13.3
Impact Factor
Theranostics 2024; 14(1):220-248. doi:10.7150/thno.87425 This issue Cite
Review
Red blood cells in biology and translational medicine: natural vehicle inspires new biomedical applications
1. Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, 310009.
2. Department of Biochemistry, Zhejiang University School of Medicine, Hangzhou, China, 310058.
3. Department of Biochemistry & Cancer Medicine, International Institutes of Medicine, the Fourth Affiliated Hospital of Zhejiang University School of Medicine, Yiwu, Zhejiang, China.
4. Institute of Translational Medicine, Zhejiang University, Hangzhou, China, 310029.
5. Cancer Center, Zhejiang University, Hangzhou, China, 310029.
6. Hon Hai Precision Industry Co., Ltd.
Received 2023-6-23; Accepted 2023-10-31; Published 2024-1-1
Abstract
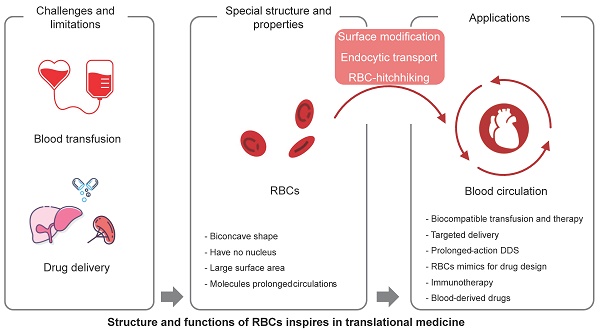
Red blood cells (RBCs) are the most abundant cell type in the blood, and play a critical role in oxygen transport. With the development of nanobiotechnology and synthetic biology, scientists have found multiple ways to take advantage of the characteristics of RBCs, such as their long circulation time, to construct universal RBCs, develop drug delivery systems, and transform cell therapies for cancer and other diseases. This article reviews the component and aging mystery of RBCs, the methods for the applied universal RBCs, and the application prospects of RBCs, such as the engineering modification of RBCs used in cytopharmaceuticals for drug delivery and immunotherapy. Finally, we summarize some perspectives on the biological features of RBCs and provide further insights into translational medicine.
Keywords: cell surface engineering, cell therapy, drug delivery, red blood cells, universal blood
Introduction
Mature human red blood cells (RBCs) are well-known for their biconcave disk shape. They contain no organelles or complete energy or genetic system, unlike normal nucleated mammalian cells. However, they have a long lifespan of approximately 120 days, traveling through arteries, veins, and capillary vessels in the circulatory system to deliver oxygen to tissues for gas exchange.
The unique properties of RBCs are essential for their function in the body. The biconcave shape of RBCs is a result of their lack of a nucleus and their high concentration of hemoglobin. The biconcave shape allows RBCs to squeeze through narrow capillaries by two mechanisms: flexibility and thinness. The flexibility of the RBC membrane allows it to deform and change shape as it passes through narrow capillaries. The thinness of the RBC membrane also helps it to squeeze through narrow capillaries. This is essential for the delivery of oxygen and nutrients to all parts of the body. What's more, the biconcave shape of RBCs also helps to increase their surface area to volume ratio. The surface area to volume ratio of a sphere is equal to 4πr²/3. The surface area to volume ratio of a biconcave disk is equal to 8πr²/3, which is greater than that of a sphere. This is important for gas exchange, as it allows the RBCs to have more surface area for oxygen and carbon dioxide to diffuse across. This is why RBCs have a biconcave shape. The increased surface area to volume ratio of RBCs allows them to carry more oxygen and carbon dioxide per unit volume. Moreover, the biconcave shape also allows RBCs to stack together in a way that minimizes their resistance to flow. This is important for the efficient transport of oxygen and nutrients throughout the body.
The cell membrane, the only structural component of erythrocytes, accounts for all of their diverse antigenic, transport, and mechanical characteristics [1]. The cell membrane is composed of a lipid bilayer, with proteins embedded in both layers. The proteins on the cell membrane provide RBCs with their antigenic properties, while the proteins in the lipid bilayers which are called hemoglobin, help to transport oxygen and carbon dioxide across the membrane. The biogenetic and mature mechanisms of erythrocytes have been investigated quite thoroughly [2],which involve the development of RBCs from stem cells in the bone marrow, and the mature mechanisms of erythrocytes involve the functions of RBCs in the bloodstream. The spleen and liver play key roles in erythrocyte metabolism. The spleen filters out old and damaged RBCs, while the liver recycles the iron from old RBCs. The spleen also produces antibodies that help to protect against infection. Bessis and Delpech et al. (1981) reviewed the priorities and credits for the discovery of RBCs [3].
Universal red blood cells (U-RBCs) are a type of RBCs that is compatible with blood from all donors, regardless of their blood type. This makes them a potentially valuable resource for blood transfusions [4], and could help to solve the problem of blood shortages and improve the safety of blood transfusions. U-RBCs are being developed based on the fundamental structure and function of RBCs. There are several different approaches to developing U-RBCs, including removing the antigens that are responsible for incompatibility; masking the antigens on the surface of RBCs so that they are not recognized by the recipient's immune system; developing artificial RBCs that do not have any antigens. There are many groups that did research on U-RBCs and made plenty of promising progress [5]. This review summarizes the work progress on developing U-RBCs, including the modification of antigen enzymes, masking of blood group antigens, ex vivo synthesis, and artificial RBCs.
It's a promising platform of RBCs for drug delivery due to their unique properties, such as their long circulation time in the bloodstream, their ability to target specific tissues, and their biodegradability. RBCs can be loaded with drugs in a number of ways, such as encapsulating the drug in a polymer coating; linking the drug to the RBC membrane; transfecting the RBC with genes that encode for the drug. Once the drug is loaded into the RBC, it can be released slowly over time, or it can be released in a targeted manner.
The use of RBCs for drug delivery has several advantages. First, RBCs' abundant and long circulation time in the bloodstream allows for a slow and sustained release of the drug. Second, easily accessible and with the ability to target specific tissues, RBCs can be used to deliver drugs to a variety of tissues, including the brain, liver, and tumors. They can also be used to deliver drugs to specific cells, such as cancer cells or immune cells. Third, RBCs are biodegradable, which means that they are broken down by the body after they have released their payload.
However, RBCs still have a limited lifespan after which they are removed from circulation by the spleen and liver. The process of RBC aging and clearance is essential for maintaining the health of the blood and preventing the accumulation of damaged RBCs, which can lead to a few health problems, including anemia, jaundice, and kidney disease. Free radicals, glycation, and apoptosis are common factors that contribute to the aging of normal cells and RBCs, while the blood is a very complex mixture of many different components, plasma, RBCs, WBCs, and platelets, electrolytes, hormones, and waste products, which made the aging and clearance process of RBCs more complex, what's more, natural antibodies, immunoglobulin G, opsonin, and the complement system, have all been proven to contribute to this process [6]. Some membrane disorders are also involved in the RBC aging and clearance process, such as band 3 clustering, phosphatidylserine (PS) exposure, the denaturation of CD47, and the cracking-off of sialic acid. In 2015, some new aspects have been taken into consideration, such as cell deformability and shape [7]. The aging and clearance process is not only playing important roles in naturally mature RBCs but also critical for drug-loaded RBCs as it can affect the RBC property, shape, and circulation life in vivo.
In this review, we discuss the mechanisms of nature-made long-circulating vehicles (RBCs) from physiochemical and biological perspectives. We also provide insights and inspiration for the development of universal red cells, man-made drug delivery systems, and cancer immunotherapy for translational applications in clinical settings.
The molecular structure of RBC membranes
In recent decades, many fundamental discoveries have been made about the structure of cell membranes. In 1972, Singer and Nicolson proposed the fluid mosaic model of the cell membrane [8]. Four years later, Marchesi and Furthmayr published a comprehensive review of the membrane of RBCs [9]. In addition to the lipid bilayer, RBC membranes also contain integrin and peripheral membrane proteins, as well as carbohydrates that are embedded in the inner leaflet. With the development of new techniques, such as freeze-fracture microscopy, scanning probe microscopy (SPM), and X-ray diffraction, the structure of RBC membranes has become increasingly clear. Figure 1 shows some details of the red cell membrane.
Lipids
Approximately 40% of the weight of the RBC membrane is composed of lipids. The main types of lipids are phospholipids (24%), unesterified cholesterol (12%), and glycerides and free fatty acids (4%). Cholesterol is an essential structural component of the RBC membrane, controlling its surface area, structural integrity, and fluidity. This allows RBCs to change shape and move around.
Membrane proteins
Mark S. Bretscher et al. (1973) summarized the general principles of membrane structure and three of the major protein components of the erythrocyte membrane in their review [10]. The three components were separated based on molecular weight using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Tektin A, which was separated from blood ghosts and named by Clarke et al. (1971), has two chains (alpha and beta) with molecular weights of 220 kDa and 240 kDa, respectively [11]. These chains are known as spectrin or band 1 (alpha chain) and band 2 (beta chain). Component A (100 kDa), also called band 3, is exposed on the surface of erythrocytes and functions in anion exchange. The major blood type epitopes are glycoproteins A, B, and C, which have molecular weights of approximately 30 kDa. These glycoproteins are located on the surface of RBCs and are recognized by the immune system to determine blood type. Blood types such as A, B, and O are determined by the presence or absence of these glycoproteins. There are many other proteins, such as band 4.1, with a molecular weight of approximately 83 kDa, and actin is approximately 43 kDa, and it's a member of the membrane skeleton. Some proteins cannot be recognized due to their low content.
A schematic representation of a red cell membrane. The plasma membrane is a composite structure composed of amphiphilic lipid molecules. A two-dimensional elastic network of skeletal proteins is embedded in the lipid bilayer through tethering sites (transmembrane proteins).
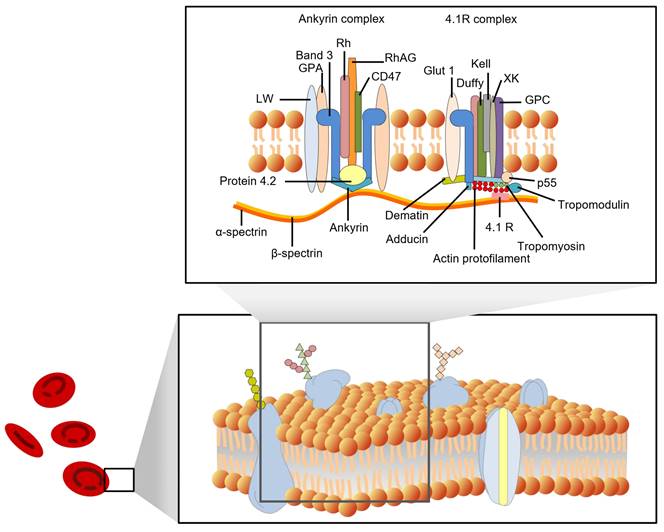
According to their position on the cell membrane, these proteins can be divided into integrated and peripheral membrane proteins. Integral membrane proteins are proteins that are embedded in the lipid bilayer of cell membranes. They are held in place by hydrophobic interactions with the lipid molecules, and they play a variety of important roles in cell function, including transport of molecules across the membrane, signaling, and energy transduction [12]. They include band 3 protein (anion exchanger 1, AE1), glycophorins (glycophorins A, B, and C), Na+/K+ ATPase, band 4.5 protein (glucose transport protein), and surface receptors; Peripheral membrane proteins are not embedded in the membrane, but are associated with it through non-covalent interactions. They include spectrin (bands 1 and 2), ankyrin (bands 2.1, 2.2, and 2.3), band 4.1, and actin (band 5). The peripheral proteins are components of the cell cytoskeleton. Spectrin is a major component of the cytoskeleton, and it forms a network of interconnected rods that help to maintain the shape of the cell membrane. Ankyrin binds to spectrin and anchors it to the membrane. Band 4.1 also binds to spectrin and helps to stabilize the cytoskeleton. Actin is a small protein that also plays a role in the cytoskeleton. The interactions between the peripheral proteins are mediated by band 4.2. Band 4.2 binds to both spectrin and ankyrin, and helps to link them together. This helps to form a strong and stable network that supports the cell membrane. Figure 1 shows a schematic diagram of the interactions between the peripheral membrane proteins.
The membrane proteins in RBCs have a variety of functions, such as transporting oxygen and carbon dioxide, maintaining the shape of RBCs, protecting the RBCs from damage, and helping to identify RBCs. Hemoglobins help to transport oxygen and carbon dioxide across the cell membrane. Other membrane proteins, such as actin, band 4.1, spectrin, ankyrin, and band 3, help maintain RBCs' biconcave shape. The membrane skeleton is a network of proteins that helps to maintain the shape of RBCs. It is also important for the deformability of RBCs.
Carbohydrates in cell membranes
Carbonate residues also are composed as a functional part of the cell membrane of RBCs, which exist in different forms of modifiers on proteins or lipids. Glycoproteins are mainly glycophorins, which are the determinants of blood types as above, and glycosphingolipids also are the antigens that define blood types. The content of sialic acid is quite high in carbonate residues, and it is mainly mannose and galactose as the main stem of carbonate residues, and fucose and sialic acid as the branches.
Sialic acid
Sialic acid is the most common negatively charged carbohydrate residue on the RBC membrane. It is present in the form of sialoglycoproteins, such as glycophorins A, B, and C. Periodic acid-Schiff stain-1 (PAS-1) and periodic acid-Schiff stain-2 (PAS-2) are two different forms of glycophorin A that can be distinguished by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). PAS-1 and PAS-2 can interconvert, but their relative proportions change with RBC age. Many researchers have reported that sialic acid, also known as N-acetylneuraminic acid, plays a significant role in the circulation time of RBCs [13]. Sialic acid helps to protect RBCs from destruction by the immune system and also helps to maintain the flexibility of the RBC membrane.
The structure of sialic acid
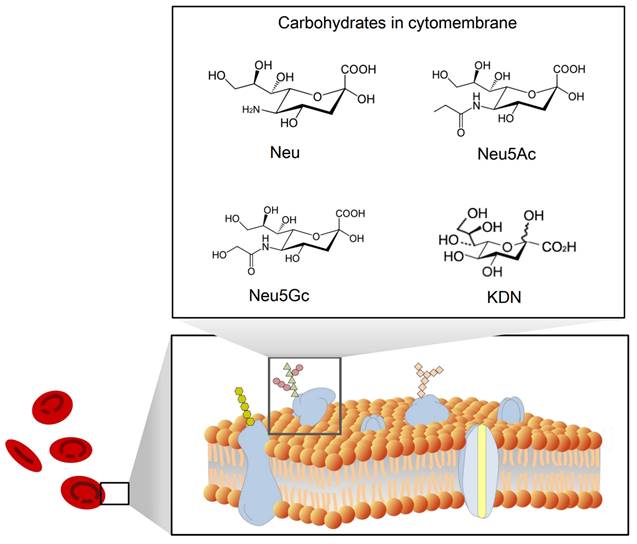
Sialic acid is a monosaccharide with a nine-carbon backbone and is widely expressed on the external surface of RBCs. It is a derivative of neuraminic acid, which can be N- or O-substituted. There are 43 derivatives in the sialic acid family, which can be classified according to their structure and configuration (Figure 2). The most common derivatives in the cell membrane are N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid (KDN) [14], and N-acetyl-9-O-acetylneuraminic acid (Neu5AcAc).
Sialic acid distribution on erythrocyte cell membranes
Sialic acids are components of the cell membrane, typically found at the terminal position of N- and O-linked glycans, as well as on glycosphingolipids expressed at the cell surface. Many secreted glycoproteins are also modified with sialic acid [16]. The sialic acid composition of erythrocytes varies among species. For example, Neu5Gc is abundant in many mammals but not in humans [11]. Sialic acid together with proteins forms glycoprotein glycophorins (A, B, and C) at the outermost surface of the membrane. Glycophorin A is a protein of 31 kDa with an unknown function in normal erythrocytes. It is the most abundant sialylated glycophorin and contains 51% carbohydrates [6, 17]. However, 50% of sialic acids in snake muscle are O-acetylated even after acid hydrolysis.
Functions of sialic acid on erythrocyte cell membranes
Sialic acid is a multifunctional molecule that regulates molecular and cellular interactions. It has been shown to play important roles in immunity, homeostasis, inflammation, and antioxidant activity [18, 19]. The zeta potential of erythrocytes is primarily determined by the sialic acid on the cell surface, which provides a high negative charge density. This negative charge has an affinity for water molecules, which helps to prevent the erythrocytes from sticking to each other and to the walls of blood vessels [20]. Sialic acid also affects the morphology, deformability, and aggregation of erythrocytes. It is also a marker of cell senescence [21]. Oxidative stress can lead to the desialylation of erythrocytes [22], which can alter their rheological properties [23]. The decrease in sialic acid content in erythrocytes may also affect their lifespan. However, the precise influence of sialic acids on the long circulation times of erythrocytes is still not fully understood.
A schematic view of the structure of sialic acid (Neu) [15] and its derivatives Neu5Ac, Neu5Gc, and KDN.
Engineered U-RBCs
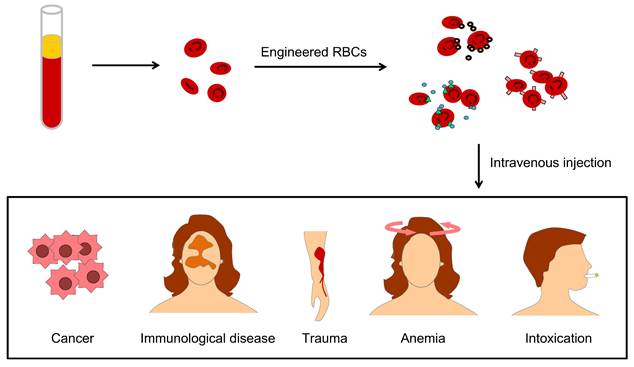
Engineering U-RBCs is a potential solution to the global blood shortage, and RBC engineering particles also override many obstacles in drug delivery, therapeutic targeting, and biological imaging due to their excellent physiochemical and biological characteristics of longer circulation time, targeting-mediated, good biocompatibility, and attenuated immune response [24]. These strengths benefit many clinical problems, such as lack of blood in cancer patients, immunological disease, large physical trauma, kinds of anemia and remove toxins from the blood (Figure 3).
U-RBCs, such as O-negative RBCs, do not have AB antigen groups and can be transfused to any recipient regardless of the kind of preexisting AB antibodies. The goal is to develop a safe and effective way to provide blood transfusions to people of all blood types. Therefore, the O-negative group and the RBCs that are modified artificially and functionally assemble into O-negative RBCs, negative for all of these antigens, and are regarded as U-RBCs, for example, enzymatically converted group O cells and stealth cells [25]. There are several ways to produce U-RBCs. One way is to remove the blood group antigens from the surface of RBCs by specific enzymes. Another way is to mask the blood group antigens using biocompatible molecules, such as PEGylation or dopamine. A third approach is to generate U-RBCs in vitro from stem cells. This involves genetically modifying the stem cells to remove the genes that code for the blood group antigens. Finally, it is also possible to create artificial U-RBCs from materials science.
Enzymatic approaches for the generation of U-RBCs
Blood group incompatibility is a major obstacle to blood transfusions for patients with massive blood loss. The enzymatic approach is to solve this problem with biochemical methods.
L. Lögdberg et al. (2005, 2011) worked on the chromosomal locations and cloning of human blood groups, and until 2010, They have identified 33 blood group systems that represent more than 300 antigens [26, 27]. The ABO blood type system was the first blood group system to be recognized in 1900. The antigens of ABO blood type system is a repeating Gal-GlcNAc disaccharide unit, which is the major polylactosamine chain structure in human RBCs, and this unit can be attached to the membrane either as a glycolipid or as an N-linked glycoprotein [28]. The specific chain structures are listed below (Table 1).
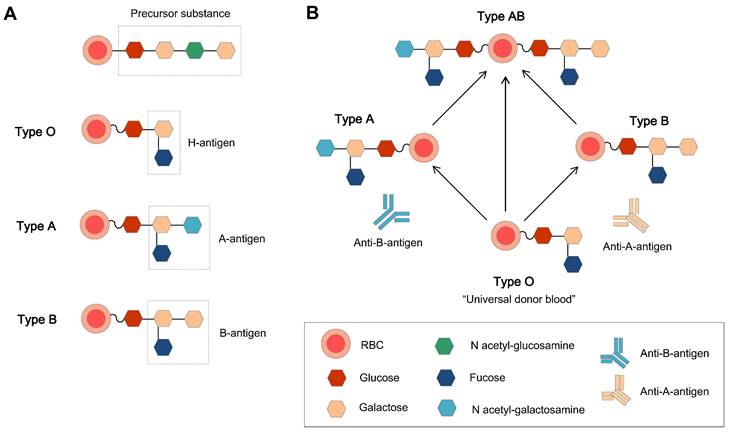
The ABO blood group system is the most important blood group system in the clinic. Each blood group has a corresponding antibody in the plasma, except for type O blood. This is why type O blood is considered the universal donor blood type. To date, homotypic principle of blood transfusion is still the main principle. The human immunodominant on the type A RBC membrane is N-acetylgalactosamine (GalNAc), and galactose (Gal) is on the membrane of type B RBCs. Type O blood is the oldest blood, which has neither A nor B type antigens, and there are A and B antibodies in the plasma. Type AB is newly oriented, and both type A and B antigens are expressed on the external surface of the RBC membrane together. Genetically, the A and B types of blood groups were inherited and transformed from type O.
The specific chain structure of human RBC antigens, antibodies, and their responsive enzymes for enzyme-converted group O (ECO) RBCs.
| Phenotypes | Structure of dominant immune epitope | Antibodies in sera | Glycosyltransferases |
|---|---|---|---|
| A type | α-3-N-acetylgalactosamine | anti-B | α-3-N-acetylgalactosaminyl |
| Subgroup A1 | A and repetitive A | anti-B | |
| Subgroup A2 | A | anti-B and anti-A1 | |
| B type | α-3-galactose | anti-A and anti-A1 | α-3-galactosyl |
| AB type | A and B | No anti-A or anti-B | |
| A1B | A, repetitive A and B | No anti-A or anti-B | |
| A2B | A and B | No anti-A or anti-B anti-A1 | |
| O type | No | anti-A and anti-B |
The applications of engineered RBCs for drug delivery include different aspects, such as lack of blood in cancer patients, immunological disease, large physical trauma, kinds of anemia, and clearance of blood toxication.
Common means to obtain universal RBCs for transfusion. (A) Preparation of enzyme-converted group O (ECO) blood cells by enzyme digestion. (B) Shielding antigens of the membrane surface from donors. (C). Inducing RBCs from iPS cells, gene editing, and adjusting the culture procedure to control the blood types.
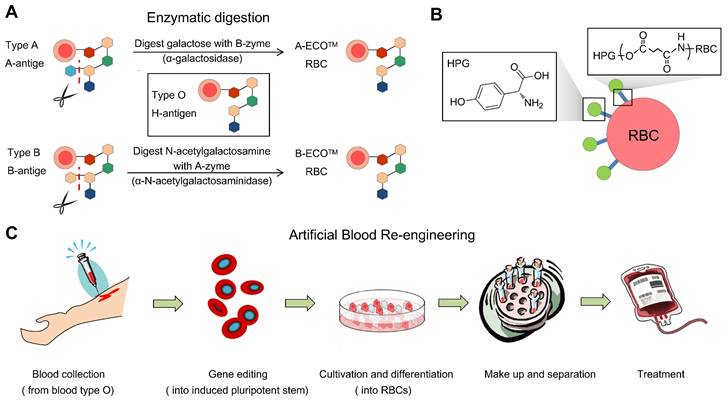
Enzyme-mediated generation of U-RBCs is to remove blood-type antigens (GalNAc and Gal), which means that an N-acetylgalactosamine transferase or a galactose transferase can remove the A and B epitopes, respectively.
Basic principles of the enzymatic approach
Antigens are molecules that can be recognized by the immune system. They can be proteins, glycoproteins, or glycolipids. The terminal residue of an antigen is the outermost sugar molecule in its carbohydrate chain, which determines the difference between type A and type B blood. To remove the terminal residue of the antigen or to remove the component on the extracellular layers of the membrane surface, the efficiency of the glycosidases and the proper procedures are essential, because cell viability and compatibility are key criteria to meet. If the glycosidases are not efficient, or if the procedures are not proper, the antigen may not be completely removed from the cell surface, and would not be accepted by the recipient's immune system.
Removal of the terminal residue of the type B antigen
Enzyme-converted group O (ECO) are RBCs that have been converted to have the same blood group antigens as type O RBCs. This is done by using enzymes to remove the terminal residues of the A and B antigens. Enzymes to cleave the terminal residue of the B antigen have been successfully used in humans, but it is slightly more difficult to remove the terminal residue of the A antigen. To convert group B RBCs to group O RBCs, the Gal residues must be removed. The enzyme for the removal of the terminal residue of the B antigen is alpha-galactosidase [31]. The relationship between the ABO group antigens is shown in Figure 5. Most enzymes used in experiments are produced in bacteria for efficiency and economy. However, in 1960, Zarnitz et al. used a-galactosidase from green coffee beans to remove the B and BP1 (nondialyzable residue from B cells) substance epitopes [32]. Eleven years later, Yatziv et al. (1971) used α-galactosidase extracted from coffee beans to treat type B RBCs and observed the disappearance of type B blood characteristics [33].
Removal of the terminal residue of the type A antigen
α-N-acetylgalactosaminidase produced by Clostridium perfringens has been used to convert type A red cells [34], but the antigens cannot be completely eliminated. This is because the A group has two subtypes, A1 and A2 [35], and it is more difficult to remove the A antigen on the RBC surface than the B antigen. Additionally, the sources of enzymes are quite limited.
Rahfeld P et al. (2019) identified an enzyme pair from the obligate anaerobe Flavonifractor plautii among the human gut microbiome that can efficiently convert types A and B red cells to type O of the same rhesus [36]. This enzyme pair is more effective than α-N-acetylgalactosaminidase and is more readily available.
New sources of enzymes need to be found to produce ECO RBCs from A1 and A2 RBCs. These enzymes should not interact with monoclonal anti-A antibodies. However, the problems with polyclonal human antibodies (i.e., cross-matching) are still in need of researchers to solve them.
Overall limitations of the enzymatic approach to generate U-RBCs
The enzymatic approach to generating U-RBCs has several limitations. One limitation is the need for proper enzymes. The enzymes used in this approach are typically extracted from bacteria [37] or coffee beans [31], and they require specific pH (pH 5.5) and temperature conditions to work properly. These conditions are often not compatible with the membrane osmotic properties of RBCs, which can damage the cells and make them unsuitable for clinical use. Antigens on RBCs determine their activities, such as type A, B, and O. The ABO blood type is the most well-known blood type and is the primary determinant for blood transfusions.
The major human alloantigen systems involve the ABO, Rhesus (Rh), MNS, Lutheran, and Kell blood groups. The Rh blood group system is a clinically significant human blood group polymorphism in alloimmunization and includes more than 50 different serologic specificities [38], making this system particularly important and challenging for biomedical scientists and clinicians. Within the Rh blood group, the D antigen is the most immunogenic and clinically important epitope [6].
The enzymatic removal of the specific antigens of groups A and B from RBCs is a feasible strategy to generate type O cells. However, this technology does not successfully produce U-RBCs. Type O-negative RBCs are considered U-RBCs for emergency conditions.
U-RBCs should not only lack the reactivity of the A or B antigens of RBCs but also lack the reactivity of the D antigen, which is highly immunogenic. Unlike the A and B antigens, the D antigen is a protein-based antigen and is closely associated with the RBC membrane [39]. Therefore, it is not possible to excise the immunogenic epitopes of the D antigen from the RBC membrane. Thus, type O-negative RBCs cannot be generated from type A-positive or B-positive RBCs via an enzymatic approach.
The relations of H, A, and B antigens and the basic structure of the ABO blood group. (A) The original ABO blood group is a precursor substance composed of glucose, galactose, and N-acetyl-galactosamine, and the H antigen (the precursor H oligosaccharide antigen) is the basic unit in the A and B antigens [40]. (B) The antigens and related antibodies of the ABO blood group, which make the type O group the natural universal donor.
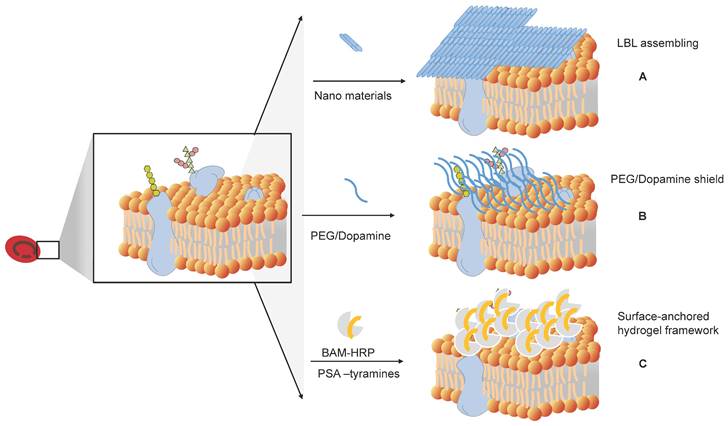
How to shield the original antigens and produce universal RBCs. (A) Layer-by-layer (LbL) assembly to mask antigens on RBCs. (B) Shielding of the original antigens of RBCs by modifying antigens on the membrane surface of RBCs, such as PEG and dopamine. (C) Surface-anchored hydrogel framework for generating RhD epitope stealth RBCs.
Masking of the blood group antigens from the cognate antibodies
Antibodies in plasma react with antigens on the RBC membrane to cause RBC aggregation. An alternative way to prevent this reaction is to block the antigens. Several methods have been developed to produce universal or stealth cells (Figure 6).
Antigen masking of RBCs by layer-by-layer (LbL) assembly
Sequential deposition of poly(ethylene glycol) (PEG) on RBCs is a practical way to create a layer-by-layer (LbL) assembly on the membranes. LbL PEGylated RBCs have a prolonged circulation time compared to both poly(lactic-co-glycolic acid) (PLGA) nanoparticles and the corresponding PEGylated nanoparticles [41].
Sania Mansouri et al. (2009, 2011) built a hybrid LbL system with different sets of polyelectrolytes: chitosan-graft-phosphorylcholine (CH-PC) and sodium hyaluronate (HA), and poly-(l-lysine)-graft-poly(ethylene glycol) (PLL-PEG) and alginate (AL). They used these polyelectrolytes to create two types of shelters on the RBC membrane. This LbL system was able to protect RBCs from hemolysis and inhibit recognition by the anti-A antibody [42].
They further tested different polyelectrolytes and found that the optimized shell was composed of four bilayers of alginate (AL) and chitosan-graft-phosphorylcholine (CH-PC) surrounded by two bilayers of alginate (AL) and poly-l-lysine-graft-polyethylene glycol (PLL-PEG) [43].
PEGylation of RBCs by covalent conjugation
PEGylation is a good way to mask blood group antigens from cognate antibodies. The molecules generally used are polyethylene glycol (PEG) and its derivatives. PEG is a nonionic, water-soluble, amphiphilic polyether with good biocompatibility. Its molecular weights (MW) vary from 100 to 8,000,000 Da, and it can be configured as a linear PEG, branched PEG (bPEG), or star PEG (sPEG). mPEG is a linear-form PEG capped with a methyl (Me) group.
The virtue of PEG solubility in water is due to the bonding of each ethylene oxide unit with three H2O molecules. Coupling reactions occur in the terminal hydroxyl group [44]. Commonly, the PEG linkers that form RBCs are cyanuric chloride (CN)-PEG, N-hydroxy succinimidyl (SP)-PEG, and benzotriazole (BTC)-PEG. S. Hashemi-Najafabadi et al. (2006) studied the optimum PEGylation conditions and found that pH 8.7 and 14 °C were the most suitable covalent conditions for PEG of 5 kDa to human RBCs [45].
Cyanuric chloride-PEG, also known as PEG-dichloro triazine (PEG-DCT), is the product of the reaction of trichloro triazine and MeO-PEG-OH (MW 5,000 Da) [46]. PEG-DCT was used to couple with the RBC membrane surface, and the PEG-coupled RBCs did not react with plasma antibodies. However, this method is not stable in lactic acidosis. Moore and colleagues studied the stability of engineered RBCs with maleimide-PEGylation and cyanuric chloride-PEGylation masking by agglutination tests with anti-D sera. They found that maleimide-PEG-RBCs showed more stability than cyanuric chloride-PEGylation under multiple conditions. They also found that critical concentrations of lactic acid caused cyanuric chloride-PEG-RBC dePEGylation [47].
Succinimidyl derivatives of PEG (SP-PEG) are similar to a decoration of PEG by succinimidyl. Succinimide derivatives have succinimide radicals or functional groups, which can help PEG gain circulation time. Extension arm facilitated PEGylation is an approach using succinimidyl derivatives of PEG [48]. PEGylation occurs by adding an extension arm on the amino groups of RBC membrane proteins. The positive charge of the extension arm successfully covers the A and D antigens of erythrocytes, and the RBCs get a mask to be immune from blood type antibodies in the serum. This method can keep the oxygen affinity of RBCs unharmed. In this approach, Mal‐Phe‐PEG‐5,000 and Mal‐Phe‐PEG‐20,000 chains are needed to mask the A and B antigens. These two molecules in combination can inhibit the agglutination of RBCs with anti-A or anti-B antibodies [49].
The stability of antigen-masked RBCs must be validated before transfusion into blood recipients. Many studies on the masking method to produce U-RBCs have shown its stability and ability to shield RBCs from immune components, and its potential for clinical use. However, Garratty et al. (2008) found that PEG is immunogenic in animals and humans, and the occurrence of PEG antibodies can shorten the circulation time of masked RBCs or proteins [44].
Antigenically shielded U-RBCs by polydopamine (PDA)-based cell surface engineering
Dopamine is a neurotransmitter and plays a key role in the neuronal system. In the meantime, it is a new star molecule that is widely used in allosteric molecular chemistry. It can tightly bond on solid surfaces and has good biocompatibility. In the bioengineering field, polydopamine-coated hemoglobin can work as an oxygen carrier. PDA is widely used as a carrier in polymeric nanodrugs after PDA-based surface modification [50]. Wang, B., et al. (2014) used PDA-based cell surface engineering [51] to produce U-RBCs with good membrane permeability, fluidity, and high oxygen capacity. The PDA coating shielded the antigenic epitopes on the RBC surface, making them compatible with all blood types. In vivo studies showed that the PDA-engineered RBCs had a long circulation time and were able to deliver oxygen effectively. Polydopamine (PDA) can also be used as an antioxidant enzyme. Liu et al. (2018) encapsulated a complex of enzyme-mimicking PDA carrying hemoglobin into the membrane of RBCs. These man-made RBCs are an oxygen-self-supplied platform that can be used to overcome the hypoxia-mediated resistance of tumors to photodynamic therapy [52].
Surface-anchored Hydrogel Framework for Generating RhD-epitope Stealth RBCs
Rhesus D (RhD) is another important blood type determinant [53]. RhD-positive blood is common in humans, which can make it difficult to find RhD-negative blood for transfusion. Zhao et al. (2020) developed a surface-anchored hydrogel framework to shield the antigens on the surface of RBCs with polysialic acid-tyramine. This completely obstructed the RhD antigens on the cell surface, and the surface-anchored framework provides an efficient method for generating U-RBCs for blood transfusion [54].
Ex vivo synthesis of U-RBCs
Blood transfusion is a cell therapy that is essential for many medical procedures and treatments. However, blood supply is not always available, and the storage of blood can be challenging due to the rapid deterioration of blood cells. Ex vivo synthesis of U-RBCs could address these challenges by providing a consistent and reliable source of blood.
General principles of the approach
Hematopoietic stem cells (CD34+ cells) in blood, bone marrow, or cord blood can generate erythroid cells. Luc Douay et al. (2007) developed a protocol for the expansion and differentiation of progenitors into mature RBCs in vivo. This approach is highly effective, but it is also expensive [55].
The Malik P. group (1998) found that adding specific combinations of growth factors in the right order to culture hematopoietic stem/progenitor cells (HSPCs) can induce erythropoiesis [56]. Neildez-Nguyen et al. (2002) cultured HSPCs (isolated from cord blood, or CB) by adding cytokines Flt3 ligand (cytokine Fms-like tyrosine kinase 3 ligand) [57], thrombopoietin, stem cell factor, erythropoietin, and insulin-like growth factor I into a culture medium in a specific order to produce RBCs from induced pluripotent stem cells (iPSCs), which are similar to embryonic stem cells (ESCs).
In addition to human hematopoietic/progenitor stem cells, human embryonic stem cells and induced pluripotent stem cells have already been used to generate functional RBCs. Blood, bone marrow, and cord blood are all possible sources of cells. Akihito Fujimi et al. (2008) obtained the large-scale generation of human RBCs from cord blood CD34+ cells by ex vivo co-culturing with macrophages [58]. RBCs generated from ESCs require the differentiation of ESCs, but the protocol for generating RBCs has limited efficacy [59].
Universal blood group-based iPSCs offer the benefits of removing blood groups, as well as HLA-ABC-deficient platelets and myeloid cells [60]. This makes it possible to produce functional RBCs in vitro on a large scale [61].
Functional properties of the ex vivo cultured RBCs
Ex vivo cultured RBCs can provide a large number of uncontaminated, mature RBCs for clinical and research use. Peptides and molecules that define erythropoiesis are important in the process of ex vivo cultured RBCs from stem cells. Enucleation is induced to promote the maturation of RBCs.
Some functional properties between normal, original RBCs and ex vivo cultured RBCs have been measured. The levels of glucose-6-phosphate dehydrogenase and pyruvate kinase are comparable to those of native RBCs, so ATP production and reduced glutathione can be provided by ex vivo cultured RBCs. Hemoglobin function and oxygen equilibrium are very close to those of native RBCs [55].
David J. Anstee et al. (2012) reviewed the RBCs generated from hematopoietic stem cells ex vivo and discussed the quality of these cells for transfusion. They concluded that all the functions of universal cells made by ex vivo hematopoietic stem cell culture meet the criteria [62], although more time is needed to meet the great shortage of transfusions.
Sabine Kupzig et al. (2017) compared the lifetime of ex vivo-generated RBCs and donor red cells using an in vivo model and showed that the former survived longer than the latter and that ex vivo-generated red cells cultured from human reticulocytes more closely met the demand of clinical transfusion.
Bioengineering of U-RBCs
The bioengineering of U-RBCs involves changing the antigens on the surface of RBCs using biological methods.
Sho-ichi Hirose et al. (2013) found that transducing c-MYC and BCL-XL genes into multipotent hematopoietic progenitor cells can overexpress these genes and induce the immortalization of erythrocyte progenitor cells [63]. This allows for the large-scale production of RBCs. Katie E. Glen et al. (2013) reported a method for the production of erythrocytes from directly isolated or Delta1 Notch ligand-expanded CD34+ hematopoietic progenitor cells [64]. This method has a high potential for manufacturing production. Jan Frayne et al. (2019) used combinatorial gene targeting to create individual and multiple blood group knockout sublines, such as ABO [Bombay], Rh [Rhnull], Kell [K0], Duffy [Fynull], and GPB [S-s-U-] [65].
With the development of culture erythroid progenitor cell lines from a variety of sources, such as adult peripheral blood mononuclear cells and even some common cell lines [66], and the reduction in the cost of culturing in vitro RBCs [67], it will be much more convenient to expand the bioengineering capacity for U-RBCs.
Artificial RBCs
Artificial RBCs are man-made membranes that encapsulate hemoglobin (Hb) and enzymes to make them resemble natural RBCs. Artificial RBCs can circulate for a longer time than natural RBCs and have similar properties, such as oxygen-carrying, flexible deformation, and semi-permeable membranes. The development of artificial RBCs is a necessity [68]. Hemoglobin-based oxygen carriers and perfluorocarbon-based products are some of the successful examples of artificial RBCs. In 2003, Chang, T.M. et al. reported artificial RBCs based on ultrathin polyethylene-glycol-polylactide (PEG-PLA) membrane nanocapsules containing Hb and enzymes. After optimization with polymerized Hb, higher molecular weight, higher concentrations of PEG-PLA, and cross-linking of the newly formed PEG-PLA Hb nanocapsules, the circulation time of Hbs dropped to 1.67 gm/dl after 41.5 hours [69].
Artificial RBCs can also be used for cancer treatment. For example, mitochondria-targeted artificial "nano-RBCs" can be used for amplified synergistic cancer phototherapy by single near-infrared region irradiation [70]. They can also relieve tumor hypoxia and enhance cancer radiotherapy with erythrocyte-membrane-enveloped perfluorocarbon [71].
With the development of bioinstrumentation and technologies, the precise structures and functions of RBCs have become more available. All of the improvements in biotechnology have led to the ex vivo synthesis of U-RBCs and artificial RBCs.
Red cell-based advanced drug delivery system
Drug delivery is a major technical challenge in drug development and clinical therapy [72-74]. A good drug delivery system should achieve the maximum therapeutic effect with the lowest side effects [75]. The drug carrier must protect the delivered cargo's effectiveness and accessibility, as well as load and release the drugs properly. Since the blood circulatory system is present throughout the body, most drugs that are injected into the bloodstream can be transported by blood [76]. Additionally, RBCs have a fascinating size and shape, an almost empty structure inside, and flexible deformation, making them the most promising drug delivery carriers [77]. Chen, S. et al. (2021) synthesized the nanomedicine poly(2-(N-oxide-N,N-diethylamino)ethyl methacrylate) (OPDEA), which reversibly attaches to RBCs and prolongs the circulation time of the anticancer drug SN38 [78].
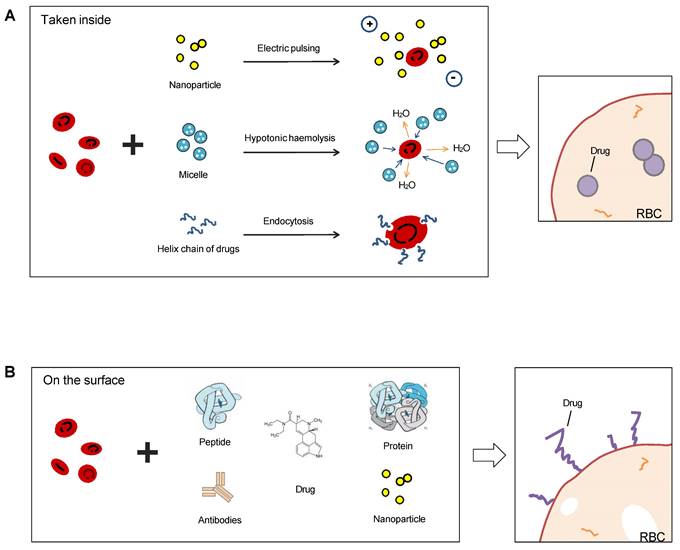
Most common methods to use RBCs as drug carriers are encapsulating the therapeutics into the cavity of the membrane or coupling them on the membrane surface (Figure 7), and RBC-hitchhiking is a new drug-loading technique (RBCH) applied nanocarriers noncovalently onto RBCs, it relies on physical forces such as van der Waals forces, hydrogen bonding, and electrostatic interactions to couple RBCs and cargoes.
The packaging of drugs or other cargoes into RBCs can be achieved before or after the RBCs mature. One approach is to use stem cell-erythroid precursors to produce long-term systemic proteins that can target specific tissues. Another approach is to couple drugs or other cargoes to the surface proteins or carbohydrate molecules of RBCs. RBCs can also be used as convenient tracers when carrying magnetic particles. In addition, RBC-mimicking synthetic biomaterial particles and erythrocyte membrane-camouflaged polymeric nanoparticles have been developed and used in practice. Carlos H. Villa et al. (2017) reviewed the pros and cons of different sources and loading types for drug delivery and pointed out four key factors that determine whether the loading was successful: the optimal loading protocol, the source of the RBCs, the production logistics, and regulatory hurdles [79]. We will analyze the different drug delivery techniques separately below.
Sketch illumination of RBCs as a drug delivery platform precedent hitchhiking. RBCs can be used for drug delivery in two ways: taking drugs inside or binding on the surface. Electric pulsing, hypotonic hemolysis, and endocytosis are common means to invade the bilayer membrane of RBCs and absorb nanoparticles or other forms of drugs in (A), and another means is linked antibodies, peptides, proteins, or chemicals directly on the surface (B).
Stem cell-derived erythroid cells mediate long-term systemic protein delivery
Erythroid precursors are born from bone marrow, and these cells have nuclei. During the differentiation and maturation of RBCs, they obtain the proper components inside and on the cells. The nuclei are released by exocytosis. Using a genome-editing technique, some scientists can use stably expressed and rationally designed proteins inside RBCs to carry specific drugs. In a mouse model of hemophilia B, Alex H. Chang et al. (2008) used gene editing technology to target human factor IX (hFIX) expression in late-stage erythropoiesis. They achieved long-term hFIX secretion (18 months) at levels tenfold higher than those obtained with an original promoter [80]. Phetcharat Phanthon et al. (2017) used pluripotent stem cell (iPSC)-derived erythroid cells as a platform to enhance β-globin gene expression in thalassemic IVS2-654 by modified U7 snRNA. Erythroblasts differentiated from transduced iPSCs expressed high levels of correctly spliced β-globin mRNA, which could be used to treat beta-thalassemia [81].
Vascular delivery of drugs encapsulated into RBC carriers
The encapsulation of drugs into RBCs can be achieved by electrical insertion or hypotonic loading. Diverse agents have been encapsulated into RBCs, such as small molecules and therapeutic proteins [82].
There are three main characteristics of encapsulated drugs in the inner volume of RBCs. First, the RBC ghost can encapsulate drug compounds that cannot pass through the plasma membrane. Second, encapsulation also helps to protect the drug from the body's immune system because the drug cargo is separated from the body. Third, the drug-loaded RBCs are only a small fraction, which is important for patients who have a low blood count [83].
The loading efficiency and release rate of different agents are important indices to be measured. Ropars C. and his colleagues (1987, 1996) found that inositol hexakisphosphate encapsulated in RBCs improved the oxygen capacity of RBCs [84]. Al-Achi A. and Greenwood R. (1998) investigated the oral administration of human insulin by human RBC encapsulation [85], and most of the projects worked well. Biologicals have also been successfully encapsulated in RBCs for antiviral interventions [86] or gene therapy [87].
Monica Piergiovanni et al. (2020) used fluidic shear stress to make the pores on the membrane of RBCs open for a short time and allowed the drug fluid (FITC-dextran, 40 kDa) to diffuse into the RBCs successfully. This experiment was beneficial to the equipment they mentioned in the study, the computational fluid dynamic model, and the microfluid channels [88].
Other pharmacological effects, such as chemotherapeutic agents in cancer chemotherapy, have also been studied [89]. For example, photosensitized erythrocytes loaded with methotrexate were found to release the drug rapidly. Kim et al. (2009) reported a quantitation method for enzyme-linked immunosorbent assay (ELISA)-based assay and a carrier-mediated antisense oligonucleotide delivery system based on erythrocyte ghosts [90]. Yew et al. (2013) successfully encapsulated phenylalanine hydroxylase into RBCs and evaluated the sustained enzymatic activity in the circulation [91]. This proves that enzyme-loaded RBCs can metabolize amino acids in the bloodstream and can be used to treat disorders of amino acid metabolism.
However, there are still some concerns about encapsulation. For example, the encapsulation procedure may damage the inner membrane or surface structure of RBCs, or the biocompatibility may decrease, leading to phagocytosis along the delivery route. Hypotonic preswelling is one of the methods to achieve intact RBC coupling, but it requires the elimination of Cremophor EL (a derivative of ethylene oxide and castor oil) and dehydrated alcohol, which have a high risk of infection and hypersensitivity reactions. In a study by Gamaleldin et al. (2004), 148.8 µg of paclitaxel was loaded per mL of erythrocytes, and as much as 81% of the loaded paclitaxel was released into the plasma in less than 48 hours. However, the coupling method led to lipid and protein oxidation in the erythrocytes [92].
Overall, the encapsulation of drugs into RBCs is a promising approach for drug delivery. However, there are still some challenges that need to be addressed, such as the preservation of the biocompatibility of RBCs, the properties of the drug and the RBCs, and the development of more efficient and controlled release methods.
Coupling therapeutic drugs to the RBC surface
As mentioned above, the RBC membrane is a complex structure with numerous residues of protein, lipids, and carbohydrate molecules. The biconcave shape of RBCs gives them a surface area that is 1.4 times larger than that of normal spherical cells, which makes them ideal for surface coupling of therapeutic drugs.
The coupling of therapeutic drugs to the RBC surface can be achieved using a variety of methods, including covalent or noncovalent affinity, absorption, adhesion, or attachment. In the early 1980s, scientists used 2-iminobiotin-avidin [93], biotin derivatives, and 125I-streptavidin to couple drugs to amino acids on the membrane surface. Sugar and lipids are also common targets for surface coupling, and many scientists have tried to couple antibody heteropolymers [94] to the RBC surface to eliminate pathogens or toxins in the circulatory system. With the coupling of complement inhibitors to the RBC surface, RBCs can be protected from complement-mediated hemolysis [95, 96].
However, most coupling agents and procedures have the potential to damage the RBC membrane and compromise its biocompatibility. Additionally, the control of drug release kinetics is a challenging problem. Despite these challenges, surface coupling technology has several advantages over other drug delivery methods, such as the ease of extraction and infusion of RBCs and the improved hygiene.
Recent advances in surface coupling technology have made it possible to develop strategies for immune-mediated disease therapy. For example, Novalia Pishesha et al. (2017) developed a method for coupling therapeutic drugs to the RBC surface that was effective in a mouse model of multiple sclerosis and type I diabetes [97].
RBC-hitchhiking (RBCH) technique
RBC hitchhiking drug delivery system (RBC-H) [24] is a novel drug delivery paradigm that uses transient coupling of nanocarriers to RBCs to deliver drugs to specific organs. This approach has great potential for the treatment of a number of diseases [98]. Nanocarriers that hitch a ride on RBCs in a non-covalent manner can travel further and more efficiently throughout the body, which can improve their therapeutic effects [99]. This is because RBCs are naturally transported to specific organs, such as the lungs and liver. The RBC-H technique is efficient and safe in delivering drugs to a variety of organs, including the brain, liver, lungs, and kidneys. It is also easier to target the lungs with RBC-H than the spleen, because the shear stress in lung capillaries dislodges particles in the lungs [100, 101]. Biodegradable drug nanoparticles assembled onto the surface of RBCs can extend the circulation time and delivery content of the drug [100].
Several studies have demonstrated the potential of RBC-H for drug delivery. For example, Yaning Ding et al. successfully generated RBC-MPSS-CSNPs, which significantly prolonged the circulation time of glucocorticoids, enabling specific delivery to the lung [102]. Junyan Li et al. utilized the RBC-hitchhiking strategy to facilitate targeted delivery and alleviate inflammatory syndrome in acute pneumonia [103]. Jacob S Brenner et al. reported nanocarriers (NCs) designed based on the RBC-hitchhiking strategy, which significantly increased the absorbance of downstream organs by orders of magnitude. This approach was successful in mice, pigs, and ex vivo human lungs without causing RBC or end-organ toxicities [101]. Anvay Ukidve et al. leveraged the ability of RBCs to present certain pathogens to antigen-presenting cells (APCs) in the spleen to develop an erythrocyte-driven immune targeting (EDIT) strategy [104]. This strategy directly presents antigenic nanoparticles to the spleen, resulting in an improved immune response to the antigen and eliciting a prophylactic effect in a tumor model.
Overall, RBC-H is a promising new drug delivery paradigm with the potential to improve the efficacy and safety of drug therapy for a variety of diseases.
RBCs as carriers in magnetic particle imaging
Loading superparamagnetic iron oxide (SPIO) nanoparticles onto RBCs is a new method of magnetic resonance imaging (MRI) for long-term monitoring of the retention time and distribution of nanoparticles in vivo. SPIO nanoparticles have superparamagnetic properties, which make them suitable for a variety of biomedical applications [105]. However, SPIO nanoparticles are rapidly cleared by the reticuloendothelial system (RES) in the liver, spleen, and lymph tissue. This limits their use in biomedical applications [106]. With SPIO-loaded RBC technology, the story is quite different. Antonelli et al. (2011) developed a method for loading SPIO nanoparticles onto RBCs. They found that the loading efficiency of the nanoparticles depended on their size, synthesis protocol, coating and/or dispersant agents [107]. The hydrodynamic diameter of the nanoparticles should be less than 60 nm to allow them to pass through the pores in the RBC membrane. He loaded several commercial SPIO nanoparticles (SHU 555A, AMI 227, and PMP-50) coated with dextran or carboxydextran and new nanomaterials (Np-1 nanoparticles dispersed in the Disperbyk®-190 agent) into RBCs and compared their loading conditions and efficiencies. The procedure of encapsulating magnetic nanoparticles into RBCs is by hypotonic dialysis and isotonic resealing, followed by reannealing with a few modifications. This method is a promising new approach for using SPIO nanoparticles in biomedical applications. It has the potential to improve the efficacy of MRI imaging and other biomedical therapies.
RBC-mimicking synthetic particles
Mimicking the biostructural and key functional features of RBCs in synthetic particles is a novel method to combine the advantages of natural and manufactured materials. Biomaterials are widely used in biomedical applications as carriers for drug/protein delivery systems, medical imaging agents, and regenerative medicine [108]. Doshi's lab has developed RBC-like particles that mimic the mechanobiological and chemobiological properties of RBCs. These particles offer the engineering control required in synthetic carriers and provide a connection between synthetic materials and biological entities [109]. Lippi et al. (2010) demonstrated the use of these RBC-mimicking particles in blood doping. The particles were able to carry a large amount of oxygen to the peripheral tissue [110], which could potentially improve athletic performance.
RBC-mimicking synthetic particles have a number of potential applications in biomedical research and medicine. They can be used as carriers for drug delivery, as contrast agents for MRI, and as cell-free models for studying the biology of RBCs. However, there are still some challenges that need to be addressed before these particles can be used in clinical applications. These challenges include ensuring the biocompatibility of the particles, developing methods for mass production, and optimizing the loading capacity of the particles. Despite these challenges, RBC-mimicking synthetic particles are a promising new class of materials with the potential to revolutionize biomedical research and medicine.
Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform
Coating biodegradable polymeric nanoparticles with natural erythrocyte membranes is a method to combine the advantages of natural complexity and manufacturing efficiency. Natural erythrocyte membranes can help nanoparticles disguise and evade the immune system [111]. RBC-derived extracellular vesicles (EVs) and microparticles (MPs) are naturally produced in both normal physiological and diseased states. RBC-derived EVs are promising delivery vehicles for therapeutic agents because they are easy to source, safe, and versatile [112]. RBC-derived MPs are a fundamental biological process of cell aging, cell survival, and cell clearance [113]. Erythrocyte nanovesicles [114] are novel transporters that can modify the functions of target cells and participate in the physiological and pathological processes of modified targets [115]. The drawback of low molecular weight (LWM) chitosan nanoparticles applied in intravascular drug delivery systems is clearance by the reticuloendothelial system. A multifunctional drug carrier composed of LWM chitosan nanoparticles and erythrocytes made by Fan W. et al. (2012) shows the compatibility of the hybrid particles [116].
RBC mimicking membranes by part of the RBC components and exhibition of erythrocyte membrane-camouflaged polymeric nanoparticles.
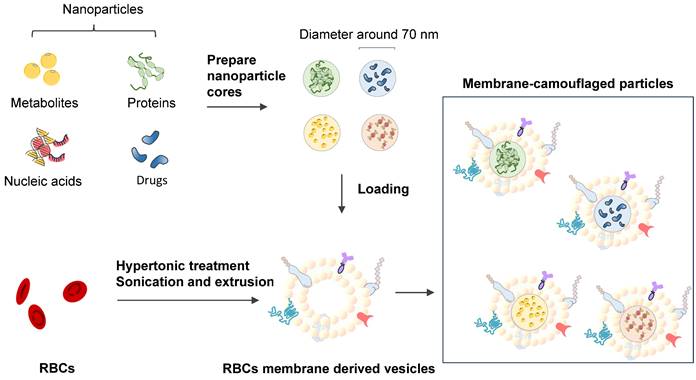
Lai P. et al. (2015) successfully constructed biomimetic stem cell membrane-camouflaged SPIO nanoparticles for theragnostic applications and analyzed the magnetic hyperthermia effect for inducing cancer cell death [118]. Nanoerythrosomes (NERs) are nanoengineered erythrocyte ghosts. One kind of drug for pulmonary arterial hypertension, Fasudil, was encapsulated in NERs by Nilesh Gupta and resulted in a 6-8-fold increase in the half-life of Fasudil [119]. Zhang et al. (2011) developed a new drug delivery platform that engrafts nanoscale RBC membrane-derived vesicles with polymeric nanoparticles made from polylactic-coglycolic acid (PLGA), an FDA-approved polymer [117] (Figure 8). Rao et al. (2015) utilized the RBC membrane as a biomimetic nanocoating material and prolonged the circulation time of Fe3O4@RBC nanoparticles due to the lack of elicitation of immune responses at either the cellular level or the humoral level [120]. This method could be exploited to reduce accelerated blood clearance by a second injection of PEG modification particles. Cao et al. (2019) used the membrane of RBCs to wrap bacteria and get the bacteria through unavoidable side effects and low treatment efficacies in therapy [121].
Cell surface coated of RBCs for blood cleaning
Small molecule toxicants can easily accumulate in blood, such as metal ions and small- and medium-molecular-weight organic substances. These toxicants can be absorbed from the in vitro environment as well as in vivo disease-damaged tissues. Removing toxicants from the blood is of great significance, as sepsis and septic toxic shock can cause more deaths than heart disease.
For decades, erythrocytes have been investigated as a carrier of specific enzymatic antidotes against chemical intoxicants. These enzymes include rhodanase for cleaning cyanide, phosphotriesterase for cleaning paraoxon, acetaldehyde dehydrogenase and alcohol oxidase for cleaning ethanol, formate dehydrogenase and δ-aminolevulinic acid dehydratase for cleaning methanol, and hydrogenase for cleaning hydrogen gas. Most of these erythrocyte-mediated enzymes have been tested in mouse models. However, this approach has limitations, as the metabolic capacity of the erythrocyte-mediated enzyme is restricted to the vascular compartment and monocyte-macrophage system [122].
A new approach to the design of next-generation nanobiomaterials is to precisely engineer their physical and chemical properties with biomimicry. This can create nanoparticles that can circulate through the blood for extended periods of time, act as detoxification devices, and improve survival in a mouse model of sepsis. For example, Elana Ben-Akiva designed biodegradable polymeric nanoparticles coated with RBC membranes for detoxification agents. These red cell membrane-coated nanoparticles enhanced the detoxification rate and decreased the death rate in a mouse model of sepsis [123]. Increased phospholipase A2 (PLA2) activity is related to many diseases in pathological conditions, such as autoimmune disorders and cancer [124]. Zhang Q. et al. (2020) used a 'lure and kill' strategy to modify RBC membranes with melittin and oleyloxyethyl phosphorylcholine (OOPC) as coverage for nanoparticles. These two key components are melittin for the "lure" and OOPC for "killing" PLA2. The lure and kill nanoparticles can eliminate the toxicity of free PLA2. The mouse model test showed that the lure and kill nanoparticles inhibited hemolysis and conferred a significant survival benefit [125]. Li C. et al. (2020) developed a Janus dendrimer amphiphile (JDA) molecule bridging to load the antidote WP6 as a supramolecular hunter and anchored the hunter on RBC membranes. In this way, the toxicant paraquat could be efficiently removed from blood, both in vitro and in vivo [126].
Overall, blood cell-related DDSs have the potential to be a promising new approach to drug delivery. However, there are a number of challenges that need to be addressed before these DDSs can be widely used [127]. More research is needed to improve the accuracy and precision of drug release, reduce the costs and time of production, and better understand the underlying mechanisms of blood cell behaviors.
RBC-inspired cancer immunotherapy
Cancer immunotherapy is a promising new approach to cancer treatment that uses the body's own immune system to fight cancer cells. RBCs play an important role in cancer immunotherapy, as they can be used to deliver drugs, genes, and other immune-stimulating agents to cancer cells. As immune engineering springs up to produce drugs with biomaterials for enhanced cancer immunotherapy [129], the delivery of these complicated drugs [130] plays a great role in treatment efficacy.
Progress of drug-loaded RBCs in immunotherapy
RBCs have several advantages for use in cancer immunotherapy. They are abundant, easy to obtain, and relatively inexpensive. Drug-loaded RBCs are also well-tolerated by the body and have a long circulation time, which allows them to deliver drugs and other agents to cancer cells over an extended period of time. Instead of labor- and fund-consuming antigen-loaded monocyte-derived DCs, antigen-loaded RBC-derived DC systems have advantages in their abundant content, biocompatibility, and circulation time. RBCs were used as artificial antigen-presenting cells [128] by Sun X. et al. (2017), and successfully stimulated T cells and the following immune response. Dr. Liu Z.'s group used DNA-assisted bottom-up self-assembly to precisely control both the lateral and vertical distributions of T cell activation ligands on the membrane of RBCs [129]. This application mimics natural antigen-presenting cells, engineers the cell membrane interface, and tunes cell-cell interactions, and it is promising for use in immunotherapy.
There are several ways to use RBCs in cancer immunotherapy. One way is to load RBCs with drugs or genes that can kill cancer cells. Another way is to engineer RBCs to express immune-stimulating molecules that can activate the body's immune system against cancer cells. A review from Anderson HL demonstrated the immunological function of RBCs, and summarized the humoral and cell-mediated immunity by binding and scavenging various inflammatory molecules to the surface of erythrocytes, involving in chemokines, nucleic acids, and pathogens [130]. Another review by Elisabeth Karsten illustrated RBCs' roles in cytokine signaling and in modulating the activity of immune cells [131]. Currently, there are several technical platforms to develop new formulations of drugs based on RBCs.
RBC-OVA system: This system uses RBCs to deliver antigens to dendritic cells. Dendritic cells are immune cells that help to activate the body's immune system against cancer cells. RBCs loaded with ovalbumin (RBC-OVA) were used as antigen carriers to target dendritic cells (DCs) [131] by Alice Banz et al. (2010), and the antigen was released by macrophage phagocytosis. Then, the OVA-specific T-cell response was activated to lyse OVA-binding cells. The antigen entrapped by RBCs can be effective for as long as 30 days. Alice Banz in another study [132] tested the utility of this delivery system in two tumor mouse models, using the E.G7-OVA and the B16F10 tumor cells. It showed that not only protein but also peptide could be efficiently entrapped in RBCs by a controlled lysis/resealing process. In both antigen models, the administration of a small quantity of antigen-loaded in RBCs combined with poly(I:C) induced an antigen-specific T-cell response and the control of tumor growth in mice, whereas the injection of the same quantity of free antigen did not. The intensity of the T-cell response was dependent on the concentrations of antigen entrapped and the treatment performed on the RBC membrane to improve antigen delivery. In summary, these results support the use of RBCs as an antigen delivery system for a powerful cancer immunotherapy approach.
Magali Cremel and colleagues (2013) investigated the effects of ovalbumin (OVA)-loaded RBCs on the humoral response in mice. They found that OVA-loaded RBCs significantly reduced the humoral response compared to OVA alone. The state of tolerance induced by RBCs was long-lasting, antigen-specific, and sufficiently robust to withstand immunization with antigen mixed with cholera toxin adjuvant [133].
Thymidine phosphorylase (TP) is an enzyme that is overexpressed in many types of cancer cells. It catalyzes the conversion of thymidine to deoxyuridine monophosphate (dUMP), which is then used to synthesize DNA. By targeting TP, it is possible to inhibit DNA synthesis and kill cancer cells. There are several ways to target TP [134]. One approach is to use TP inhibitors, which are molecules that bind to TP and prevent it from catalyzing the conversion of thymidine to dUMP. TP inhibitors are currently being developed as cancer drugs. Another approach is to use TP-deficient cells as cancer vaccines. These cells are engineered to lack TP, which makes them unable to survive in the body. When they are injected into a patient, they are recognized by the immune system as foreign cells and are killed. This triggers the production of antibodies against TP, which can then kill cancer cells that express TP.
The study of erythrocyte-encapsulated thymidine phosphorylase (EE-TP) in treating mitochondrial neuro gastrointestinal encephalomyopathy (MNGIE) involved three adult patients with MNGIE who received intravenous EE-TP at increasing doses over a period of 4 weeks. The results showed that EE-TP was well tolerated and resulted in significant reductions in the levels of thymidine and deoxyuridine, two of the harmful metabolites associated with MNGIE [135]. Preclinical studies were conducted to assess the safety of EE-TP. The studies involved mice and dogs that were administered EE-TP by intravenous bolus injection [136]. The results showed that EE-TP was well tolerated in both species. However, some infusion-related reactions were observed in dogs, including transient clinical signs, such as lethargy and decreased activity. Orphan Technologies utilizes thymidine phosphorylase to significantly improve the cure of rare homocysteine and related diseases.
WTX-212, this product is a novel erythrocyte-anti-PD-1 antibody conjugate that is being developed by Westlake Therapeutics. WTX-212 is being developed based on the REDx technology platform. REDx is a technology that allows for the precise control of the distribution of T cell activation ligands on the membrane of RBCs. With antitumor efficacy in models of acquired resistance to immune checkpoint inhibitors. The therapy of WTX-212 comprises autologous engineered RBCs administered as an infusion. WTX-212 is being developed based on the RBC drug technology platform REDx and acts by expressing serine protein kinase B-Raf-g469v mutated protein and it is now in clinical trial Phase 1.
Dex 21-P, this product uses RBC low permeability load technology to load dexamethasone sodium phosphate into patient RBCs, to cure ataxia telangiectasia and is in phase III clinical trials by EryDel (Italy) [137]. This platform extends the circulation time of drugs to 86 days.
Eryaspase, the comerial name of GRASPA (L-asparaginase) [138], this product uses enzymes to attach drugs or genes to the surface of RBCs from ERYCAPS platform, which is another Erytech company. This allows the drugs or genes to be delivered to cancer cells more effectively. The drug eryaspase is designed to treat acute myeloid leukemia, acute lymphocytic leukemia, pancreatic cancer, and non-Hodgkin's lymphoma. ERYCAPS technology can package a wide range of molecules from 1 kD to 500 kD.
CD34+ hematopoietic stem cells, Rubius used bioengineering technology on CD34+ hematopoietic stem cells from healthy O-type donors for cell therapy products, such as RTX-134 for PKU (phenylketonuria), RTX-240 for AML, and RTX-321 (RTX-aAPC) for HPV 16+ cancer. Rubius Therapeutics has developed in-stock RTX-240 through I/II phase clinical trials for r/r AML (refractory and recurrent AML) patients and for solid tumors. RTX-240 coexpresses 4-1BBL (4-1 BB ligand) and IL-15TP on RBCs, and these two ligands can induce the immune system of patients. 4-1BBL drives NK cell and T cell proliferation and activation and produces IFNγ, while as the fusion of IL-15 and IL-15 receptor α, IL-15TP can bridge innate immunity and acquired immunity. However, Rubius Therapeutics cut off the remaining research on RTX-240 and RTX-224 due to insufficient curacy.
In addition, Anokion technology takes advantage of the specific protein glycophorin A on the surface of RBCs, and is links with self-immune disease-related antigens such as OVA, these antigen-combined RBCs modulate immune tolerance to avoid unnecessary immune responses [139]. This allows the antigens to be delivered to cancer cells more effectively. Cello Therapeutics develops biomimetic cell membrane-coated nanoparticles that can be used to deliver cancer drugs, including chemotherapy agents, immunotherapy agents, or mRNA. This technology uses the membrane of RBCs to enwrap nanoparticles and increase the drug payload and possibilities of targeted delivery. This allows the nanoparticles to be delivered to cancer cells more effectively. Nanoparticle-based drug carriers have obtained great therapeutic potential [140].
CD47-inspired cancer immunotherapy
CD47 is a cell-surface molecule that is highly expressed in RBCs and in cancer cells [141, 142]. It can send a message of "do not eat me" to hide immune system surveillance and promote immune evasion by engaging signal-regulatory protein alpha (SIRPα), because CD47-SIRPa originally serves as an inhibitory receptor on macrophages [143]. Kong F. et al. (2016) [141] discussed the promising therapeutic application of anti-CD47 antibodies for eliminating tumor cells. Vonderheide R H. et al. (2015) reviewed the blockage of CD47 as an immunotherapy target not only by phagocytosis of macrophages but also by T cells and dendritic cells [144]. A report on the immune response by McCracken M N et al. (2015) showed that monotherapy focused on CD47: SIRP-α engagement can modulate antitumor T cells in vivo [145]. Weiskopf K et al. (2016) used the CD47 immunotherapy method to treat small lung cancer patients [146].
Sialic acid-inspired cancer immunotherapy
Carbohydrate vaccines for cancer immunotherapy are based on self-antigens that are expressed on cancer cells and can be recognized by the immune system. These vaccines can induce an immune response that can kill cancer cells [147]. Sialyl Tn (sTn) antigen is a sialylated disaccharide that is expressed on some cancer cells. It can be conjugated to keyhole limpet hemocyanin (KLH), which is a carrier protein that helps the immune system to recognize the antigen. Two types of sTn-KLH conjugates have been developed: N-iso-butanoyl sTn-KLH and N-phenylacetyl sTn-KLH. Both conjugates induce an immune response dependent on T cells. However, N-phenylacetyl sTn-KLH is more suitable for developing cancer immunotherapy, especially vaccines [148]. sTn is expressed on three of 10 types of normal epithelia. However, it is overexpressed in some cancer cells, such as prostate cancer cells. This makes it a good target for cancer immunotherapy. Zhang et al. (1998) studied the methods that could be used to select antigens for cancer immunotherapy. They found that sTn was a good target for prostate cancer because it was overexpressed on these cells and it induced an immune response that could kill cancer cells [149]. And Jicheng Wu et al. developed tumor-targeting molecules that can selectively remove sialoglycans from cancer cells. They found that this process, called desialylation, triggers CAR-iMac cells to become activated and more effective at killing cancer cells. This suggests that cancer cell desialylation is a promising new approach to improving the effectiveness of solid tumor cellular immunotherapy [150].
Erythrocyte-driven immune targeting (EDIT)
Erythrocyte-driven immune targeting (EDIT) is a strategy that presents nanoparticles from the surface of erythrocytes to the antigen-presenting cells (APCs) in the spleen. Researchers take advantage of the natural antigen-presenting function of RBCs in capturing and presenting pathogens in the blood. This innate immune function of RBCs can be leveraged to enhance the immune response against antigenic nanoparticles adsorbed on the erythrocyte surface. For example, EDIT has been shown to improve antibody response against the antigen, increase central memory T cell response, and decrease regulatory T cell response, which can slow down tumor progression [104]. However, senescent or apoptotic erythrocytes can also be used to achieve antigen-specific immune tolerance. This is because these cells are not as effective at presenting antigens to APCs as healthy erythrocytes. This makes it crucial to control the type of erythrocytes used in EDIT for tumor immunotherapy [99].
Clinical progress of drug-loaded RBCs
RBCs have been the subject of extensive research as drug delivery systems for the past 5 decades due to their potential to modulate the pharmacokinetic, pharmacodynamic, and biological properties of the drugs they deliver. In recent years, a small number of RBC-based drug delivery systems (DDSs) have been introduced to clinical trials [77]. The review from Siyu Wang et al. summarized RBC-NPs used for delivering chemotherapy therapeutics for cancer treatment [151]. The clinical trials summarized in Table 2 demonstrate the use of RBCs as drug delivery systems (DDSs) for both interventional and diagnostic applications.
We can see from the table that more efforts are made in the clinic to cure diseases, while only a few attempts are made for diagnosis. The success rate for new applications is very rare. However, the ultimate goal of discovering and developing drug delivery systems (DDSs) is to bring them to the clinical stage and achieve large-scale industrial production. Most studies on drug-loaded RBCs are still in the preclinical stage, but some formulations have undergone clinical research and may be used to treat patients in the near future. The development of DDSs is a long and complex process, but it has the potential to revolutionize the way we treat diseases.
RBCs are a promising platform for DDSs, but there are still many challenges that need to be addressed before they can be used in the clinic. Despite the challenges, the development of DDSs is an exciting field with the potential to improve the lives of millions of people.
The factors affecting the aging and clearance of RBCs
This process of RBC aging and clearance is essential for maintaining the health of the blood and preventing the accumulation of damaged RBCs.There are a number of factors that contribute to cell aging. As a kind of cell in the bloodstream, RBCs are constantly exposed to oxygen, which can lead to the production of free radicals. Free radicals are unstable molecules that can damage RBCs and other cells in the body. Another factor that contributes to RBC aging is glycation. Glucose can bind to proteins and lipids in RBCs, forming compounds called advanced glycation end products (AGEs). AGEs can damage RBCs and make them more susceptible to clearance by the spleen and liver.
Circulating around the blood vessels and carrying oxygen are prone to turn out oxidation for RBCs, as the reduced forms of nicotinamide-adenine dinucleotide (NAD) and glutathione (GSH) are involved in their energy sources, and excess O- affects the structure of the membrane and causes physical damage to the spleen, liver, and narrow capillaries. The membrane change of normal RBCs can lead to recognition failure affinity by autologous immunoglobulin G (IgG) and complements involved in the phagocytosis of RBCs, as well as abnormal hemoglobin, Fe2+, band 3, and phosphatidylserine (PS) levels that influence RBC structure and functions. Lysed membrane, released hemoglobin, and heme, a coordination complex consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands, can be cleared up by an immune complex, but effete RBCs (senescent RBCs and oxidatively damaged RBCs) have different phagocytosis mechanisms. Now, we summarize the related factors that lead to RBC aging and phagocytosis.
Formation and exposure of clearance-related molecules
Band 3 (anion channel) is the major erythrocyte membrane-spanning protein and the integrating membrane protein. It is also known as AE1 (anion exchanger) and is anchored to the membrane skeleton as a tetramer. The mobile fraction of band 3 forms dimers and transports bicarbonate (HCO3-) from tissues around the body to the lungs. Several articles have reviewed naturally occurring antibodies (NAbs) (also called autologous IgG) that have an affinity for band 3 [152, 153]. When conditions become harsh, such as with oxidative damage, band 3 dimers can form oligomers. NAbs can easily bind to band 3 oligomers and then induce the immune system to phagocytose the cells. Band 3 protein clustering on human erythrocytes also promotes the binding of NAbs and anti-spectrin antibodies [154].
List of clinical trials for RBC DDS (retrieved from www.clinicaltrials.gov).
| Study Type | Interventional | ||||||
|---|---|---|---|---|---|---|---|
| Product | Carried drugs | Loading method | Conditions | Study Status | Sponsor | Phases | ClinicalTrials.gov Identifier |
| Eryaspase | GRASPA(L-asparaginase) | Encapsulated-Hypotonic dialysis | Acute Lymphoblastic Leukemia | Completed | ERYtech Pharma | Phase 2 | NCT01523782 |
| Eryaspase | GRASPA(L-asparaginase) | Encapsulated-Hypotonic dialysis | Acute Lymphoblastic Leukemia, in Relapse | Completed | ERYtech Pharma | Phase 2/3 | NCT01518517 |
| Eryaspase | GRASPA(L-asparaginase) | Encapsulated-Hypotonic dialysis | Acute Myeloid Leukemia | Completed | ERYtech Pharma | Phase 2 | NCT01810705 |
| Eryaspase | GRASPA(L-asparaginase) | Encapsulated-Hypotonic dialysis | Triple Negative Breast Cancer | Terminated | ERYtech Pharma | Phase 2/3 | NCT03674242 |
| Eryaspase | GRASPA(L-asparaginase) | Encapsulated-Hypotonic dialysis | Pancreatic Adenocarcinoma | Completed | ERYtech Pharma | Phase 3 | NCT03665441 |
| Eryaspase | GRASPA(L-asparaginase) | Encapsulated-Hypotonic dialysis | Locally advanced pancreatic ductal adenocarcinoma, metastatic pancreatic ductal adenocarcinoma | Active, not recruiting | ERYtech Pharma | Phase 1 | NCT04292743 |
| Dex 21-p | Dexamethasone 21-Phosphate | Osmotic loading | Atherosclerosis, Acute-Phase Reaction | Unknown | University of Rome Tor Vergata | Phase 4 | NCT00484965 |
| Dex 21-p | Dexamethasone 21-Phosphate | Osmotic loading | Ulcerative Colitis | Unknown | Casa Sollievo della Sofferenza IRCCS | Phase 2 | NCT01171807 |
| EryDex | Dexamethasone 21-Phosphate | Osmotic loading | Crohn's Disease | Terminated | EryDel | Phase 3 | NCT01277289 |
| EryDex | Dexamethasone 21-Phosphate | Osmotic loading | Healthy | Completed | EryDel | Phase 1 | NCT01925859 |
| EryDex | Dexamethasone 21-Phosphate | Osmotic loading | Ataxia telangiectasia | Recruiting | EryDel | Phase 3 | NCT03563053 |
| IEDAT01 | Dexamethasone 21-Phosphate | Osmotic loading | Nervous System Disorder, Genetic Syndrome | Completed | Erydel | Phase 2 | NCT01255358 |
| WTX212 | engineered RBCs with PD-1 inhibitor pembrolizumab | Genetic engineering | Cancer, Solid Tumor, Hematologic Malignancy | Recruiting | Westlake Therapeutics | Phase 1 | NCT05707325 |
| Thymidine phosphorylase | Thymidine phosphorylase | Encapsulated-Freely diffuse | Mitochondrial neurogastrointestinl encephalomyopathy | Active, not recruiting | St George's, University of London | Phase 2 | NCT03866954 |
| RTX-134 | allogeneic human RBCs expressing the AvPAL | Genetic engineering | Phenylketonuria | Terminated on 11/30/22 | Rubius therapeutics | Phase 1 | NCT04110496 |
| RTX-240 | Engineered RBCs co-expressing 4-1BBL and IL-15TP | Genetic engineering | Solid Tumor, AML Adult | Terminated on 11/30/22 | Rubius Therapeutics | Phase 1/2 | NCT04372706 |
| RTX-321 | Engineered RBCs co-expressing 4-1BBL, IL-12, and HPV-16 Antigen | Genetic engineering | Cervical Cancer, Head and Neck Cancer, Anal Cancer | Terminated on 11/30/22 | Rubius Therapeutics | Phase 1 | NCT04672980 |
| RTX-224 | Engineered RBCs co-expressing both 4-1BBL and IL-12 | Genetic engineering | Non-Small Cell Lung Cancer, Cutaneous Melanoma, Head and Neck Squamous Cell Carcinoma, Urothelial Carcinoma, Triple-Negative Breast Cancer | Terminated on 11/30/22 | Rubius Therapeutics | Phase 1/2 | NCT05219578 |
| SQZ-AAC-HPV | Immunogenic epitopes of human papillomavirus strain 16+ | Microfluidic-based cell squeeze | Metastatic HPV16 + solid tumors | Recruiting | SQZ Biotechnologies | Phase 1 | NCT04892043 |
| Ery-apoB | Erythrocyte-bound apolipoprotein B | Transgenic expression | Hyperlipidemia, Atherosclerosis | Completed | Sint Franciscus Gasthuis | Not Applicable | NCT01634906 |
| MTX | Autologous Erythrocytes Derived Microparticles Packaging Methotrexate | Encapsulated-Hypotonic dialysis | Malignant Ascites | Unknown | Hui ting Xu, MD | Phase 1/2 | NCT03230708 |
| Study Type | Diagnostic | ||||||
| indocyanine green (ICG)-loaded erythrocytes | ICG | Encapsulated-Hypotonic dialysis | Retinal Disease | Withdrawn | Northwell Health | Not Applicable | NCT02445001 |
| Biotin-Labeled RBCs | Biotin | Bio-Labeled | Healthy Volunteers | Terminated | Gladwin, Mark, MD | Phase 2 | NCT03364686 |
| Biotin-Labeled RBCs | Biotin | Bio-Labeled | Anemia | Completed | Emory University | Phase 1 | NCT02757898 |
| FDG-labeled Human Erythrocytes | FDG | Bio-Labeled | Breast Cancer|Breast Cancer, Female|Breast Cancer, Male|Breast Neoplasms | Withdrawn | H. Lee Moffitt Cancer Center and Research Institute | Early Phase 1 | NCT03295695 |
Note: The specific information can be searched in https://clinicaltrials.gov by ClinicalTrials.gov Identifier
Erythrocytes help phagocytose complements, erythrocytes, and complement-opsonized particles interact through C3b/C4b (CR1) and are phagocytosed by macrophages, and erythrocytes are decomposed by phagosomes to release iron, porphyrin, globin, and HO (heme oxygenase) for recycling.
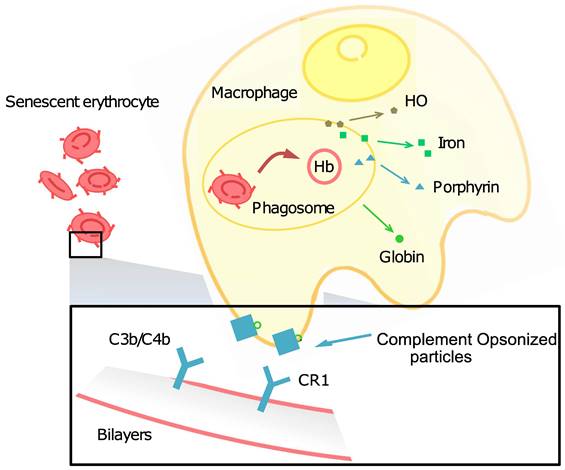
The complement system also has important effects on the clearance of RBCs. H.U. Lutz et al. (2004) reviewed detailed information on this topic in 2004 [155]. In addition to oxidative stress, band 3 clustering can also be caused by hemoglobin and exoplasmic crosslinking. Hemochrome is formed when hemoglobin is denatured. Hemochrome can then crosslink band 3 proteins into clusters. These clusters become the recognition sites for cell-senescent antibodies. Diffusion-mediated oligomerization can also lead to exoplasmic crosslinking, band 3 clustering, and bivalent binding of anti-band 3 naturally occurring antibodies (NAbs). Complement proteins can then deposit on these clusters.
RBCs circulating around the body not only participate in gas exchange but also play an immune adherence role for later phagocytosis by interacting with complement receptor 1 (CR1, CD35), also known as complement 3b/4b (C3b/C4b). RBCs use CR1 to bind circulating complement-opsonized particles [156]. RBCs use CR1 to bind circulating complement-opsonized particles, such as microbes, immune complexes, and self-generated inflammatory particle adherent complexes. They then remove and transfer these particles to resident macrophages in the liver and spleen for clearance [17] (Figure 9).
Phosphatidylserine (PS) is an important phospholipid membrane component. Normal phospholipids can move freely within the membrane, but PS is restricted to the inner leaflet by flippases. Exposure of PS on the outer leaflet of RBCs, accompanied by elevated intracellular calcium (Ca2+), results in opsonization and engulfment by the mononuclear phagocyte system, leading to RBC clearance [157].
Loss or conformational change of self-marker molecules
CD47 as a self-marker molecule
CD47 is an integrin-associated protein that is associated with human Rhesus antigens. It has a molecular weight of 47-52 kDa. The cytosolic soluble segment of CD47 on the surface of the membrane can interact with the inhibitory receptor signal regulatory protein alpha (SIRPα) on macrophages to confirm self-identity. This interaction prevents phagocytosis, so CD47 is called a self-marker protein on mammalian RBCs. When CD47 is oxidized, it can crosslink into dimers. These dimers can interact with SIRPα on macrophages, which sends a signal that triggers phagocytosis. This is why CD47-/- RBCs (RBCs that lack CD47) are cleared more rapidly than wild-type RBCs. Oldenborg et al. (2000, 2001) studied CD47-/- RBCs in wild and CD47-/- mice. They found that CD47-/- RBCs were cleared very rapidly in wild mice, and that CD47-/- aggravated anemia in CD47-/-C57BL/6 mice [143]. H.U. Lutz (2004) concluded that increased opsonization and a lower amount of CD47 enhanced phagocytosis. This means that if RBCs are coated with antibodies or other proteins (opsonization), or if they have fewer CD47 molecules, they are more likely to be phagocytosed [155].
CD47 is generally lost as RBCs circulate around the body. The inhibition of phagocytosis by CD47 molecules may have a dose-dependent effect, meaning that the amount of CD47 on the RBCs affects the effectiveness of its inhibitory signal [158]. For example, senescent RBCs show a very low content of CD47 on the membrane. CD47 is also affected by RBC aging and oxidation during circulation [159]. Additionally, the inhibition efficiency depends on the binding strength of the CD47-SIRPα interaction.
The "do not eat me" signal sent by CD47 can inhibit the actomyosin cytoskeleton of macrophages, which is a network of proteins that helps the macrophage to engulf its target. Nisha G. Sosale et al. (2015) identified that phagocytosis may be driven by the typical rigidity of microbes and many synthetic materials. This insight has already been applied to basic research on how macrophages engulf cancer cells or enhance the circulation time of nanoparticles in the body [160]. The adhesive process of macrophage engulfment is mediated by myosin-II, a protein that helps to contract the cytoskeleton. Activation of myosin II makes the macrophage phagocytic synapses rigid. If CD47 sends the "do not eat me" signal, myosin will be inhibited and the macrophage will not be able to engulf its target. However, the rigidity of phagocytosed RBCs can hyperactivate myosin, overwhelming the "self" signal of CD47 to macrophages and causing them to engulf the RBCs.
Sialic acid gets influenced by RBCs aging
Sialic acids on the RBC membrane are mostly conjugated to galactose (Gal) residues. These sialic acids act as modifiers of proteins, lipids, and glycoproteins. If sialic acids are stripped off, the Gal residues are exposed to various molecules. This can lead to the sequestration of RBCs from the circulation system by macrophages [161].
Siglecs are lectins that belong to the IgG superfamily and bind to N-acetylneuraminic acid residues that link alpha 3 or alpha 6 to galactose. Human RBCs bind to immunoreceptor tyrosine-based inhibitory motif (ITIM)-carrying siglecs in macrophages and prevent the engulfment of sialic acid-carrying cells, which makes sialic acid a self-marker molecule [155].
Aminoff et al. (1988) defined senescent RBCs as "RBCs do show the greatest number of time-dependent changes" [162], and D. Bratosin et al. (1998) depicted it more vividly: "senescent RBCs represent the erythrocyte population which has been condemned to death and which thus has one foot in the grave". When treated with neuraminidase, phagocytosed and adherent RBCs by monocytes sustained a higher percentage than young RBCs and more membrane-bound IgG [163]. Mohammad and his colleagues (2012) also found that the sialic acid content in the erythrocyte membrane decreases significantly during RBC aging in human blood, while hydroperoxides can lead to a significant increase in lipid peroxidation [21]. In most cases, desialylation of sialoglycoprotein directly leads to exposure to galactoside, which can be recognized by galactoside receptors in the liver. Loss of glycocalyx (i.e., loss of negative charges) leads to the increased sodium sensitivity of erythrocytes [20], and lack of sialic acid in RBCs is prone to affinity to complement and autologous IgG, and then activates the phagocytic process. However, Biondi et al. (2003) found that desialylation was not involved in the increased removal of erythrocytes observed in elderly individuals [164].
Other membrane proteins related to RBC clearance
Another cluster of differentiation (CD) molecules related to RBC clearance is CD44 on the erythrocyte membrane. CD44 is a phagocytic receptor [165] and a surface marker in human erythrocytes. It is involved in the inflammatory response, helping to recruit immune cells to the site of inflammation. Oxidative modification of membrane-cytoskeleton proteins can change the levels of CD44 and its characteristics under the changed physicochemical parameters of the cellular environment. This may be due to changes in the protein-protein interactions in the cell membrane. In drug-loaded RBCs, CD44 plays a key role in maintaining the stability of the cell membrane [166]. Damage to the cell membrane can affect the ability of RBCs to bind to other cells and tissues, leading to problems such as anemia, rheumatoid arthritis, and multiple sclerosis. CD44 of desialylated or aged erythrocytes interacting with hyaluronic acid on vascular endothelia can facilitate the sequestration of RBCs., as in drug-loaded RBCs, CD44 plays a key role in the stability of cell membrane [167].
Glycophorin A (GPA) is the major transmembrane sialoglycoprotein of RBCs [168] and specific to erythrocyte membranes [169]. It has been shown to contribute to the expression of the MN and Wright blood group antigens, to act as a receptor for the malaria parasite Plasmodium falciparum [170] and Sendai virus, and along with the anion transporter, band 3, may contribute to the mechanical properties of the RBC membrane. GPA protein is involved in the transport of oxygen and carbon dioxide in the blood. It is also involved in the immune response, helping to protect the body from infection by identifying and targeting foreign cells. GPA protein is also involved in the clearance of damaged or infected RBCs from the blood [171]. Without GPA protein, the immune system would not be able to function properly. The GPA-targeted ERY1 ligand enhances binding without impacting cellular function [172].
Change of membrane properties with blood rheology
Blood rheology is also considered to be related to RBC aging. The rheology of blood is influenced by two important factors in erythrocytes: aggregability and deformability. These two factors reflect the key determinants of blood viscosity at high and low shear rates. Simmonds et al. (2013) found that blood rheology and aging cooperated together [173]. Fens and Storm et al. (2010) measured the phagocytosis of opsonized erythrocytes with reduced deformability by angiogenic growth factor-activated endothelial cells and found a significant increase in whole-blood viscosity (WBV) [174]. Harisa et al. (2015) acclaimed an oxidative stress-mediated mechanism, and oxidative stress led to less WBV [23]. The reduced deformability of RBCs over time was associated with a poor outcome in septic patients. This was due to changes in the inner constitutions of band 3, outer exposed phosphatidylserine (PS), and lactadherin-bridging molecules. Li et al. (2010) studied the factors associated with increasing blood viscosity [175]. Malondialdehyde (MDA) is a lipid oxidation product that is used as a biochemical indicator to measure the degree of lipid oxidation. MDA is formed when free radicals attack polyunsaturated fatty acids in cell membranes. This can lead to the oxidation of membrane proteins and glycoproteins, with a high level of MDA and a low level of GSH indicating increased oxidative stress, which can damage cells and tissues [176].
Less deformability may occur in effete RBCs, and it may affect the deformability of surrounding RBCs' [177]. Effects RBCs are old, worn-out RBCs that have lost their ability to deform. They are removed from the circulation through a process called mechanical trapping in the spleen. Deformability is also positively affected by complement receptor 1 (CR1). A CR1-dependent increase in membrane deformability could facilitate the transfer of CR1-bound particles from RBCs to hepatic and splenic phagocytes [156]. Angiogenic endothelial cells enhance erythrophagocytosis by loss of erythrocyte deformability [174].
The clearance of RBCs is affected by their shape. Experiments have shown that rigid but more rounded RBC stomatocytes signaled "self" more than rigid RBC discocytes, highlighting the effect of shape. The physical properties of phagocytic targets can thus modulate "self" signaling, as they seem to be relevant to the splenic clearance of rigid RBCs after storage or the clearance of rigid pathological cells such as sickle and thalassemic RBCs [160]. While CD47-/- cells are not inherently responsible for abnormalities in cell shape [21], sphericity and surface loss can increase splenic entrapment in Plasmodium falciparum-infected RBCs [178].
RBCs phagocytosis mainly in liver and spleen
Sequestration of senescent RBCs is thought to take place primarily in reticuloendothelial cells (RE cells) in the spleen, liver, and bone marrow. RE cells are cells that phagocytose (engulf and destroy) foreign particles and cells.
The liver is a large immune organ whose sinusoidal cells are often referred to as Kupffer cells. Kupffer cells in the liver are major residential hepatic immune cells; approximately 25% of nonhepatocyte cells are Kupffer cells, second to endothelial cells (approximately 50% of nonhepatocyte cells in the whole liver) [179], and Kupffer cells are a distinct population of RBC-phagocytizing cells in the liver [180]. Blood in the liver will go through the portal vein. As galectins in the liver have an affinity for desialylated gal residues, gal-exposing RBCs are phagocytosed by Kupffer cells. Therefore, the liver is the primary organ to remove damaged RBCs.
The spleen serves as the largest filter of blood in the human body. The structure of the spleen not only adapts to remove older erythrocytes from the circulation but also efficiently removes blood-borne microorganisms and cellular debris [181]. The structure and function of the spleen make special contributions to blood circulation. Blood in the spleen needs to undergo two kinds of circulation: one is that RBCs traverse venous sinuses bypassing the red pulp, so-called "closed circulation". During this process, the meshwork in the red pulp ascertains the structural and mechanical quality of RBCs, retains young and normal RBCs, and removes old and abnormal RBCs; the other is called “open circulation”, during which the remaining blood goes through the reticular meshwork, then the microcirculation [182]. Safeukei and colleagues (1996, 2008) designed a method for perfusing recently removed human spleens with tagged RBCs called the "eosin-maleimide test" [183]. This test is expected to determine the key determinate that makes the spleen clear the effete RBCs, and the "eosin-5-maleimide test" (eosin-5-maleimide binding is a diagnostic test for hereditary spherocytosis) has been applied in clinical practice [184].
The clearance of drug delivery RBCs
The properties of RBCs are easily altered by drug loading [185, 186], which can affect the clearance process and lead to unprecedented changes in the pharmacokinetics, pharmacodynamics, and immunogenicity of the drugs. The clearance of drug delivery RBCs will affect the circulation time and target site of the drugs, which have a significant impact on the way that the drugs are metabolized, distributed, and eliminated by the body. Accordingly, some specific, salient parameters controlling behavior of RBC/DDS are defined in the body [83].
Despite transmitting infections or choosing the wrong blood type, for it is a complex and sterile process will lead to clearance, there are many factors that contribute to the clearance of drug delivery RBCs. These factors include the type of drug that is being delivered, the method used to load the drug onto the RBCs, the properties of the RBC membrane, the immune response to the drug-loaded RBCs.
The type of drug that is being delivered can affect the clearance of drug delivery RBCs in a number of ways. For example, drugs that are more easily metabolized by the body tend to be cleared more quickly than drugs that are not easily metabolized. This is because the body can break down and remove these drugs more easily.
The method used to load the drug onto the RBCs can also affect the clearance of drug delivery RBCs. In order to make sure the high quality of RBCs, any crude method for preparation should be avoid, otherwise, these drug-delivery RBCs will rapidly degrade in the bloodstream. For example, drug-delivery RBCs that are loaded using a physical method, such as encapsulation, tend to be cleared more slowly than drug-delivery RBCs that are loaded using a chemical method, such as conjugation. This is because physical methods of loading drugs onto RBCs do not damage the RBC membrane as much as chemical methods [187]. Damage to the RBC membrane can make the RBCs more susceptible to clearance by the immune system.
The properties of the RBC membrane can also affect the clearance of drug-delivery RBCs. Normally, RBCs with a more negatively charged membrane tend to be cleared more slowly than RBCs with a less negatively charged membrane. This is because the negatively charged membrane repels the immune cells that would otherwise clear the RBCs. The function of sialic acids is very important in this regard. Sialic acids are negatively charged molecules that are found on the surface of RBCs. They help to protect RBCs from being cleared by the immune system.
The immune response to the drug-loaded RBCs can also affect their clearance. The drug-loaded on RBCs that are rejected by the immune system will be cleared more quickly.
Understanding the factors that affect clearance can help researchers develop methods to prolong the circling time of drug delivery RBCs. Researchers are also working on developing drugs that can suppress the immune response to drug-delivery RBCs. This would help to prevent the drug delivery RBCs from being cleared by the immune system too quickly and make them more effective for delivering drugs to patients.
In addition, we have analyzed key molecules on the membrane, such as CD47 and sialic acids. These molecules are important for maintaining the compatibility of RBCs. CD47 is a "don't eat me" molecule that helps to prevent immune cells from attacking RBCs. Sialic acids are negatively charged molecules that help to repel immune cells (see Section “The membrane”). There will be more choices to mimic when the biology and structure characteristics of RBCs are fully understood.
Conclusion and perspectives
RBC engineering provides numerous ways to optimize clinical curation and diagnosis. With cancer immunotherapy being widely used, RBCs as a vector will be increasingly popular due to their compatibility and long circulation time. RBCs could help to solve many difficulties in the biotechnology and biomedical fields in the future. We reviewed different methods that have been used to load drugs onto RBCs, and the applications of RBC-based DDS. Furthermore, the challenges and future directions of RBC-based DDS are key points to explore.
The research on U-RBCs is a promising field with the potential to revolutionize blood transfusion medicine. U-RBCs could also have other benefits. For example, they could be used to treat anemia, sickle cell disease, and other blood disorders. They could also be used to deliver drugs and other therapies to the body. However, with the discovery of new blood group antigen profiles from different geographic regions [188], clinics need to further develop rational preparation of whole blood or blood components and efficient bioprocesses [189]. With continued research, it is possible that these products could be available for clinical use in the near future.
The application of RBCs in drug delivery system takes advantage of the quality that RBCs are abundant, easily accessible, and have a long circulation time in the blood. In addition, RBCs are able to target specific tissues and cells, making them ideal for the delivery of drugs to hard-to-reach areas. Despite DDS of RBCs improved the loading efficiency and traveling time of drug, as well as the dose and efficacy of drug release, DDS of RBCs still have challenges in maintaining the function and properties of RBCs, avoiding aggregation and blood clotting, decreasing fatigue, senescence, and damage. Many researchers investigated on loading and relaxation dynamics of an RBC, when mechanical load on the membrane, the deformation and rotational contributions were induced and Guglietta F et al. figured out the imposition/cessation of external mechanical loads on RBCs [190]. The viscoelastic response of RBCs during loading is involved in traits like reduced deformability, increased membrane viscosity, and change in cell shape, causing substantial changes in the overall hemodynamics [191].
Drug-loaded RBCs are most commonly administered via vascular injection, but they have also been studied for oral administration [192]. Studies on oral administration of drug-loaded RBCs are still in their early stages. Oral administration of drug-loaded RBCs could potentially change the immune response to xenogeneic cells [193, 194]. Oral administration of drug-loaded RBCs could benefit patients who cannot tolerate injections or need to take multiple medications. However, there are challenges such as protecting RBCs from the harsh environment of the stomach like varying pH (highly acidic stomach) and the presence of enzymes, and controlling the release of drugs to avoid toxicity, as well as the first-pass effect in the liver and the intestinal barrier to drug absorption [195]. In addition, another novel application of RBCs for therapy is RBC-based hydrogel. RBC hydrogel is a gel derived from RBCs and utilized in applications such as tissue regeneration and cancer photo-immunotherapy. This hydrogel capitalizes on the platelet activation and photothermal properties of RBCs. Ziying Fei et al. developed an injectable RBC-based gel for cancer therapy. Upon exposure to near-infrared (NIR) laser light, this hydrogel effectively releases tumor-associated antigens (TAAs), thereby triggering a sustained immune response that leads to the eradication of tumor [196].
We list above many factors that contribute to effete RBCs, and the determinates of senescent RBCs, while the long circulation time in vivo benefits from the structural completeness of the cell membrane and high deformability, as well as biconcave shape; and the marker of self-molecules such as CD47 and sialic acids get its passport all along the way. Some antioxidant complexes also sustain membrane completeness. It is known to us all that effete RBCs have multiple regressive characteristics, which will aggressively affect the traveling time of RBCs. While senescent cells are phagocytosed and cleared by the liver, spleen, and other reticuloendothelial systems. The targeting of senescent cells in the liver and spleen is a potential therapeutic strategy for a variety of diseases. Researchers are currently investigating ways to target senescent cells to certain organs such as the liver and spleen [104].
Mathematical models show surface area and membrane shear modulus are important parameters to evaluate the aging status [197]. Travis Nemkov et al. (2020) found an alternative way for some RBCs to overcome stress conditions, by decoding the metabolic landscape [198]. The use of these models, techniques, and research on the membrane property index will make it more convenient to evaluate the state of RBCs and drug-loaded RBCs. It will also be easier to find optimal conditions for sustaining the suitable condition for RBCs as a vector for drug delivery. With these models, techniques, and research on the content of the membrane, the evaluation on the state of RBCs and drug-loaded RBCs will be convenient, and easy to find optimal conditions for sustaining the suitable condition for RBCs as a vector for drug delivery. Future studies could use Uncertainty quantification (UQ) techniques and computational model updating to improve the predictive capacity of models and assist in more reliable treatment planning and outcome assessment [199].
Acknowledgements
This study was supported by the National Key R&D Program of China (2022YFC3401600 and 2022YFE0121600), the National Natural Science Foundation of China (22277107 and 82188102), the Natural Science Foundation of Zhejiang Province (LZ21H160002), and the Fundamental Research Funds for the Central Universities of China (226-2022-00168).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939-48
2. Harris JW. The red cell production, metabolism, destruction: normal and abnormal. Acad Med. 1963;38:617
3. Bessis M, Delpech G. Discovery of the red blood cell with notes on priorities and credits of discoveries, past, present and future. Blood Cells. 1981;7:447-80
4. Barty RL, Pai M, Liu Y, Arnold DM, Cook RJ, Zeller MP. et al. Group O RBCs: where is universal donor blood being used. Vox Sang. 2017;112:336-42
5. Rahfeld P, Withers SG. Toward universal donor blood: enzymatic conversion of A and B to O type. J Biol Chem. 2020;295:325-34
6. Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC. et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173-95
7. Sosale NG, Rouhiparkouhi T, Bradshaw AM, Dimova R, Lipowsky R, Discher DE. Cell rigidity and shape override CD47's "self"-signaling in phagocytosis by hyperactivating myosin-II. Blood. 2015;125:542-52
8. Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720-31
9. Marchesi VT, Furthmayr H, Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667-98
10. Bretscher MS. Membrane structure: some general principles. Science. 1973;181:622-9
11. Clarke M. Isolation and characterization of a water-soluble protein from bovine erythrocyte membranes. Biochem Biophys Res Commun. 1971;45:1063-70
12. Whitelegge JP. Integral membrane proteins and bilayer proteomics. Anal Chem. 2013;85:2558-68
13. Aminoff D, Bruegge W, Bell WC, Sarpolis K, Williams R. Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc Natl Acad Sci U S A. 1977;74:1521-4
14. Inoue S, Lin SL, Chang T, Wu SH, Yao CW, Chu TY. et al. Identification of free deaminated sialic acid (2-keto-3-deoxy-D-glycero-D-galacto-nononic acid) in human red blood cells and its elevated expression in fetal cord red blood cells and ovarian cancer cells. J Biol Chem. 1998;273:27199-204
15. Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357-92
16. Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255-66
17. Bratosin D, Mazurier J, Tissier JP, Slomianny C, Estaquier J, Russo-Marie F. et al. Molecular mechanisms of erythrophagocytosis. Characterization of the senescent erythrocytes that are phagocytized by macrophages. C R Acad Sci III. 1997;320:811-8
18. Byrne B, Donohoe GG, O'Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov Today. 2007;12:319-26
19. Ogasawara Y, Namai T, Yoshino F, Lee M-C-i, Ishii K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007;581:2473-7
20. Kliche K, Gerth U, Pavenstädt H, Oberleithner H. Recharging red blood cell surface by hemodialysis. Cell Physiol Biochem. 2015;35:1107-15
21. Mehdi MM, Singh P, Rizvi SI. Erythrocyte sialic acid content during aging in humans: correlation with markers of oxidative stress. Dis Markers. 2012;32:179-86
22. Izabella Z A Pawluczyk MGN, Samita Patel, Priyanka Desai, Dipti Vashi, Moin A Saleem, Peter S Topham. Sialic acid attenuates puromycin aminonucleoside-induced desialylation and oxidative stress in human podocytes. Exp Cell Res. 2014;320:258-68
23. Harisa GI. Blood viscosity as a sensitive indicator for paclitaxel induced oxidative stress in human whole blood. Saudi Pharm J. 2015;23:48-54
24. Zhang E, Phan P, Algarni HA, Zhao Z. Red blood cell inspired strategies for drug delivery: emerging concepts and new advances. Pharm Res. 2022;39:2673-98
25. Lublin DM. Universal RBCs. Transfusion. 2000;40:1285-9
26. Lögdberg L, Reid ME, Lamont RE, Zelinski T. Human blood group genes 2004: chromosomal locations and cloning strategies. Transfus Med Rev. 2005;19:45-57
27. Lögdberg L, Reid ME, Zelinski T. Human blood group genes 2010: chromosomal locations and cloning strategies revisited. Transfus Med Rev. 2011;25:36-46
28. Green C. The ABO, Lewis and related blood group antigens; a review of structure and biosynthesis. FEMS Microbiol Immunol. 1989;1:321-30
29. Schachter H, Michaels MA, Tilley CA, Crookston MC, Crookston JH. Qualitative differences in the N-acetyl-D-galactosaminyltransferases produced by human A1 and A2 genes. Proc Natl Acad Sci U S A. 1973;70:220-4
30. Clausen H, Levery SB, Nudelman E, Tsuchiya S, Hakomori S. Repetitive A epitope (type 3 chain A) defined by blood group A1-specific monoclonal antibody TH-1: chemical basis of qualitative A1 and A2 distinction. Proc Natl Acad Sci U S A. 1985;82:1199-203
31. Goldstein J, Siviglia G, Hurst R, Lenny L, Reich L. Group B erythrocytes enzymatically converted to group O survive normally in A, B, and O individuals. Science. 1982;215:168-70
32. Zarnitz ML, Kabat EA. Immunochemical studies on blood groups. 1 XXV. The action of coffee bean α-Galactosidase on blood group B and BP1 substances. J Am Chem Soc. 1960;82:3953-7
33. Yatziv S, Flowers HM. Action of -galactosidase on glycoprotein from human B-erythrocytes. Biochem Biophys Res Commun. 1971;45:514-8
34. Levy GN, Aminoff D. Purification and properties of alpha-N-acetylgalactosaminidase from Clostridium perfringens. J Biol Chem. 1980;255:11737-42
35. Olsson ML. Stripped red cells-efforts to eliminate A and B antigens from the blood supply:SI14. Transfus Med. 2006 16
36. Rahfeld P, Sim L, Moon H, Constantinescu I, Morgan-Lang C, Hallam SJ. et al. An enzymatic pathway in the human gut microbiome that converts A to universal O type blood. Nat Microbiol. 2019;4:1475-85
37. Liu QP, Sulzenbacher G, Yuan H, Bennett EP, Pietz G, Saunders K. et al. Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol. 2007;25:454-64
38. Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95:375-87
39. Salomao M, Zhang X, Yang Y, Lee S, Hartwig JH, Chasis JA. et al. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. PNAS. 2008;105:8026-31
40. Garratty G. Progress in modulating the RBC membrane to produce transfusable universal/stealth donor RBCs. Transfus Med Rev. 2004;18:245-56
41. Yan J, Yu J, Wang C, Gu Z. Red blood cells for drug delivery. Small Methods. 2017;1:1700270
42. Mansouri S, Fatisson J, Miao Z, Merhi Y, Winnik FM, Tabrizian M. Silencing red blood cell recognition toward Anti-A antibody by means of polyelectrolyte layer-by-layer assembly in a two-dimensional model system. Langmuir. 2009;25:14071-8
43. Mansouri S, Merhi Y, Winnik FM, Tabrizian M. Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules. 2011;12:585-92
44. Garratty G. Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sang. 2008;94:87-95
45. Hashemi-Najafabadi S, Vasheghani-Farahani E, Shojaosadati SA, Rasaee MJ, Armstrong JK, Moin M. et al. A method to optimize PEG-coating of red blood cells. Bioconjug Chem. 2006;17:1288-93
46. Falchi Taddei. PEG-dichlorotriazine (PEG-DCT): A new soluble polymer-supported scavenger for alcohols, thiols, phosphines, and phosphine oxides. Org Lett. 2000;2:3429-31
47. Moore MS, Okelberry E, Cordingley K, Drake A, Robinett Z. DePEGylation studies: PEG-RBC stability in conditions consistent with massive transfusion. Clin Lab Sci. 2011;24:227-32
48. Nacharaju P, Manjula BN, Acharya SA. Thiolation mediated pegylation platform to generate functional universal red blood cells. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:107-18
49. Nacharaju P, Boctor FN, Manjula BN, Acharya SA. Surface decoration of red blood cells with maleimidophenyl-polyethylene glycol facilitated by thiolation with iminothiolane: an approach to mask A, B, and D antigens to generate universal red blood cells. Transfusion. 2005;45:374-83
50. Park J, Brust TF, Lee HJ, Lee SC, Watts VJ, Yeo Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano. 2014;8:3347-56
51. Wang B, Wang G, Zhao B, Chen J, Zhang X, Tang R. Antigenically shielded universal red blood cells by polydopamine-based cell surface engineering. Chem Sci. 2014;5:3463-8
52. Liu W, Liu T, Zou M, Yu W, Li C, He Z. et al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater. 2018;30:e1802006
53. Flegel WA, Wagner FF. RHD epitope density profiles of RHD variant red cells analyzed by flow cytometry. Transfus Clin Biol. 1996;3:429-31
54. Zhao Y, Fan M, Chen Y, Liu Z, Shao C, Jin B. et al. Surface-anchored framework for generating RhD-epitope stealth red blood cells. Sci Adv. 2020;6:eaaw9679
55. Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: what is the future in transfusion? Transfus Med Rev. 2007 21
56. Malik P, Fisher TC, Barsky LL, Zeng L, Izadi P, Hiti AL. et al. An in vitro model of human red blood cell production from hematopoietic progenitor cells. Blood. 1998;91:2664-71
57. Neildez-Nguyen TMA, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana M-C. et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467-72
58. Fujimi A, Matsunaga T, Kobune M, Kawano Y, Nagaya T, Tanaka I. et al. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008;87:339-50
59. Lu S-J, Feng Q, Park JS, Vida L, Lee B-S, Strausbauch M. et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475-84
60. Feng Q, Shabrani N, Thon JN, Huo H, Thiel A, Machlus KR. et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014;3:817-31
61. Hansen M, von Lindern M, van den Akker E, Varga E. Human-induced pluripotent stem cell-derived blood products: state of the art and future directions. FEBS Lett. 2019;593:3288-303
62. Anstee DJ, Gampel A, Toye AM. Ex-vivo generation of human red cells for transfusion. Curr Opin Hematol. 2012;19:163-9
63. Hirose S-I, Takayama N, Nakamura S, Nagasawa K, Ochi K, Hirata S. et al. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Rep. 2013;1:499-508
64. Glen KE, Workman VL, Ahmed F, Ratcliffe E, Stacey AJ, Thomas RJ. Production of erythrocytes from directly isolated or Delta1 Notch ligand expanded CD34+ hematopoietic progenitor cells: process characterization, monitoring and implications for manufacture. Cytotherapy. 2013;15:1106-17
65. Daniels DE, Downes DJ, Ferrer-Vicens I, Ferguson DCJ, Singleton BK, Wilson MC. et al. Comparing the two leading erythroid lines BEL-A and HUDEP-2. Haematologica. 2020;105:e389-e94
66. Kurita R, Funato K, Abe T, Watanabe Y, Shiba M, Tadokoro K. et al. Establishment and characterization of immortalized erythroid progenitor cell lines derived from a common cell source. Exp Hematol. 2019;69:11-6
67. Poldee S, Metheetrairut C, Nugoolsuksiri S, Frayne J, Trakarnsanga K. Optimization of an erythroid culture system to reduce the cost of production of red blood cells. MethodsX. 2018;5:1626-32
68. T. KB. Blood substitutes: where do we stand today? J Intern Med. 2003;253(5):495-7
69. Chang TMS, Powanda D, Yu WP. Analysis of polyethylene-glycol-polylactide nano-dimension artificial red blood cells in maintaining systemic hemoglobin levels and prevention of methemoglobin formation. Artif Cells Blood Substit Immobil Biotechnol. 2003;31:231-47
70. Zhang L, Wang D, Yang K, Sheng D, Tan B, Wang Z. et al. Mitochondria-targeted artificial "Nano-RBCs" for amplified synergistic cancer phototherapy by a single NIR irradiation. Adv Sci (Weinh). 2018;5:1800049
71. Gao M, Liang C, Song X, Chen Q, Jin Q, Wang C. et al. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor Hypoxia and Enhance Cancer Radiotherapy. Adv Mater. 2017 29
72. Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118:145-60
73. Rossi L, Serafini S, Pierigé F, Antonelli A, Cerasi A, Fraternale A. et al. Erythrocyte-based drug delivery. Expert Opin Drug Deliv. 2005;2:311-22
74. Li Y, Raza F, Liu Y, Wei Y, Rong R, Zheng M. et al. Clinical progress and advanced research of red blood cells based drug delivery system. Biomaterials. 2021;279:121202
75. Nguyen PHD, Jayasinghe MK, Le AH, Peng B, Le MTN. Advances in drug delivery systems based on red blood cells and their membrane-derived nanoparticles. ACS Nano. 2023;17:5187-210
76. Sun Y, Su J, Liu G, Chen J, Zhang X, Zhang R. et al. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci. 2017;96:115-28
77. Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016 106
78. Chen S, Zhong Y, Fan W, Xiang J, Wang G, Zhou Q. et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat Biomed Eng. 2021;5:1019-37
79. Carlos H Villa DBC, Don L Siegel, Vladimir Muzykantov. Erythrocytes as carriers for drug delivery in blood transfusion and beyond. Transfus Med Rev. 2017;31:26-35
80. Chang AH, Stephan MT, Lisowski L, Sadelain M. Erythroid-specific human factor IX delivery from in vivo selected hematopoietic stem cells following nonmyeloablative conditioning in hemophilia B mice. Mol Ther. 2008;16:1745-52
81. Phanthong P, Borwornpinyo S, Kitiyanant N, Jearawiriyapaisarn N, Nuntakarn L, Saetan J. et al. Enhancement of β-globin gene expression in thalassemic IVS2-654 induced pluripotent stem cell-derived erythroid cells by modified U7 snRNA. Stem Cells Transl Med. 2017;6:1059-69
82. Hambÿe AS, Verbeke KA, Vandermeiren RP, Joosens EJ, Verbruggen AM, De Roo MJ. Comparison of modified technetium-99m albumin and technetium-99m red blood cells for equilibrium ventriculography. J Nucl Med. 1997;38:1521-8
83. Glassman PM, Villa CH, Ukidve A, Zhao Z, Smith P, Mitragotri S. et al. Vascular drug delivery using carrier red blood cells: focus on RBC surface loading and pharmacokinetics. Pharmaceutics. 2020 12
84. Teisseire B, Ropars C, Villeréal MC, Nicolau C. Long-term physiological effects of enhanced O2 release by inositol hexaphosphate-loaded erythrocytes. PNAS. 1987;84:6894-8
85. Al-Achi A, Greenwood R. Erythrocytes as oral delivery systems for human insulin. Drug Dev Ind Pharm. 1998;24:67-72
86. Grimaldi S, Lisi A, Pozzi D, Santoro N. Attempts to use liposomes and RBC ghosts as vectors in drug and antisense therapy of virus infection. Res Virol. 1997;148:177-80
87. Byun HM, Suh D, Yoon H, Kim JM, Choi HG, Kim WK. et al. Erythrocyte ghost-mediated gene delivery for prolonged and blood-targeted expression. Gene Ther. 2004;11:492-6
88. Piergiovanni M, Casagrande G, Taverna F, Corridori I, Frigerio M, Bianchi E. et al. Shear-induced encapsulation into red blood cells: a new microfluidic approach to drug delivery. Ann Biomed Eng. 2020;48:236-46
89. Flynn G, McHale L, McHale AP. Methotrexate-loaded, photosensitized erythrocytes: a photo-activatable carrier/delivery system for use in cancer therapy. Cancer Lett. 1994;82:225-9
90. Kim S-H, Kim E-J, Hou J-H, Kim J-M, Choi H-G, Shim C-K. et al. Opsonized erythrocyte ghosts for liver-targeted delivery of antisense oligodeoxynucleotides. Biomaterials. 2009;30:959-67
91. Yew NS, Dufour E, Przybylska M, Putelat J, Crawley C, Foster M. et al. Erythrocytes encapsulated with phenylalanine hydroxylase exhibit improved pharmacokinetics and lowered plasma phenylalanine levels in normal mice. Mol Genet Metab. 2013;109:339-44
92. Harisa GI, Ibrahim MF, Alanazi F, Shazly GA. Engineering erythrocytes as a novel carrier for the targeted delivery of the anticancer drug paclitaxel. Saudi Pharm J. 2014;22:223-30
93. GA O. The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components. J Biol Chem. 1981;256(2):761-6
94. Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am J Pathol. 1987;128:276-85
95. Hill A, Ridley SH, Esser D, Oldroyd RG, Cullen MJ, Kareclas P. et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood. 2006;107:2131-7
96. Muzykantov VR, Zaltsman AB, Smirnov MD, Samokhin GP, Morgan BP. Target-sensitive immunoerythrocytes: interaction of biotinylated red blood cells with immobilized avidin induces their lysis by complement. Biochim Biophys Acta. 1996;1279:137-43
97. Pishesha N, Bilate AM, Wibowo MC, Huang N-J, Li Z, Deshycka R. et al. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. PNAS. 2017;114:3157-62
98. Brenner JS, Mitragotri S, Muzykantov VR. Red blood cell hitchhiking: a novel approach for vascular delivery of nanocarriers. Annu Rev Biomed Eng. 2021;23:225-48
99. Wang F, Zong R, Chen G. Erythrocyte-enabled immunomodulation for vaccine delivery. J Control Release. 2022;341:314-28
100. Zhao Z, Ukidve A, Gao Y, Kim J, Mitragotri S. Erythrocyte leveraged chemotherapy (ELeCt): nanoparticle assembly on erythrocyte surface to combat lung metastasis. Sci Adv. 2019;5:eaax9250
101. Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun. 2018;9:2684
102. Ding Y, Lv B, Zheng J, Lu C, Liu J, Lei Y. et al. RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release. 2022;341:702-15
103. Li J, Ding Y, Cheng Q, Gao C, Wei J, Wang Z. et al. Supramolecular erythrocytes-hitchhiking drug delivery system for specific therapy of acute pneumonia. J Control Release. 2022;350:777-86
104. Ukidve A, Zhao Z, Fehnel A, Krishnan V, Pan DC, Gao Y. et al. Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function. PNAS. 2020;117:17727-36
105. Antonelli A, Sfara C, Rahmer J, Gleich B, Borgert J, Magnani M. Red blood cells as carriers in magnetic particle imaging. Biomed Tech (Berl). 2013;58:517-25
106. Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem. 2004;14:2161-75
107. Antonelli A, Sfara C, Manuali E, Bruce IJ, Magnani M. Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents. Nanomedicine (Lond). 2011;6:211-23
108. Stupp SI. Biomaterials for regenerative medicine. MRS Bull. 2005;30:546-53
109. Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S. Red blood cell-mimicking synthetic biomaterial particles. PNAS. 2009;106:21495-9
110. Lippi G, Franchini M, Banfi G. Red blood cell-mimicking synthetic biomaterial particles: the new frontier of blood doping? Int J Sports Med. 2010;31:75-6
111. Fang RH, Hu C-MJ, Zhang L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin Biol Ther. 2012;12:385-9
112. Yang L, Huang S, Zhang Z, Liu Z, Zhang L. Roles and applications of red blood cell-derived extracellular vesicles in health and diseases. Int J Mol Sci. 2022 23
113. Westerman M, Porter JB. Red blood cell-derived microparticles:an overview. Blood Cells Mol Dis. 2016;59:134-9
114. Harisa GI, Badran MM, Alanazi FK. Erythrocyte nanovesicles: Biogenesis, biological roles and therapeutic approach: Erythrocyte nanovesicles. Saudi Pharm J. 2017 25
115. Han X, Shen S, Fan Q, Chen G, Archibong E, Dotti G. et al. Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci Adv. 2019;5:eaaw6870
116. Fan W, Yan W, Xu Z, Ni H. Erythrocytes load of low molecular weight chitosan nanoparticles as a potential vascular drug delivery system. Colloids Surf B Biointerfaces. 2012;95:258-65
117. Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. PNAS. 2011;108:10980-5
118. Lai P, Huang R, Lin S, Lin Y, Chang C. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5:98222-30
119. Gupta N, Patel B, Ahsan F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: preparation and characterization. Pharm Res. 2014;31:1553-65
120. Rao L, Bu L-L, Xu J-H, Cai B, Yu G-T, Yu X. et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11:6225-36
121. Cao Z, Cheng S, Wang X, Pang Y, Liu J. Camouflaging bacteria by wrapping with cell membranes. Nat Commun. 2019;10:3452
122. Bax BE. Erythrocytes as carriers of therapeutic enzymes. Pharmaceutics. 2020 12
123. Ben-Akiva E, Meyer RA, Yu H, Smith JT, Pardoll DM, Green JJ. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci Adv. 2020;6:eaay9035
124. Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233-55
125. Zhang Q, Fang RH, Gao W, Zhang L. A biomimetic nanoparticle to “lure and kill” phospholipase A2. Angew Chem Int Ed Engl. 2020;59:10461-5
126. Li C, Xie Z, Chen Q, Zhang Y, Chu Y, Guo Q. et al. Supramolecular hunter stationed on red blood cells for detoxification based on specific molecular recognition. ACS Nano. 2020;14:4950-62
127. Wang S, Han K, Ma S, Qi X, Guo L, Li X. Blood cells as supercarrier systems for advanced drug delivery. Med Drug Discov. 2022;13:100119
128. Sun X, Han X, Xu L, Gao M, Xu J, Yang R. et al. Surface-engineering of red blood cells as artificial antigen presenting cells promising for cancer immunotherapy. Small. 2017 13
129. Sun L, Shen F, Xu J, Han X, Fan C, Liu Z. DNA-edited ligand positioning on red blood cells to enable optimized T cell activation for adoptive immunotherapy. Angew Chem Int Ed Engl. 2020;59:14842-53
130. Anderson HL, Brodsky IE, Mangalmurti NS. The evolving erythrocyte: red blood cells as modulators of innate immunity. J Immunol. 2018;201:1343-51
131. Karsten E, Herbert BR. The emerging role of red blood cells in cytokine signalling and modulating immune cells. Blood Rev. 2020;41:100644
132. Banz A, Cremel M, Mouvant A, Guerin N, Horand F, Godfrin Y. Tumor growth control using red blood cells as the antigen delivery system and poly(I:C). J Immunother. 2012;35:409-17
133. Cremel M, Guérin N, Horand F, Banz A, Godfrin Y. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int J Pharm. 2013;443:39-49
134. Bax BE, Levene M, Bain MD, Fairbanks LD, Filosto M, Kalkan Uçar S. et al. Erythrocyte encapsulated thymidine phosphorylase for the treatment of patients with mitochondrial neurogastrointestinal encephalomyopathy: study protocol for a multi-centre, multiple dose, open label trial. J Clin Med. 2019 8
135. Levene M, Bain MD, Moran NF, Nirmalananthan N, Poulton J, Scarpelli M. et al. Safety and efficacy of erythrocyte encapsulated thymidine phosphorylase in mitochondrial neurogastrointestinal encephalomyopathy. J Clin Med. 2019 8
136. Levene M, Coleman DG, Kilpatrick HC, Fairbanks LD, Gangadharan B, Gasson C. et al. Preclinical toxicity evaluation of erythrocyte-encapsulated thymidine phosphorylase in BALB/c mice and beagle dogs: an enzyme-replacement therapy for mitochondrial neurogastrointestinal encephalomyopathy. Toxicol Sci. 2013;131:311-24
137. Tan TK, Gopal Subramaniam A, Hau R. Effects of combined dexamethasone and tranexamic acid in lower limb total arthroplasty: a systematic review and meta-analysis of randomized clinical trials. Eur J Orthop Surg Traumatol. 2023
138. Hammel P, Fabienne P, Mineur L, Metges JP, Andre T, De La Fouchardiere C. et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: an open-label, randomized Phase Iib trial. Eur J Cancer. 2020;124:91-101
139. Kontos S, Kourtis IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. PNAS. 2013;110:E60-E8
140. Luk BT, Fang RH, Hu C-MJ, Copp JA, Thamphiwatana S, Dehaini D. et al. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6:1004-11
141. Kong F, Gao F, Li H, Liu H, Zhang Y, Zheng R. et al. CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol. 2016;18:1051-5
142. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051-4
143. Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855-62
144. H VR. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21(10):1122-3
145. McCracken MN, Cha AC, Weissman IL. Molecular pathways: Activating T cells after cancer cell phagocytosis from blockade of CD47 "Don't eat me" signals. Clin Cancer Res. 2015;21:3597-601
146. Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL. et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126:2610-20
147. Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418-28
148. Wu J, Guo Z. Improving the antigenicity of sTn antigen by modification of its sialic acid residue for development of glycoconjugate cancer vaccines. Bioconjug Chem. 2006;17:1537-44
149. Zhang S, Zhang HS, Reuter VE, Slovin SF, Scher HI, Livingston PO. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295-302
150. Wu J, Wang X, Huang Y, Zhang Y, Su S, Shou H. et al. Targeted glycan degradation potentiates cellular immunotherapy for solid tumors. PNAS. 2023;120:e2300366120
151. Wang S, Wang Y, Jin K, Zhang B, Peng S, Nayak AK. et al. Recent advances in erythrocyte membrane-camouflaged nanoparticles for the delivery of anti-cancer therapeutics. Expert Opin Drug Deliv. 2022;19:965-84
152. Lutz HU. Homeostatic roles of naturally occurring antibodies: an overview. J Autoimmun. 2007;29:287-94
153. Hornig R, Lutz HU. Band 3 protein clustering on human erythrocytes promotes binding of naturally occurring anti-band 3 and anti-spectrin antibodies. Exp Gerontol. 2000;35:1025-44
154. Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, Muthana S. et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011;71:3352-63
155. Lutz H. Innate immune and non-immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy-le-grand). 2004;50:107-16
156. Glodek AM, Mirchev R, Golan DE, Khoory JA, Burns JM, Shevkoplyas SS. et al. Ligation of complement receptor 1 increases erythrocyte membrane deformability. Blood. 2010;116:6063-71
157. Boas F E FL, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. PNAS. 1998;95(6):3077-81
158. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J. et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94
159. Khandelwal S, van Rooijen N, Saxena RK. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion. 2007;47:1725-32
160. Sosale N, Bradshaw A, Agudo J, Dimova R, Lipowsky R, Discher DE. Physics vs biology of phagocytosis: cell rigidity and shape override CD47 'self'signaling in phagocytosis by hyperactivating myosin-II. Biophys J. 2015;108:180a
161. Galili U, Korkesh A, Kahane I, Rachmilewitz EA. Demonstration of a natural antigalactosyl IgG antibody on thalassemic red blood cells. Blood. 1983;61:1258-64
162. Aminoff D. The role of sialoglycoconjugates in the aging and sequestration of red cells from circulation. Blood Cells. 1988;14:229-57
163. Ensinck A, Biondi CS, Marini A, García Borrás S, Racca LL, Cotorruelo CM. et al. Effect of membrane-bound IgG and desialysation in the interaction of monocytes with senescent erythrocytes. Clin Exp Med. 2006;6:138-42
164. Biondi C, Cotorruelo C, Garcia Borrás S, Rocca L, Ensinck A, Marini A. et al. Study of phagocytosis of senescent erythrocytes in young and elderly individuals. Clin Exp Med. 2003;2:197-8
165. Vachon E, Martin R, Plumb J, Kwok V, Vandivier RW, Glogauer M. et al. CD44 is a phagocytic receptor. Blood. 2006;107:4149-58
166. Zemlianskykh NG. Effect of substances with cryoprotective properties on surface marker CD44 in human erythrocytes. Tsitol Genet. 2016;50:66-79
167. Kerfoot SM, McRae K, Lam F, McAvoy EF, Clark S, Brain M. et al. A novel mechanism of erythrocyte capture from circulation in humans. Exp Hematol. 2008;36:111-8
168. de Isla NG, Riquelme BD, Rasia RJ, Valverde JR, Stoltz JF. Quantification of glycophorin A and glycophorin B on normal human RBCs by flow cytometry. Transfusion. 2003;43:1145-52
169. Yunoki K, Naruko T, Komatsu R, Ehara S, Shirai N, Sugioka K. et al. Enhanced expression of haemoglobin scavenger receptor in accumulated macrophages of culprit lesions in acute coronary syndromes. Eur Heart J. 2009;30:1844-52
170. Blackall DP, Armstrong JK, Meiselman HJ, Fisher TC. Polyethylene glycol-coated red blood cells fail to bind glycophorin A-specific antibodies and are impervious to invasion by the plasmodium falciparum malaria parasite. Blood. 2001;97:551-6
171. Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:387
172. Sahoo K, Koralege RS, Flynn N, Koteeswaran S, Clark P, Hartson S. et al. Nanoparticle attachment to erythrocyte via the glycophorin A targeted ERY1 ligand enhances binding without impacting cellular function. Pharm Res. 2016;33:1191-203
173. Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. J Geriatr Cardiol. 2013;10:291-301
174. Marcel H A M Fens GS, Ralf C M Pelgrim, Anton Ultee, Annette T Byrne, Carlo A Gaillard, Wouter W van Solinge, Raymond M Schiffelers. Erythrophagocytosis by angiogenic endothelial cells is enhanced by loss of erythrocyte deformability. Exp Hematol. 2010;38:282-91
175. Li G, He H, Yan H, Zhao Q, Yin D. Does carbonyl stress cause increased blood viscosity during storage? Clin Hemorheol Microcirc. 2010;44:145-54
176. Richards RS, Nwose EU. Blood viscosity at different stages of diabetes pathogenesis. Br J Biomed Sci. 2010;67:67-70
177. Nuchsongsin F, Chotivanich K, Charunwatthana P, Omodeo-Salè F, Fausta O-S, Taramelli D. et al. Effects of malaria heme products on red blood cell deformability. Am J Trop Med Hyg. 2007;77:617-22
178. Safeukui I, Buffet PA, Perrot S, Sauvanet A, Aussilhou B, Dokmak S. et al. Surface area loss and increased sphericity account for the splenic entrapment of subpopulations of Plasmodium falciparum ring-infected erythrocytes. PLoS One. 2013;8:e60150
179. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54-S62
180. Gao B, Jeong W-I, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729-36
181. Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606-16
182. Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. Retention of erythrocytes in the spleen: A double-edged process in human malaria. Curr Opin Hematol. 2009;16:157-64
183. Blackman SM, Cobb CE, Beth AH, Piston DW. The orientation of eosin-5-maleimide on human erythrocyte band 3 measured by fluorescence polarization microscopy. Biophys J. 1996;71:194-208
184. Aljurf M, Ma L, Angelucci E, Lucarelli G, Snyder LM, Kiefer CR. et al. Abnormal assembly of membrane proteins in erythroid progenitors of patients with beta-thalassemia major. Blood. 1996;87:2049-56
185. Alvarez FJ, Herráez A, Murciano JC, Jordán JA, Díez JC, Tejedor MC. In vivo survival and organ uptake of loaded carrier rat erythrocytes. J Biochem. 1996;120:286-91
186. Kravtzoff R, Ropars C, Laguerre M, Muh JP, Chassaigne M. Erythrocytes as carriers for L-asparaginase. Methodological and mouse in-vivo studies. J Pharm Pharmacol. 1990;42:473-6
187. Tzounakas VL, Karadimas DG, Papassideri IS, Seghatchian J, Antonelou MH. Erythrocyte-based drug delivery in transfusion medicine: wandering questions seeking answers. Transfus Apher Sci. 2017;56:626-34
188. Nagervadze M, Tsintsadze I, Akhvlediani L, Koiava T, Tskvitinidze S, Khukhunaishvili R. et al. ABO system combination with Rh, Kell and MN group in Georgian blood donors. Am J Blood Res. 2021;11:132-9
189. Sivalingam J, Su EY, Lim ZR, Lam ATL, Lee AP, Lim HL. et al. A scalable suspension platform for generating high-density cultures of universal red blood cells from human induced pluripotent stem cells. Stem Cell Rep. 2021;16:182-97
190. Guglietta F, Behr M, Falcucci G, Sbragaglia M. Loading and relaxation dynamics of a red blood cell. Soft Matter. 2021;17:5978-90
191. Hareendranath S, Sathian SP. Dynamic response of red blood cells in health and disease. Soft Matter. 2023;19:1219-30
192. Lorant T, Wilton J, Olausson M, Tufveson G, Johnsson C. Oral administration of xenogeneic erythrocytes induces production of antibodies that are capable of inducing hyperacute rejection of concordant vascularized xenografts. Transplantation. 2004;77:1100-3
193. Weiner HL, Friedman A, Miller A, Khoury SJ, al-Sabbagh A, Santos L. et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809-37
194. Niimi M, Shirasugi N, Hamano K, Esato K, Matsumoto K, Ikeda Y. et al. Oral delivery of xeno-antigen combined with non-depleting anti-CD4 monoclonal antibody induces significantly prolonged survival of concordant skin xenograft. Xenotransplantation. 2001;8:75-9
195. Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017 9
196. Fei Z, Fan Q, Dai H, Zhou X, Xu J, Ma Q. et al. Physiologically triggered injectable red blood cell-based gel for tumor photoablation and enhanced cancer immunotherapy. Biomaterials. 2021;271:120724
197. Wei Q, Wang X, Zhang C, Dao M, Gong X. Evolution of surface area and membrane shear modulus of matured human red blood cells during mechanical fatigue. Sci Rep. 2023;13:8563
198. Nemkov T, Qadri SM, Sheffield WP, D'Alessandro A. Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells. Blood Transfus. 2020;18:130-42
199. Economides A, Arampatzis G, Alexeev D, Litvinov S, Amoudruz L, Kulakova L. et al. Hierarchical bayesian uncertainty quantification for a model of the red blood cell. Phys Rev Appl. 2021;15:034062
Author contact
![]() Corresponding authors: Ben Wang: Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, 310009. Tel: 86-0571-86971790, Mail: benwangedu.cn; Yan Luo: Department of Biochemistry, Zhejiang University School of Medicine, Hangzhou, China, 310058; Mail: luoyan2011edu.cn.
Corresponding authors: Ben Wang: Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, 310009. Tel: 86-0571-86971790, Mail: benwangedu.cn; Yan Luo: Department of Biochemistry, Zhejiang University School of Medicine, Hangzhou, China, 310058; Mail: luoyan2011edu.cn.
 Global reach, higher impact
Global reach, higher impact