13.3
Impact Factor
Theranostics 2024; 14(1):363-378. doi:10.7150/thno.89433 This issue Cite
Research Paper
The plasticity of neuropeptide Y-Y1 receptor system on Tac2 neurons contributes to mechanical hyperknesis during chronic itch
1. Shanghai Key Laboratory of Anesthesiology and Brain Functional Modulation, Clinical Research Center for Anesthesiology and Perioperative Medicine, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, No.1481, Xinshi North Road, Shanghai 200434, China.
2. Department of Anesthesiology and Perioperative Medicine, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, No. 1279, Sanmen Road, Shanghai 200434, China.
Abstract
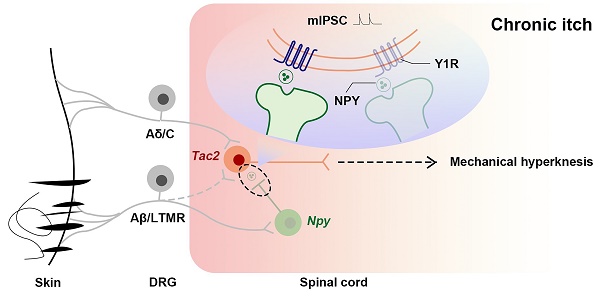
Rationale: In the physiological states, the act of scratching protects the person from harmful substances, while in certain pathological conditions, the patient suffers from chronic itch, both physically and mentally. Chronic itch sufferers are more sensitive to mechanical stimuli, and mechanical hyperknesis relief is essential for chronic itch treatment. While neuropeptide Y-Y1 receptor (NPY-Y1R) system is known to play a crucial role in modulating mechanical itch in physiological conditions, it is elusive how they are altered during chronic itch. We hypothesize that the negative regulatory effect of Y1Rs on Tac2 neurons, the key neurons that transmit mechanical itch, declines during chronic itch.
Methods: We combined transgenic mice, chemogenetic manipulation, immunofluorescence, rabies virus circuit tracing, and electrophysiology to investigate the plasticity of Y1Rs on Tac2 neurons during chronic itch.
Results: We found that Tac2 neurons receive direct input from Npy neurons and that inhibition of Npy neurons induces activation of Tac2 neurons. Moreover, the expression of Y1Rs on Tac2 neurons is reduced, and the regulatory effect is also reduced during chronic itch.
Conclusion: Our study clarifies the plasticity of Y1Rs on Tac2 neurons during chronic itch and further elucidates the mechanism by which NPY-Y1R system is responsible for modulating mechanical itch. We highlight Y1Rs as a promising therapeutic target for mechanical hyperknesis during chronic itch.
Keywords: itch, spinal cord, neuropeptide, NPY, Y1 receptor
 Global reach, higher impact
Global reach, higher impact