13.3
Impact Factor
Theranostics 2023; 13(14):4885-4904. doi:10.7150/thno.86695 This issue Cite
Research Paper
3D autofluorescence imaging of hydronephrosis and renal anatomical structure using cryo-micro-optical sectioning tomography
1. Britton Chance Center for Biomedical Photonics, Wuhan National Laboratory for Optoelectronics, MoE Key Laboratory for Biomedical Photonics, School of Engineering Sciences, Innovation Institute, Huazhong University of Science and Technology, Wuhan 430074, China.
2. Research Unit of Multimodal Cross Scale Neural Signal Detection and Imaging, Chinese Academy of Medical Sciences, HUST-Suzhou Institute for Brainmatics, JITRI, Suzhou 215123, China.
3. School of Biomedical Engineering, Hainan University, Haikou, 570228, China.
Abstract
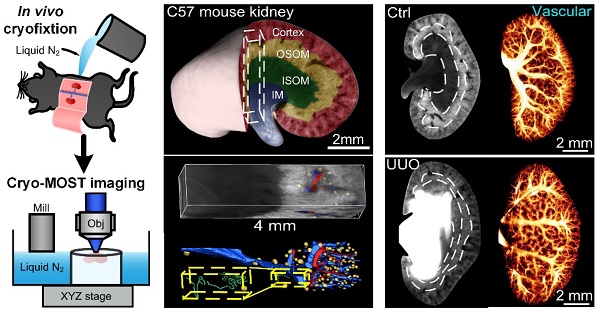
Rationale: Mesoscopic visualization of the main anatomical structures of the whole kidney in vivo plays an important role in the pathological diagnosis and exploration of the etiology of hydronephrosis. However, traditional imaging methods cannot achieve whole-kidney imaging with micron resolution under conditions representing in vivo perfusion.
Methods: We used in vivo cryofixation (IVCF) to fix acute obstructive hydronephrosis (unilateral ureteral obstruction, UUO), chronic spontaneous hydronephrosis (db/db mice), and their control mouse kidneys for cryo-micro-optical sectioning tomography (cryo-MOST) autofluorescence imaging. We quantitatively assessed the kidney-wide pathological changes in the main anatomical structures, including hydronephrosis, renal subregions, arteries, veins, glomeruli, renal tubules, and peritubular functional capillaries.
Results: By comparison with microcomputed tomography imaging, we confirmed that IVCF can maintain the status of the kidney in vivo. Cryo-MOST autofluorescence imaging can display the main renal anatomical structures with a cellular resolution without contrast agents. The hydronephrosis volume reached 26.11 ± 6.00 mm3 and 13.01 ± 3.74 mm3 in 3 days after UUO and in 15-week-old db/db mouse kidneys, respectively. The volume of the cortex and inner stripe of the outer medulla (ISOM) increased while that of the inner medulla (IM) decreased in UUO mouse kidneys. Db/db mice also showed an increase in the volume of the cortex and ISOM volume but no atrophy in the IM. The diameter of the proximal convoluted tubule and proximal straight tubule increased in both UUO and db/db mouse kidneys, indicating that proximal tubules were damaged. However, some renal tubules showed abnormal central bulge highlighting in the UUO mice, but the morphology of renal tubules was normal in the db/db mice, suggesting differences in the pathology and severity of hydronephrosis between the two models. UUO mouse kidneys also showed vascular damage, including segmental artery and vein atrophy and arcuate vein dilation, and the density of peritubular functional capillaries in the cortex and IM was reduced by 37.2% and 49.5%, respectively, suggesting renal hypoxia. In contrast, db/db mouse kidneys showed a normal vascular morphology and peritubular functional capillary density. Finally, we found that the db/db mice displayed vesicoureteral reflux and bladder overactivity, which may be the cause of hydronephrosis formation.
Conclusions: We observed and compared main renal structural changes in hydronephrosis under conditions representing in vivo perfusion in UUO, db/db, and control mice through cryo-MOST autofluorescence imaging. The results indicate that cryo-MOST with IVCF can serve as a simple and powerful tool to quantitatively evaluate the in vivo pathological changes in three dimensions, especially the distribution of body fluids in the whole kidney. This method is potentially applicable to the three-dimensional visualization of other tissues, organs, and even the whole body, which may provide new insights into pathological changes in diseases.
Keywords: hydronephrosis, UUO, db/db mice, autofluorescence imaging, cryo-imaging
 Global reach, higher impact
Global reach, higher impact