105. Younis MA, Sato Y, Elewa YHA, Kon Y, Harashima H. Self-homing nanocarriers for mRNA delivery to the activated hepatic stellate cells in liver fibrosis. J Control Release. 2023;353:685-98
106. Yu X, Liu S, Cheng Q, Wei T, Lee S, Zhang D. et al. Lipid-Modified Aminoglycosides for mRNA Delivery to the Liver. Adv Healthc Mater. 2020;9:1901487
107. Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D. et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci U S A. 2014;111:3955-60
108. Knapp CM, Guo P, Whitehead KA. Lipidoid Tail Structure Strongly Influences siRNA Delivery Activity. Cell Mol Bioeng. 2016;9:305-14
109. Hajj KA, Ball RL, Deluty SB, Singh SR, Strelkova D, Knapp CM. et al. Branched-Tail Lipid Nanoparticles Potently Deliver mRNA In Vivo due to Enhanced Ionization at Endosomal pH. Small. 2019;15:1805097
110. Hajj KA, Melamed JR, Chaudhary N, Lamson NG, Ball RL, Yerneni SS. et al. A Potent Branched-Tail Lipid Nanoparticle Enables Multiplexed mRNA Delivery and Gene Editing In Vivo. Nano Lett. 2020;20:5167-75
111. Kopac T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int J Biol Macromol. 2021;169:290-301
112. Ren J, Andrikopoulos N, Velonia K, Tang H, Cai R, Ding F. et al. Chemical and Biophysical Signatures of the Protein Corona in Nanomedicine. J Am Chem Soc. 2022;144:9184-205
113. Johnson LT, Zhang D, Zhou K, Lee SM, Liu S, Dilliard SA. et al. Lipid Nanoparticle (LNP) Chemistry Can Endow Unique In Vivo RNA Delivery Fates within the Liver That Alter Therapeutic Outcomes in a Cancer Model. Mol Pharm. 2022;19:3973-86
114. Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X. et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc Natl Acad Sci U S A. 2021;118:e2020401118
115. Qiu M, Tang Y, Chen J, Muriph R, Ye Z, Huang C. et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119:e2116271119
116. Zhang X, Zhao W, Nguyen GN, Zhang C, Zeng C, Yan J. et al. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci Adv. 2020;6:eabc2315
117. Zhang X, Li B, Luo X, Zhao W, Jiang J, Zhang C. et al. Biodegradable Amino-Ester Nanomaterials for Cas9 mRNA Delivery in Vitro and in Vivo. ACS Appl Mater Interfaces. 2017;9:25481-7
118. Luo X, Zhao W, Li B, Zhang X, Zhang C, Bratasz A. et al. Co-delivery of mRNA and SPIONs through amino-ester nanomaterials. Nano Res. 2018;11:5596-603
119. Rui Y, Wilson DR, Tzeng SY, Yamagata HM, Sudhakar D, Conge M. et al. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci Adv. 2022;8:eabk2855
120. Jarzębińska A, Pasewald T, Lambrecht J, Mykhaylyk O, Kümmerling L, Beck P. et al. A Single Methylene Group in Oligoalkylamine-Based Cationic Polymers and Lipids Promotes Enhanced mRNA Delivery. Angew Chem Int Ed Engl. 2016;55:9591-5
121. Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci U S A. 2021;118:e2109256118
122. Wei T, Cheng Q, Min Y-L, Olson EN, Siegwart DJ. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 2020;11:3232
123. Zhao X, Chen J, Qiu M, Li Y, Glass Z, Xu Q. Imidazole-Based Synthetic Lipidoids for In Vivo mRNA Delivery into Primary T Lymphocytes. Angew Chem. 2020;59:20083-9
124. Kowalski PS, Capasso Palmiero U, Huang Y, Rudra A, Langer R, Anderson DG. Ionizable Amino-Polyesters Synthesized via Ring Opening Polymerization of Tertiary Amino-Alcohols for Tissue Selective mRNA Delivery. Adv Mater. 2018;30:1801151
125. Fenton OS, Kauffman KJ, McClellan RL, Appel EA, Dorkin JR, Tibbitt MW. et al. Bioinspired Alkenyl Amino Alcohol Ionizable Lipid Materials for Highly Potent In Vivo mRNA Delivery. Adv Mater. 2016;28:2939-43
126. Fenton OS, Kauffman KJ, Kaczmarek JC, McClellan RL, Jhunjhunwala S, Tibbitt MW. et al. Synthesis and Biological Evaluation of Ionizable Lipid Materials for the In Vivo Delivery of Messenger RNA to B Lymphocytes. Adv Mater. 2017;29:1606944
127. Fenton OS, Kauffman KJ, McClellan RL, Kaczmarek JC, Zeng MD, Andresen JL. et al. Customizable Lipid Nanoparticle Materials for the Delivery of siRNAs and mRNAs. Angew Chem Int Ed Engl. 2018;57:13582-6
128. Gomi M, Sakurai Y, Sato M, Tanaka H, Miyatake Y, Fujiwara K. et al. Delivering mRNA to Secondary Lymphoid Tissues by Phosphatidylserine-Loaded Lipid Nanoparticles. Adv Healthc Mater. 2023;12:2202528
129. Li B, Luo X, Deng B, Giancola JB, McComb DW, Schmittgen TD. et al. Effects of local structural transformation of lipid-like compounds on delivery of messenger RNA. Sci Rep. 2016;6:22137
130. Cao Y, He Z, Chen Q, He X, Su L, Yu W. et al. Helper-Polymer Based Five-Element Nanoparticles (FNPs) for Lung-Specific mRNA Delivery with Long-Term Stability after Lyophilization. Nano Lett. 2022;22:6580-9
131. Tombácz I, Laczkó D, Shahnawaz H, Muramatsu H, Natesan A, Yadegari A. et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol Ther. 2021;29:3293-304
132. Ni H, Hatit MZC, Zhao K, Loughrey D, Lokugamage MP, Peck HE. et al. Piperazine-derived lipid nanoparticles deliver mRNA to immune cells in vivo. Nat Commun. 2022;13:4766
133. Sago CD, Lokugamage MP, Paunovska K, Vanover DA, Monaco CM, Shah NN. et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc Natl Acad Sci U S A. 2018;115:E9944-52
134. Kaczmarek JC, Kauffman KJ, Fenton OS, Sadtler K, Patel AK, Heartlein MW. et al. Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 2018;18:6449-54
135. Kaczmarek JC, Patel AK, Rhym LH, Palmiero UC, Bhat B, Heartlein MW. et al. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials. 2021;275:120966
136. Yan Y, Xiong H, Zhang X, Cheng Q, Siegwart DJ. Systemic mRNA Delivery to the Lungs by Functional Polyester-based Carriers. Biomacromolecules. 2017;18:4307-15
137. Gu W, An J, Li Y, Yang Y, Wang S, Shan H. et al. Tuning the Organ Tropism of Polymersome for Spleen-Selective Nanovaccine Delivery to Boost Cancer Immunotherapy. Adv Mater. 2023 2301686
138. Herrera-Barrera M, Ryals RC, Gautam M, Jozic A, Landry M, Korzun T. et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci Adv. 2023;9:eadd4623
139. Xue L, Gong N, Shepherd SJ, Xiong X, Liao X, Han X. et al. Rational Design of Bisphosphonate Lipid-like Materials for mRNA Delivery to the Bone Microenvironment. J Am Chem Soc. 2022;144:9926-37
140. Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L. et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91-6
141. Rodrigues AF, Rebelo C, Simões S, Paulo C, Pinho S, Francisco V. et al. A Polymeric Nanoparticle Formulation for Targeted mRNA Delivery to Fibroblasts. Adv Sci. 2022;10:2205475
142. Johnsen KB, Burkhart A, Thomsen LB, Andresen TL, Moos T. Targeting the transferrin receptor for brain drug delivery. Prog Neurobiol. 2019;181:101665
143. Ma F, Yang L, Sun Z, Chen J, Rui X, Glass Z. et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci Adv. 2020;6:eabb4429
144. dos Santos Rodrigues B, Kanekiyo T, Singh J. In vitro and in vivo characterization of CPP and transferrin modified liposomes encapsulating pDNA. Nanomedicine. 2020;28:102225
145. Ramalho MJ, Loureiro JA, Coelho MAN, Pereira MC. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics. 2022;14:279
146. Tu Z, Timashev P, Chen J, Liang X-J. Ferritin-based drug delivery system for tumor therapy. BMEMat. 2023 e12022
147. Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release. 2020;317:273-81
148. Li W, Qiu J, Li X-L, Aday S, Zhang J, Conley G. et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci Adv. 2021;7:eabd6889
149. Zhou Z, Li M. Targeted therapies for cancer. BMC Med. 2022;20:90
150. Wang X, Liu S, Sun Y, Yu X, Lee SM, Cheng Q. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat Protoc. 2022;18:265-91
151. Kumar B, Singh S, Skvortsova I, Kumar V. Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr Med Chem. 2017;24:4729-52
152. Kedmi R, Veiga N, Ramishetti S, Goldsmith M, Rosenblum D, Dammes N. et al. A modular platform for targeted RNAi therapeutics. Nat Nanotechnol. 2018;13:214-9
153. Veiga N, Goldsmith M, Granot Y, Rosenblum D, Dammes N, Kedmi R. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat Commun. 2018;9:4493
154. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396-401
155. Setten RL, Rossi JJ, Han S-P. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421-46
156. Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83-112
157. Chow MYT, Qiu Y, Lam JKW. Inhaled RNA Therapy: From Promise to Reality. Trends Pharmacol Sci. 2020;41:715-29
158. Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM. et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898-907
159. Patel S, Ryals RC, Weller KK, Pennesi ME, Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release. 2019;303:91-100
160. Ryals RC, Patel S, Acosta C, McKinney M, Pennesi ME, Sahay G. The effects of PEGylation on LNP based mRNA delivery to the eye. PloS One. 2020;15:e0241006
161. Zheng M, Tao W, Zou Y, Farokhzad OC, Shi B. Nanotechnology-Based Strategies for siRNA Brain Delivery for Disease Therapy. Trends Biotechnol. 2018;36:562-75
162. Eygeris Y, Gupta M, Kim J, Sahay G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc Chem Res. 2022;55:2-12
163. Melamed JR, Yerneni SS, Arral ML, LoPresti ST, Chaudhary N, Sehrawat A. et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci Adv. 2023;9:eade1444
164. Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145-53
165. Loughrey D, Dahlman JE. Non-liver mRNA Delivery. Acc Chem Res. 2022;55:13-23
166. Liang F, Lindgren G, Lin A, Thompson EA, Ols S, Röhss J. et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol Ther. 2017;25:2635-47
167. Luozhong S, Yuan Z, Sarmiento T, Chen Y, Gu W, McCurdy C. et al. Phosphatidylserine Lipid Nanoparticles Promote Systemic RNA Delivery to Secondary Lymphoid Organs. Nano Lett. 2022;22:8304-11
168. Kim J, Jozic A, Lin Y, Eygeris Y, Bloom E, Tan X. et al. Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of mRNA through Inhalation. ACS Nano. 2022;16:14792-806
169. Newman SP. Drug delivery to the lungs: challenges and opportunities. Ther Deliv. 2017;8:647-61
170. Lokugamage MP, Vanover D, Beyersdorf J, Hatit MZC, Rotolo L, Echeverri ES. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5:1059-68
171. Devoldere J, Peynshaert K, Dewitte H, Vanhove C, De Groef L, Moons L. et al. Non-viral delivery of chemically modified mRNA to the retina: Subretinal versus intravitreal administration. J Control Release. 2019;307:315-30
172. You Y, Tian Y, Yang Z, Shi J, Kwak KJ, Tong Y. et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat Biomed Eng. 2023:1-14
173. McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2:89-96
174. Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51:8529-33
175. Xiong H, Liu S, Wei T, Cheng Q, Siegwart DJ. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J Control Release. 2020;325:198-205
176. Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B. et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165-70
177. Piotrowski-Daspit AS, Kauffman AC, Bracaglia LG, Saltzman WM. Polymeric vehicles for nucleic acid delivery. Adv Drug Deliv Rev. 2020;156:119-32
178. Zhang F, Trent Magruder J, Lin Y-A, Crawford TC, Grimm JC, Sciortino CM. et al. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J Control Release. 2017;249:173-82
179. Liu MA, Zhou T, Sheets RL, Meyer H, Knezevic I. WHO informal consultation on regulatory considerations for evaluation of the quality, safety and efficacy of RNA-based prophylactic vaccines for infectious diseases, 20-22 April 2021. Emerg Microbes Infect. 2022;11:384-91
180. Patel S, Ashwanikumar N, Robinson E, Xia Y, Mihai C, Griffith JP. et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun. 2020;11:983
181. Lee S-Y, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20:495101
182. Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM-C. et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41:2312-8
183. Devarajan PV, Jindal AB, Patil RR, Mulla F, Gaikwad RV, Samad A. Particle shape: a new design parameter for passive targeting in splenotropic drug delivery. J Pharm Sci. 2010;99:2576-81
184. Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M. et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011;108:586-91
185. Curreri A, Sankholkar D, Mitragotri S, Zhao Z. RNA therapeutics in the clinic. Bioeng Transl Med. 2023;8:e10374
186. Burris H, Patel M, Cho D, Clarke J, Gutierrez M, Zaks T. et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J Clin Oncol. 2019;37:2523-2523
187. Bauer T, Patel M, Jimeno A, Wang D, McDermott J, Zacharek S. et al. Abstract CT210: A Phase I, open-label, multicenter, dose escalation study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral injection alone and in combination with immune checkpoint blockade. Cancer Res. 2019;79:CT210
188. Anttila V, Saraste A, Knuuti J, Jaakkola P, Hedman M, Svedlund S. et al. Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol Ther Methods Clin Dev. 2020;18:464-72
189. Ariceta G, Barrios K, Brown BD, Hoppe B, Rosskamp R, Langman CB. Hepatic Lactate Dehydrogenase A: An RNA Interference Target for the Treatment of All Known Types of Primary Hyperoxaluria. Kidney Int Rep. 2021;6:1088-98
190. Hoppe B, Martin-Higueras C. Improving Treatment Options for Primary Hyperoxaluria. Drugs. 2022;82:1077-94
191. Mandrile G, Beck B, Acquaviva C, Rumsby G, Deesker L, Garrelfs S. et al. Genetic assessment in primary hyperoxaluria: why it matters. Pediatr Nephrol. 2022;38:625-34
192. Lai C, Pursell N, Gierut J, Saxena U, Zhou W, Dills M. et al. Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria. Mol Ther. 2018;26:1983-95
193. Hoppe B, Koch A, Cochat P, Garrelfs SF, Baum MA, Groothoff JW. et al. Safety, pharmacodynamics, and exposure-response modeling results from a first-in-human phase 1 study of nedosiran (PHYOX1) in primary hyperoxaluria. Kidney Int. 2022;101:626-34
194. Friedrich M, Aigner A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. Biodrugs. 2022;36:549-71
195. Crooke ST, Baker BF, Crooke RM, Liang X. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20:427-53
196. Fernandez-Prado R, Perez-Gomez MV, Ortiz A. Pelacarsen for lowering lipoprotein(a): implications for patients with chronic kidney disease. Clin Kidney J. 2020;13:753-7
197. Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T. et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J. 2020;42:192-201
198. Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J. et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. 2021;42:178-88
199. Moore PJ, Tarran R. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease. Expert Opin Ther Targets. 2018;22:687-701
200. Drevinek P, Pressler T, Cipolli M, De Boeck K, Schwarz C, Bouisset F. et al. Antisense oligonucleotide eluforsen is safe and improves respiratory symptoms in F508DEL cystic fibrosis. J Cyst Fibros. 2020;19:99-107
Author contact
![]() Corresponding author: Shuai Liu, Email: shuailiuedu.cn.
Corresponding author: Shuai Liu, Email: shuailiuedu.cn.
 Global reach, higher impact
Global reach, higher impact