13.3
Impact Factor
Theranostics 2023; 13(14):4858-4871. doi:10.7150/thno.86770 This issue Cite
Research Paper
Phase I clinical evaluation of 99mTc-labeled Affibody molecule for imaging HER2 expression in breast cancer
1. Department of Nuclear Therapy and Diagnostic, Cancer Research Institute, Tomsk National Research Medical Center, Russian Academy of Sciences, Tomsk, Russia.
2. Research Centrum for Oncotheranostics, Research School of Chemistry and Applied Biomedical Sciences, Tomsk Polytechnic University, Tomsk, Russia.
3. Department of Pharmaceutical Analysis, Siberian State Medical University, 634050 Tomsk, Russia.
4. Department of Immunology, Genetics and Pathology, Uppsala University, 752 37 Uppsala, Sweden.
5. Affibody AB, Solna, Sweden.
6. Department of Medicinal Chemistry, Uppsala University, Uppsala, Sweden.
7. Radiology and Nuclear Medicine, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden.
Abstract
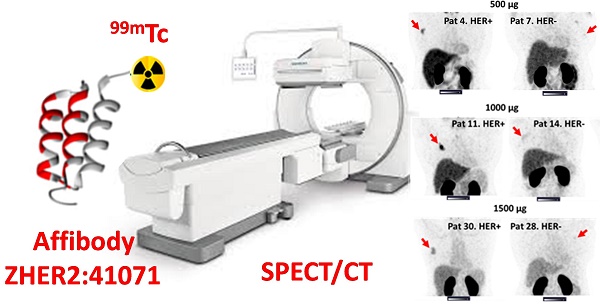
The determination of tumor human epidermal growth factor receptor type 2 (HER2) status is of increasing importance with the recent approval of more efficacious HER2-targeted treatments. There is a lack of suitable methods for clinical in vivo HER2 expression assessment. Affibody molecules are small affinity proteins ideal for imaging detection of receptors, which are engineered using a small (molecular weight 6.5 kDa) nonimmunoglobulin scaffold. Labeling of Affibody molecules with positron emitters enabled the development of sensitive and specific agents for molecular imaging. The development of probes for SPECT would permit the use of Affibody-based imaging in regions where PET is not available. In this first-in-human study, we evaluated the safety, biodistribution, and dosimetry of the 99mTc-ZHER2:41071 Affibody molecule developed for SPECT/CT imaging of HER2 expression.
Methods: Thirty-one patients with primary breast cancer were enrolled and divided into three cohorts (injected with 500, 1000, or 1500 µg ZHER2:41071) comprising at least five patients with high (positive) HER2 tumor expression (IHC score 3+ or 2+ and ISH positive) and five patients with low (IHC score 2+ or 1+ and ISH negative) or absent HER2 tumor expression. Patients were injected with 451 ± 71 MBq 99mTc-ZHER2:4107. Planar scintigraphy was performed after 2, 4, 6 and 24 h, and SPECT/CT imaging followed planar imaging 2, 4 and 6 h after injection.
Results: Injections of 99mTc-ZHER2:41071 were well tolerated and not associated with adverse events. Normal organs with the highest accumulation were the kidney and liver. The effective dose was 0.019 ± 0.004 mSv/MBq. Injection of 1000 µg provided the best standard discrimination between HER2-positive and HER2-low or HER2-negative tumors 2 h after injection (SUVmax 16.9 ± 7.6 vs. 3.6 ± 1.4, p < 0.005). The 99mTc-ZHER2:41071 uptake in HER2-positive lymph node metastases (SUVmax 6.9 ± 2.4, n = 5) was significantly (p < 0.05) higher than that in HER2-low/negative lymph nodes (SUVmax 3.5 ± 1.2, n = 4). 99mTc-ZHER2:41071 visualized hepatic metastases in a patient with liver involvement.
Conclusions: Injections of 99mTc-ZHER2:41071 appear safe and exhibit favorable dosimetry. The protein dose of 1000 µg provides the best discrimination between HER2-positive and HER2-low/negative expression of HER2 according to the definition used for current HER2-targeting drugs.
Keywords: HER2, Affibody, 99mTc, ZHER2:41071, SPECT, Phase I
 Global reach, higher impact
Global reach, higher impact