13.3
Impact Factor
Theranostics 2024; 14(1):379-391. doi:10.7150/thno.89495 This issue Cite
Review
Endosialin in Cancer: Expression Patterns, Mechanistic Insights, and Therapeutic Approaches
1. Xi'an Key Laboratory of Stem Cell and Regenerative Medicine, Institute of Medical Research, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China.
2. Department of Urology, Xijing Hospital, Fourth Military Medical University, Xi'an, Shaanxi 710032, China.
† These authors contributed equally to this work.
Received 2023-8-25; Accepted 2023-10-26; Published 2024-1-1
Abstract
Endosialin, also known as tumor endothelial marker 1 (TEM1) or CD248, is a single transmembrane glycoprotein with a C-type lectin-like domain. Endosialin is mainly expressed in the stroma, especially in cancer-associated fibroblasts and pericytes, in most solid tumors. Endosialin is also expressed in tumor cells of most sarcomas. Endosialin can promote tumor progression through different mechanisms, such as promoting tumor cell proliferation, adhesion and migration, stimulating tumor angiogenesis, and inducing an immunosuppressive tumor microenvironment. Thus, it is considered an ideal target for cancer treatment. Several endosialin-targeted antibodies and therapeutic strategies have been developed and have shown preliminary antitumor effects. Here, we reviewed the endosialin expression pattern in different cancer types, discussed the mechanisms by which endosialin promotes tumor progression, and summarized current therapeutic strategies targeting endosialin.
Keywords: endosialin, TEM1, CD248, tumor progression, angiogenesis, tumor microenvironment, targeted therapy
1. Introduction
Although the incidence of some cancer types has decreased in recent years, cancer is still a serious threat to human health. Most tumors are difficult to discover in the early stage, while in the late stage, tumors may progress rapidly, and metastasis may occur [1, 2]. Current strategies for cancer treatment cannot meet clinical requirements, and the antitumor effect of some treatment strategies is not satisfactory. Therefore, it is important to elucidate the mechanisms of tumor development and progression and identify novel tumor-specific markers for early diagnosis and treatment.
The tumor microenvironment (TME) is composed of various cellular and acellular components, including immune cells, stroma cells, blood vessels, extracellular matrix (ECM) proteins and many secretory molecules [3]. Among them, cancer-associated fibroblasts (CAFs) have been found to promote tumor progression through direct mechanisms (by cell-to-cell communication) or indirect mechanisms (through secretion of soluble factors or extracellular vesicles) [4-8]. Thus, CAFs are considered suitable therapeutic targets for cancer treatment. However, until now, no ideal markers have been identified for CAF-specific targeting. Furthermore, CAFs can be divided into different subtypes, and some subtypes may even have antitumor functions [9, 10]. Therefore, it is important to identify biomarkers that are specifically expressed in CAFs and elucidate whether they are important for tumor progression, which will contribute to the development of novel promising therapeutic strategies.
Endosialin, also known as tumor endothelial marker 1 (TEM1) or CD248, is a type I transmembrane glycoprotein that belongs to the C-type lectin domain (CTLD) group 14 family of transmembrane glycoproteins [11]. Endosialin was first identified as a tumor stromal antigen by using FB5 antibody, which was generated through immunization with human fibroblasts fused with myeloma cells. It was named endosialin and was found to be selectively expressed in tumor endothelial cells but not in normal blood vessels or other adult tissues [12]. The human endosialin gene was cloned in 2001 and was found to have the same sequence as TEM1. Endosialin is composed of a signal leader peptide, five globular extracellular domains (including a C-type lectin domain, one domain with similarity to the Sushi/ccp/scr pattern, and three EGF repeats), followed by a mucin-like region, a transmembrane segment, and a short cytoplasmic tail [13]. In 2001, when St Croix et al. tried to identify the differentially expressed genes in tumor endothelial cells, they compared the gene expression patterns in endothelial cells derived from blood vessels of normal and malignant colorectal tissues and found that endosialin was one of the genes that was specifically expressed in tumor endothelial cells but barely expressed in normal tissues [14].
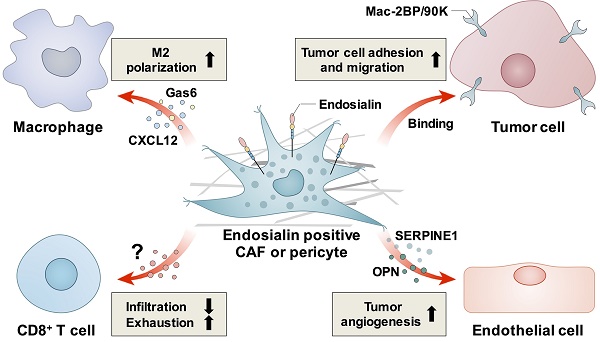
Until now, several molecules have been identified to bind with endosialin; these include MMRN2, Mac-2BP/90K, galectin-3, CD68, and some ECM proteins, such as fibronectin (FN), collagen types I and IV (Col I and Col IV) [11, 15]. Studies have revealed that endosialin can promote tumor progression through multiple mechanisms, such as promoting tumor cell proliferation, adhesion and migration, stimulating tumor angiogenesis and inducing an immunosuppressive TME. Because of its tumor-promoting function, endosialin is considered an ideal target for cancer treatment.
In this review, we summarized the expression pattern of endosialin in different cancer types, elucidated its tumor-promoting mechanism, and discussed the research progress regarding endosialin-targeted therapeutic strategies.
2. Endosialin expression in different cancer types
2.1 Endosialin is highly expressed in CAFs and pericytes in epithelial cell-derived tumors and correlated with patient prognosis
Although endosialin was initially found to be selectively expressed in endothelial cells of various tumors, subsequent studies clarified that endosialin was mainly expressed in the tumor stroma, especially CAFs and pericytes. For example, Rouleau et al. examined endosialin expression in 250 clinical specimens of human cancer, including 20 cancer subtypes, and found that endosialin was mainly expressed in stromal cells and perivascular cells in carcinomas [16]. By using four newly generated antibodies, MacFadyen et al. demonstrated that endosialin was predominantly expressed in fibroblasts and a subset of tumor vessel-associated pericytes but not in the tumor endothelium [17]. Christian et al. also found that endosialin was expressed in CAFs and tumor vessel-associated mural cells but not endothelial cells [18].
Other studies have also examined endosialin expression in different cancer types. In melanoma, Kiyohara et al. found that 70% (46/66) of stage III or stage IV melanoma specimens and 86% (117/136) of stage IV specimens had endosialin expression, mainly in pericytes and stromal fibroblasts, while no expression was detected in 29 normal tissue controls [19]. Huber et al. reported that in cutaneous melanoma metastases and squamous cell carcinomas, endosialin was predominantly expressed either in tumor blood vessels or both tumor blood vessels and stromal fibroblasts [20].
In brain tumor, several studies reported inconsistent results. Carson-Walter et al. found that endosialin expression was upregulated in primary and metastatic human brain tumors, and it was primarily localized to the tumor vasculature and a subset of tumor stromal cells [21]. Brady et al. found that endosialin was mainly expressed in highly invasive glioblastoma multiforme, anaplastic astrocytomas and metastatic carcinomas; and endosialin was localized to the endothelium of small and large vessels, Thy-1-positive fibroblast-like cells and α-smooth muscle actin (α-SMA)-positive cells [22]. However, Simonavicius et al. demonstrated that endosialin expression was upregulated in high-grade gliomas and was mainly expressed in tumor-associated pericytes but not endothelial cells [23]. While in another study, Rouleau, et al. examined endosialin expression in neuroblastoma, small cell lung cancer and melanoma, and they observed vascular endosialin staining in all three kinds of tumors. Interestingly, they found that tumor cells also expressed endosialin, and the expression was highest in neuroblastoma, weak in melanoma and rare in small cell lung cancer [24].
In gastric cancer, Fujii et al. examined endosialin expression in a tissue microarray that contained 945 tumor tissues and found that endosialin was specifically expressed in CAFs, and its expression was significantly correlated with recurrence-free survival, overall survival, cancer-related overall survival, scirrhous subtype, tumor depth, nodal status, distant metastasis, serosal invasion, lymphatic or venous vessel infiltration and pTMN stage [25]. In colorectal cancer (CRC), O'Shannessy et al. found that stromal expression of endosialin had prognostic value, and signature combining endosialin expression score with other compartment-specific expression scores (endosialin stroma, endosialin tumor vessel, HIF2α stromal vessel, Col IV tumor, and FN stroma) had even better prognostic value, specifically in stage II CRC patients [26].
In breast cancer, Davies et al. found that patients with recurrent disease or those who died of breast cancer had a significantly elevated expression of endosialin, and the elevated endosialin level was associated with nodal involvement and disease progression [27]. In ovarian cancer, when Kuk et al. tried to identify potential biomarkers from soluble ascites, they found that endosialin was among the 52 new proteins that deserve further clinical validation [28]. In RCC, Xu et al. found that endosialin-positive CAFs were correlated with poor prognosis and an immunosuppressive TME [29].
In addition to its specific expression on CAFs and pericytes, endosialin was also found to be expressed in the serum of cancer patients. For example, Pietrzyk et al. found that serum level of endosialin was significantly higher in CRC patients than in healthy controls, and high endosialin level was associated with CRC progression and a poor prognosis [30]. However, in another study, O'Shannessy et al. examined the serum level of endosialin using novel antibodies they generated, yet they found that the serum level of endosialin was not different between CRC patients and healthy individuals [31]. Thus, whether it could be used as a serum biomarker for cancer needs to be further evaluated.
2.2 Endosialin is also highly expressed in tumor cells of mesenchymal cell-derived sarcomas
In addition to its expression in the tumor stroma of epithelial cell-derived cancer types, endosialin was also found to be highly expressed in the tumor cells of mesenchymal cell-derived sarcomas. In Rouleau's study, they found that in sarcoma tissue, not only tumor stromal cells and perivascular cells, but also tumor cells had endosialin expression [16]. Guo et al. analyzed endosialin expression in 19 human sarcoma subtypes (203 specimens) and found that endosialin was expressed in 96% of human sarcomas, among which 81% had endosialin expression in both tumor cells and tumor vasculature [32]. O'Shannessy et al. examined endosialin expression in a cohort of 94 sarcoma patients and found that endosialin was highly expressed and that its expression was positively correlated with the expression level of platelet-derived growth factor receptor-β (PDGFR-β) [33].
Thway et al. examined endosialin expression in 514 human soft tissue sarcomas and found that endosialin was expressed in 89% of undifferentiated pleomorphic sarcomas (104/117), 77% of fibrosarcomas (20/26), 62% of synovial sarcomas (37/60), 51% of leiomyosarcoma (94/185) and 31% of rhabdomyosarcoma (39/126). These findings indicate that endosialin could be used to distinguish undifferentiated and poorly differentiated sarcomas [34]. De Gooyer et al. found that strong endosialin expression was common (88.2%) in myxofibrosarcoma and that endosialin could be used as a suitable target for tumor-targeted imaging [35].
Some studies have also found that endosialin expression is closely correlated with tumor malignancy. For example, Kondo et al. found that in 10 cases of nonmetastatic osteosarcoma (OS), only one had endosialin expression, while 7 of the 8 metastatic OSs had endosialin expression, indicating that high endosialin expression was correlated with metastasis [36]. Rouleau et al. also conducted a retrospective analysis of clinical specimens and found that all high-grade and metastatic sarcomas had higher endosialin expression than low-grade sarcomas [37].
The side population (SP) is considered to have stem cell-like properties, and Rouleau et al. found that endosialin was expressed in the SP of sarcoma cell lines [38]. Sun et al. also found that primary human OS samples contained approximately 3.9% SP cells, which are responsible for therapy failure and tumor recurrence. These endosialin-positive SP cells were able to regenerate the tumor population and had high invasive potential, indicating that endosialin-positive SP cells might be a potential target to prevent OS recurrence after chemotherapy [39].
Therefore, in epithelial cell-derived cancers, endosialin was mainly expressed in stromal cells, especially CAFs and pericytes, while in mesenchymal cell-derived sarcomas, endosialin was also expressed in tumor cells. Because of its specific high expression in different cancer types, endosialin is considered to be an effective therapeutic target for cancer treatment [40].
3. Mechanisms of how endosialin promotes tumor progression
3.1 Promoting tumor cell proliferation, adhesion and migration
The tumor-promoting function of endosialin was first demonstrated in endosialin knockout (KO) mice. It was shown that there was no difference in the growth of subcutaneously inoculated tumors in endosialin KO mice; however, tumor growth, invasiveness and metastasis were significantly inhibited in endosialin KO mice when tumor cells were transplanted in abdominal sites, indicating that endosialin has an anatomical site-specific tumor-promoting function or that the local microenvironment might be involved in endosialin-mediated tumor progression [41]. In endosialin transgenic mice, which express endosialin lacking its cytoplasmic domain, the growth of T241 fibrosarcoma and Lewis lung carcinoma was significantly reduced compared with wild-type (WT) mice. In addition, compared with WT fibroblasts, conditioned medium from fibroblasts from the same transgenic mice showed impaired supportive function for tumor cell survival, indicating that the cytoplasmic domain of endosialin is critical for its tumor-promoting function, possibly through other intracellular interacting proteins and/or downstream signaling pathways [42].
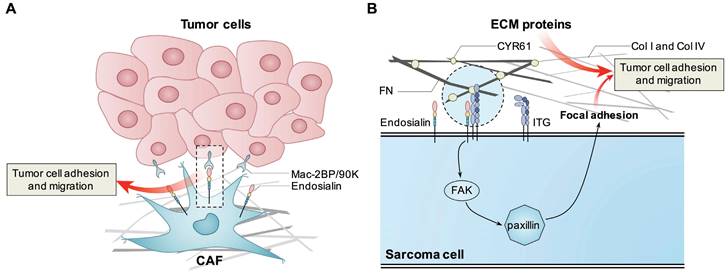
Since endosialin is highly expressed in the tumor stroma, it is speculated that endosialin may promote cell adhesion and migration of epithelial cell-derived tumors through cell-cell or cell-ECM interactions. One study showed that stromal fibroblast-expressed endosialin could bind with Mac-2BP/90K, which is highly expressed in tumor cells; thus, endosialin might promote tumor cell adhesion and migration through interaction with Mac-2BP/90K [43]. Another study showed that endosialin-expressing pericytes could promote tumor cell intravasation in a cell contact-dependent manner, thus facilitating distant metastasis. They also showed that in breast cancer patients, upregulated endosialin levels were significantly correlated with increased metastasis and poor prognosis [44]. For ECM proteins, endosialin was found to bind with FN, Col I and Col IV, and the interaction could be blocked by the anti-endosialin antibody MORAb-004; overexpression of endosialin in CHO cells enhanced cell adhesion to FN and promoted cell migration in Matrigel, indicating that endosialin may promote tumor progression and invasion [45].
In colon cancer, Park et al. found that endosialin could regulate cell migration and drug resistance; overexpression of endosialin could promote cell migration, while downregulation of endosialin resulted in increased cell apoptosis in chemotherapy-resistant cells [46]. In breast cancer, Huang et al. constructed a bone metastasis-specific regulatory network based on prognostic stemness-related signatures (PSRSs), their upstream transcription factors (TFs) and downstream pathways and found that MAF may positively regulate endosialin expression and that endosialin may influence breast cancer bone metastasis via the apical junction pathway [47].
Endosialin expressed in mesenchymal cell-derived sarcoma cells has also been shown to promote cell proliferation and migration. It was found that overexpression of endosialin in endosialin-negative osteosarcoma MG63 cells could significantly promote cell proliferation and migration [48]. Lu et al. found that knockdown of endosialin significantly inhibited OS cell migration, invasion and lung metastasis but had no obvious effect on cell proliferation in vitro or tumor growth in vivo. Mechanistic study showed that CD248 could promote the interaction between ITGB1 and ECM proteins and activate the FAK-paxillin pathway to promote the formation of focal adhesion and metastasis of OS cells [49]. Since the humanized endosialin antibody MORAb-004 could effectively inhibit the migration of sarcoma cells but had no obvious effect on cell proliferation, endosialin was considered to be involved in the metastatic process of OS [36].
Thus, endosialin can promote tumor progression through different mechanisms. In epithelial cell-derived tumors, endosialin may promote tumor cell proliferation, adhesion and migration through cell-cell or cell-ECM interactions, while in mesenchymal cell-derived sarcomas, endosialin may promote tumor cell migration and invasion through intracellular pathways such as the FAK-paxillin pathway (summarized in Figure 1). However, the detailed molecular mechanisms have not been fully elucidated, and more studies are still needed.
3.2 Stimulating tumor angiogenesis
In addition to the regulation of tumor cells, endosialin was also found to be involved in tumor angiogenesis [50]. For example, Bagley et al. found that endosialin was expressed in tumor vasculature pericytes and that an endosialin antibody could inhibit pericyte tube formation and migration in vitro, indicating that endosialin was involved in active angiogenesis during tumor development [51]. In addition, they also found that endosialin was also highly expressed in endothelial precursor cells (EPCs) than in mature endothelial cells, and anti-endosialin antibodies inhibited EPC migration and tube formation in vitro and decreased the number of circulating murine EPCs in tumor-bearing mice. These findings indicated that endosialin is involved in the earlier stages of tumor angiogenesis [52].
The proangiogenic function of endosialin was also demonstrated in KO and knock-in mice. Nanda et al. found that in endosialin KO mice, wound healing was normal, indicating that endosialin is not required for neovascularization during wound repair.
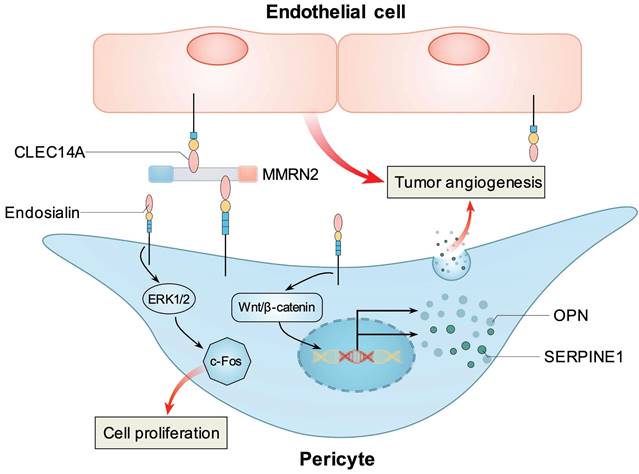
However, tumor vessels failed to efficiently mature, leading to decreased numbers of medium and large vessels and a compensatory increase in small vessels, indicating that endosialin is needed for efficient maturation of vessels within tumors [41]. By using an orthotopic lung cancer model, Hong et al. also found that in endosialin KO mice, tumor volume, the density of vessels and pericytes, and the functionality of tumor vessels were all decreased. Mechanistically, they found that endosialin could activate Wnt/β-catenin signaling and upregulate two angiogenic factors, OPN and SERPINE1, in pericytes, resulting in enhanced angiogenesis and lung cancer growth [53]. In human endosialin knock-in mice, Rybinski et al. found that MORAb-004 could induce the internalization of endosialin into pericytes and impair tumor microvasculature maturation, thus inhibiting tumor growth and metastasis [54].
Known mechanisms how endosialin promotes tumor cell proliferation, adhesion and migration. Endosialin expressed in CAFs binds with Mac-2BP/90K expressed in tumor cells to promote tumor cell adhesion and migration. Tumor cell expressed endosialin could bind with ECM proteins like FN, Col I and Col IV to enhance cell adhesion and migration, or promote the interaction between ITGB1 and ECM proteins, activate the FAK-paxillin pathway, and promote the formation of focal adhesion and metastasis.
In renal cell carcinoma (RCC), endosialin was found to be specifically expressed in blood vessels, and its expression level was found to be correlated with microvascular density [55]. In urothelial carcinoma of the bladder (UCB), endosialin was also found to be specifically expressed in blood vessels, and its expression was closely associated with a poor prognosis in UCB patients [56]. By using a mouse model composed of tumor cells and endosialin-expressing endothelial cells, Li et al. demonstrated that the endosialin antibody MORAb-004 could inhibit tumor angiogenesis, indicating that endosialin was involved in tumor vasculature [57].
In pancreatic cancer, endosialin was demonstrated to bind with MMRN2, which is a unique endothelial-specific ECM protein that has been implicated in angiogenesis and tumor progression. Endosialin and CLEC14A, which is another C-type lectin domain-containing group 14 family member, could simultaneously bind with MMRN2 at the interface between the endothelium and pericytes in human pancreatic cancer and were speculated to promote tumor angiogenesis [58]. In addition, Tomkowicz et al. found that endosialin played a role in PDGF-induced proliferation of vascular pericytes. When endosialin was knocked down, PDGF-BB-induced proliferation, ERK1/2 phosphorylation, and c-Fos expression were significantly suppressed. Thus, targeting endosialin and the PDGF/ERK1/2/c-Fos pathway may provide novel strategies to inhibit tumor angiogenesis [59]. Interestingly, Hong et al. reported that during wound healing, endosialin expression was highly upregulated, and endosialin could bind with PDGFR to enhance the mitogenic and chemoattractive effects of PDGF-BB and collagen deposition in myofibroblasts, thus promoting wound healing [60]. In another study, Brett et al. treated mice with dermal wounds with the sorted endosialin positive stromal vascular fraction (SVF) from human lipoaspirate, and found that wounds healed significantly faster than the endosialin negative or unsorted SVF groups. These data also indicates that endosialin has pro-angiogenic function [61].
These findings indicate that endosialin is involved in tumor angiogenesis, and known mechanisms how endosialin stimulates tumor angiogenesis were summarized in Figure 2. Because of its role in tumor angiogenesis, endosialin is also considered to be an effective target for antiangiogenic therapy in different cancer types [62, 63].
Known mechanisms how endosialin stimulates tumor angiogenesis. Pericyte expressed endosialin and endothelial cell expressed CLEC14A simultaneously bind with MMRN2 at the interface between the endothelium and pericytes to promote tumor angiogenesis. Endosialin could activate ERK1/2/c-Fos pathway to promote pericytes proliferation. Endosialin could activate Wnt/β-catenin signaling and upregulate two angiogenic factors, OPN and SERPINE1, in pericytes to promote tumor angiogenesis.
3.3 Inducing an immunosuppressive tumor microenvironment
Cancer progression depends on the surrounding TME. Endosialin is expressed in CAFs and pericytes, which are important components of the TME and contribute to the formation of an immunosuppressive TME, thus promoting tumor progression.
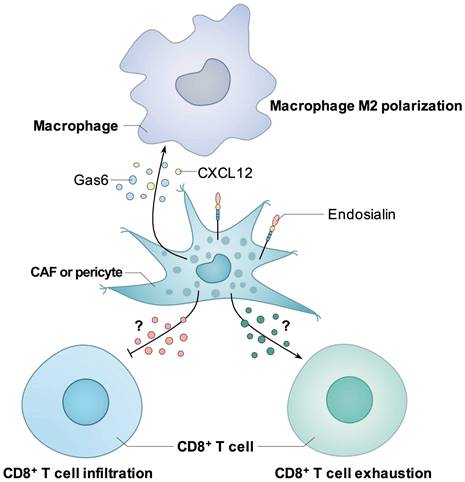
In hepatocellular carcinoma (HCC), Yang et al. found that endosialin-positive CAFs could recruit macrophages through interaction with CD68 and promote M2 polarization by inducing CAFs to secrete Gas6, thus promoting HCC progression [64]. In non-small cell lung cancer (NSCLC), Wu et al. reported that endosialin-positive CAFs could secrete CXCL12 to mediate the M2 polarization of macrophages both in vitro and in vivo, thus promoting NSCLC progression [65].
In RCC, Zhang et al. found that high endosialin expression was closely associated with patients' poor prognosis and immunosuppressive TME, such as increased infiltration of regulatory T cells (Treg) and upregulated immune checkpoint molecules such as PD-1, CTLA-4 and LAG-3 [66]. Further study confirmed that the number of endosialin-positive CAFs was closely correlated with patients' poor prognosis and immunosuppressive TME, as indicated by increased exhausted T cells and M2 macrophages [29]. Lu et al. found that high endosialin expression was associated with low cytotoxic T lymphocyte (CTL) infiltration in RCC tissues in both clinical patients and endosialin KO mice, and antibody blockade of endosialin promoted CTL infiltration and inhibited RCC growth in vivo. By using co-culture assay, they showed that knockdown of endosialin in pericytes or antibody blockade could promote T cell migration, thus they concluded that endosialin positive pericytes could promote tumor progression through inhibiting CTL infiltration. In addition, they also found that combined treatment with endosialin antibody and PD-1 antibody could enhance the antitumor efficacy of PD-1 antibody in an RCC xenograft model [67].
In glioma, Ochs et al. found that FACS-sorted human malignant glioma-derived pericytes (HMGPs) specifically express endosialin. These pericytes accumulated in human gliomas, and the levels of HMGPs and CD8+ T cells were negatively correlated. In addition, HMGPs could suppress T-cell responses in vitro, indicating that they could promote local immunosuppression in glioma [68].
Known mechanisms how endosialin induces immunosuppressive tumor microenvironment. Endosialin-positive CAFs could secrete Gas6 or CXCL12 to mediate the M2 polarization of macrophages. Endosialin-positive CAFs or pericytes could inhibit the infiltration or induce the exhaustion of CD8+ T cell through unknown mechanisms.
Thus, endosialin can induce an immunosuppressive TME, probably through the regulation of the infiltration and exhaustion of CD8+ T cells or the recruitment and polarization of M2 macrophages (summarized in Figure 3). However, further studies are still needed to elucidate the detailed mechanisms.
4. Endosialin-targeted antitumor therapeutic strategies
Because of its tumor-promoting function, endosialin is considered an effective antitumor target. To date, a variety of endosialin-specific antibodies have been developed, and different endosialin-targeted strategies have been designed (Table 1).
4.1 Endosialin-specific antibodies
MORAb-004 (also called ontuxizumab), the humanized version of the mouse anti-human endosialin antibody Fb5, was the first antibody that was applied for cancer therapy in clinical trials. In 2014, the first phase I trial was conducted in 36 patients with treatment-refractory solid tumors. Patients were treated at 10 dose levels (ranging from 0.0625 to 16 mg/kg) once a week in 4-week cycles, and it was found that MORAb-004 was safe when the dose was up to 12 mg/kg, and preliminary antitumor activity was observed [69]. Norris et al. conducted a phase I trial in children with relapsed or refractory solid tumors and found that MORAB-004 was well tolerated when administered weekly at 12 mg/kg [70]. Doi et al. performed another phase I trial in Japanese patients with solid tumors who had failed standard chemotherapy, and they observed long-term disease stabilization in gastric cancer (GC) and extraskeletal chondrosarcoma, and tumor shrinkage in gastrointestinal stromal tumor (GIST) and HCC. The maximum tolerated dose (MTD) was not reached, and they recommended 8 mg/kg weekly or 12 mg/kg biweekly for further application [71].
In 2018, D'Angelo et al. performed a phase II trial in 76 patients with metastatic melanoma. Patients were given 2 or 4 mg/kg MORAb-004 weekly, and MORAb-004 was found to be well tolerated at both doses. The 24-week progression-free survival (PFS) value was 11.4% among all treated patients, and the overall response rate (ORR) was 3.1% at the 4 mg/kg dose, with clinical benefit achieved in 42.4% of response evaluable patients [72]. Another phase II trial was performed in 126 chemorefractory metastatic colorectal cancer patients. Patients were given intravenous MORAb-004 (8 mg/kg) weekly or placebo plus best supportive care. Although MORAb-004 was found to be well tolerated, there were no significant differences between MORAb-004 monotherapy and placebo in terms of PFS, overall survival or ORR [73]. In another phase I and randomized controlled phase II trial, Robin et al. examined the safety and efficacy of the combined treatment of MORAb-004 with gemcitabine and docetaxel (G/D) in 209 patients with metastatic soft-tissue sarcomas. They found that although the combination of MORAb-004 plus G/D was generally well tolerated, no significant difference was observed in either PFS or median overall survival between the MORAb-004 plus G/D group and the placebo plus G/D group [74].
It seemed that MORAb-004 alone was not sufficient to elicit an effective antitumor effect. The inefficiency of MORAb-004 might be caused by the fact that MORAb-004 does not have antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) effects, which are important for the antitumor effects of many antibodies [54]. However, since it can internalize into endosialin-positive cells, it is possible to elicit antitumor effects if using this antibody to deliver cytotoxic drugs via different strategies, such as antibody‒drug conjugates (ADCs). For example, the CD30 antibody showed limited activity as a single agent, but after conjugation with monomethyl auristatin E (MMAE) (named Brentuximab Vedotin), the ADC showed potent antitumor effects and has been approved by the FDA for clinical application [75, 76]. Another option for improving its antitumor effect is to combine it with other therapeutic treatments. For example, the combination of rituximab with CHOP chemotherapy showed an increased complete response rate and prolonged event-free and overall survival, without a significant increase in toxicity compared to the CHOP alone group [77]. Since specific expression of endosialin in the tumor stroma of many cancers and also tumor cells in sarcomas have been well demonstrated, we believe that other endosialin-specific antibodies or antibody-based therapeutic strategies deserve further investigation.
Currently, in addition to MORAb-004, several other antibodies targeting endosialin have also been developed. For example, Zhao et al. obtained an endosialin-specific single chain antibody fragment (scFv78) by screening novel paired display-secretory yeast libraries. The binding affinity, specificity, and in vivo distribution of scFv78 were validated by flow cytometry, ELISA and immunofluorescence staining, which indicates that it could be used for endosialin-targeted imaging and therapy [78]. Fierle et al. screened two new endosialin scFvs (1C1m and 7G22) from a phage display antibody library, and their group examined their application in different therapeutic strategies (discussed in the following section) [79].
Endosialin specific antibodies and antibody based endosialin-targeted anti-tumor therapeutic strategies.
| Antibody name | Antibody type | Antibody source | Current stage | Therapeutic strategies | Patients or xenograft models | Refs |
|---|---|---|---|---|---|---|
| MORAb-004 (Ontuxizumab) | humanized antibody (IgG1) | Mouse immunized with human endosialin protein | Phase I | Antibody alone (dose ranging from 0.0625 to 16 mg/kg) | Treatment-refractory solid tumors | 69 |
| Antibody alone (12 mg/kg) | Relapsed or refractory solid tumors (children) | 70 | ||||
| Antibody alone (8 mg/kg weekly or 12 mg/kg biweekly) | Solid tumors (Japanese patients) | 71 | ||||
| Phase II | Antibody alone (2 or 4 mg/kg, weekly) | Metastatic melanoma | 72 | |||
| Antibody (8 mg/kg) plus best supportive care | Chemo-refractory metastatic colorectal cancer | 73 | ||||
| Antibody (8 mg/kg) combined with gemcitabine and docetaxel (G/D) | Metastatic soft-tissue sarcomas | 74 | ||||
| 1C1m-Fc | Fully human scFv + Fc | Radioimmunotherapy (radiolabeled with 125I or 177Lu) | Ewing's sarcoma A-673 or neuroblastoma SK-N-AS xenograft model | 80-82 | ||
| hMP-E-8.3 | Humanized antibody | Mouse immunized with extracellular endosialin peptides | Preclinical | ADC (conjugated with duocarmycin derivative) | Osteosarcoma SJSA-1 xenograft model | 83 |
| Not named anti-endosialin | Fully human antibody | Humanized transgenic mouse | Preclinical | ADC (conjugated with MMAE) | Neuroblastoma SK-N-AS and Ewing's sarcoma A-673 xenograft model | 84 |
| 1C1m, 7G22 | Fully human scFv | Phage display library | Preclinical | CAR-T, TriBiTEs | Ewing's sarcoma A-673 xenograft model | 79 |
| scFv78-Fc | Fully human scFv + Fc | Yeast display library | Preclinical | Immunotoxin (78Fc and saporin) | Osteosarcoma SJSA-1 and Ewing's sarcoma A-673 xenografts model | 32 |
| PLGA nanoparticles (loaded with shikonin) | Endosialin-expressing MS1 and TC1 xenograft model | 86 | ||||
| ScFv-CM6 | Fully human scFv | Phage display library | Preclinical | Immunoliposome (loaded with cytotoxic drug) | Endosialin-expressing IMR-32 xenograft model | 85 |
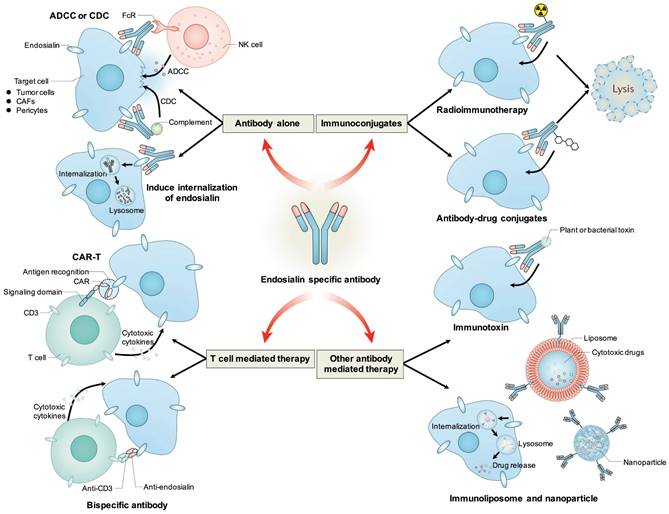
Antibody based therapeutic strategies targeting endosialin. Antibody alone could mediate ADCC or CDC effects to induce cytotoxicity to tumor cells, CAFs or pericytes, or induce the internalization of endosialin to inhibit its tumor-promoting function. Antibody could be conjugated with radioisotope or cytotoxic drugs and deliver them to endosialin positive cells to mediate specific cell killing. Antibody variable regions could be used to retarget immune effector cells towards endosialin positive cells through CAR-T or bispecific antibody. Antibody could also be fused with plant or bacterial toxin, or coupled to liposomes or nanoparticles to deliver toxin or cytotoxic drug to induce specific cell killing.
O'Shannessy et al. immunized female Lewis rats and generated a series of novel monoclonal antibodies (mAbs) that can recognize different extracellular domains of endosialin. Through pairing different antibodies to setup an electrochemiluminescence (ECL) assay, they demonstrated that these antibodies could be used to detect soluble endosialin/TEM-1 (sEND) in the serum of CRC patients and healthy individuals [31]. In addition, other endosialin-specific antibodies, such as ScFv-CM6 and hMP-E-8.3, were also generated and applied in different strategies (shown in the following section).
4.2 Other endosialin-targeted therapeutic strategies
MORAb-004 was the only endosialin antibody that was applied in clinical trials; however, it seemed that it could not elicit an effective antitumor effect when applied alone; thus, researchers have tried to pursue other endosialin-targeted therapeutic strategies, such as radioimmunotherapy, ADCs, CAR-T (chimeric antigen receptor T cell), BiTEs (bispecific T-cell engagers), immunotoxins, immunoliposomes, nanoparticles, and even DNA vaccines.
For radioimmunotherapy, D'Onofrio et al. evaluated a new panel of endosialin antibody fragments obtained from a phage display antibody library. They identified that the 125I radiolabeled antibody fragment 1C1m-Fc, which contains the endosialin-specific scFv (1C1m) and Fc, had high affinity for both human and mouse endosialin, could be effectively internalized into endosialin-positive cells, and could be specifically distributed in A673 xenografts in mice. Thus, it could be a promising candidate for the development of endosialin-targeted radioimmunoconjugates [80]. Delage et al. conjugated 1C1m-Fc to p-SCN-Bn-DOTA and labeled it with 177Lu and confirmed that it could be specifically taken up by endosialin-positive tumors through biodistribution and single-photon emission SPECT/CT imaging studies [81]. Later, they also examined the best DOTA per 1C1m-Fc ratio for theranostic applications and found that one DOTA per 1C1m-Fc gave the best pharmacokinetic behavior for future application of 177Lu-1C1m-Fc in patients [82].
For ADCs, Capone et al. generated a kind of ADC that contained the endosialin antibody hMP-E-8.3 (a humanized antibody) and a duocarmycin derivative. The ADC showed powerful, specific and target-dependent killing activity in vitro and led to long-lasting tumor growth inhibition in a cell line-based human osteosarcoma model. These results demonstrated that endosialin is an attractive target in sarcoma and that endosialin-specific ADC has the potential to be developed into a biotherapy agent for these malignancies [83]. Rouleau et al. conjugated a human anti-endosialin antibody with the anti-neoplastic agent MMAE through the maleimidocaproyl-valine-citrulline-p-aminobenzylcarbamate linker (so-called endosialin-MC-VC-PABC-MMAE) and demonstrated that the ADC had selective cytotoxicity to endosialin-positive cells in vitro and achieved profound and durable antitumor efficacy in human tumor xenograft models [84].
For T-cell-mediated immunotherapy, Fierle et al. constructed two types of CAR-T cells and soluble bispecific trivalent engagers, which they termed TriloBiTEs (tBs), by using two endosialin scFvs (1C1m and 7G22). They demonstrated that the two types of CAR-T cells could specifically recognize endosialin-positive target cells and be activated; the tBs could redirect T cells toward endosialin-positive target cells, and systemic delivery of 1C1m-tB could effectively prevent the establishment of Ewing sarcoma tumors in a xenograft model. These data further confirmed that endosialin could be used as a promising target for T-cell-mediated immunotherapy [79].
For immunotoxin, Guo et al. generated an immunotoxin through conjugating the anti-endosialin 78Fc, which contains scFv78 and Fc fragment, with saporin (78Fc-Sap) and confirmed that 78Fc-Sap was effective in killing endosialin-positive sarcoma cells in vitro and could eliminate human sarcoma xenografts without apparent toxicity in vivo [32].
For immunoliposomes, Marty et al. isolated a single chain antibody fragment (scFv-CM6) that specifically binds to the extracellular part of endosialin using phage display technology. They further functionalized and coupled ScFv-CM6 to liposomes to generate immunoliposomes and loaded them with cytotoxic drugs, which showed increased binding affinity and up to 80% higher cytotoxic activity toward endosialin-expressing IMR-32 tumor cells compared with control liposomes [85].
For nanoparticles, Matthaiou et al. developed PEGylated poly (lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) and functionalized them with the anti-endosialin antibody fragment 78Fc and loaded them with the necroptosis-inducing agent shikonin (SHK) (78Fc-PLGA-SHK NPs). They found that these NPs had significant toxicity in vitro, could effectively inhibit the growth of endosialin-positive tumors, and could induce an obvious immune response in vivo [86].
In addition to antibody-based therapy, endosialin was also targeted for cancer therapy through DNA vaccination. Facciponte et al. immunized immunocompetent mice with endosialin cDNA fused to the minimal domain of the C fragment of tetanus toxoid (referred to as the Tem1-TT vaccine) and demonstrated that Tem1-TT vaccination could elicit CD8+ and/or CD4+ T-cell responses, reduce tumor vascularity, increase CD3+ T-cell infiltration, and control the progression of established tumors. In addition, prophylactic immunization can prevent or delay tumor formation in several murine tumor models [87]. Furthermore, combinatorial treatment may enhance the therapeutic effect of DNA-based cancer vaccines. For example, combination with chemotherapy may enhance immunogenicity, and combination with approaches to alleviate myeloid-derived suppressor cell (MDSC)- and Treg cell-induced immunosuppressive TME may also enhance the therapeutic effect of DNA vaccines [88].
4.3 Imaging and diagnostic strategies targeting endosialin
Because of its specific expression in tumor stroma in many cancers and also tumor cells in sarcomas, endosialin has also been used in tumor imaging or diagnosis. For example, Yuan et al. evaluated the potential application of scFv78 as a tool for tumor molecular imaging and found by optical imaging that scFv78 was specifically localized in tumors in a tumor mouse model that had highly endogenous mouse endosialin expression in the vasculature [89]. Li et al. also confirmed that the radiolabeled fusion protein 78Fc could distinguish mouse- or human-endosialin-expressing tumor grafts from normal organs and control grafts in vivo. Thus, it could be further developed and optimized as an endosialin-targeted imaging agent for clinical application [90]. Cicone et al. conjugated scFv78-Fc with the chelator p-SCN-Bn-CHX-A"-DTPA and then labeled the product with indium-111 to generate 111In-CHX-DTPA-scFv78-Fc. They demonstrated that it was stable in serum and could specifically bind with endosialin-positive cells and target endosialin-positive xenografts in tumor-bearing mice, providing translation potential for the diagnosis of sarcoma [91].
Chacko et al. radiolabeled MORAb-004 with 124I and confirmed its specific and sensitive binding with endosialin-positive cells and demonstrated that radiolabeled MORAb-004 could be specifically and sensitively taken up by endosialin-positive tumors in vivo, suggesting that it could be clinically used for immuno-PET to assess endosialin-positive tumor status [92]. By using a tumor model that contained tumor cells and endosialin-expressing endothelial cells, Li et al. also demonstrated that 124I-labeled MORAb-004-PET could be used to visualize tumors enriched with endosialin-positive vasculature with high specificity and sensitivity [57].
These studies confirmed that endosialin is a promising target for the treatment and diagnosis of endosialin-positive sarcomas and multiple kinds of tumors with endosialin-positive stroma or vasculature.
5. Conclusion and perspectives
The identification of tumor-specific biomarkers could help to develop therapeutic strategies for targeted therapy, thus improving antitumor efficacy with minimal side effects. As a transmembrane glycoprotein, endosialin was found to be specifically and highly expressed in the stroma of various epithelial cell-derived tumors and both stroma and tumor cells of mesenchymal cell-derived sarcomas. Endosialin could promote tumor cell proliferation, adhesion and migration, stimulate tumor angiogenesis, and induce an immunosuppressive TME; thus, it is considered to be an ideal therapeutic target for cancer treatment.
Several anti-endosialin-specific antibodies have been developed, and various endosialin-targeted therapeutic strategies have been designed, some of which have shown preliminary antitumor effects. However, for the application of these endosialin antibodies or antibody-based therapeutic strategies in cancer treatment, several aspects need to be further validated. First, although the specific expression and tumor-promoting function of endosialin are relatively clear, the detailed mechanisms by which it promotes tumor progression and whether endosialin antibodies or antibody-based therapeutic strategies inhibit these mechanisms still need to be further elucidated. Second, some studies have found that endosialin could be detected in the serum of cancer patients, especially colon cancer patients, and whether patients with other cancer types also have serum endosialin expression and whether serum endosialin may influence the therapeutic effect of antibody-based therapies also need to be examined. In addition, the factors that regulate endosialin expression are not clear; although it has been found that the transcription factors HIF-2 and SP1 could regulate endosialin expression under hypoxia and at high cell density, the detailed mechanisms by which endosialin is regulated also need to be elucidated [93, 94]. The clarification of its tumor-promoting mechanism and expression regulation mechanism will help to develop more efficient and specific endosialin-targeted therapeutic and diagnostic strategies and more effective combined therapeutic strategies for cancer treatment.
Abbreviations
ADC: antibody-drug conjugates; α-SMA: α-smooth muscle actin; BiTEs: bispecific T-cell engagers; CAFs: cancer associated fibroblasts; CAR-T: chemeric antigen receptor T-cell; Col I: collagen type I; CRC: colorectal cancer; CTL: cytotoxic T lymphocyte; DOTA: dodecane tetraacetic acid; ECM: extracellular matrix; EPC: endothelial precursor cells; FN: fibronectin; GC: gastric cancer; HCC: hepatocellular carcinoma; MDSCs: myeloid-derived suppressor cells; NPs: nanoparticles; NSCLC: non-small cell lung cancer; ORR: overall response rate; OS: osteosarcoma; PDGFR-β: platelet-derived growth factor receptor-β; PEG: polyethylene glycol; PET: positron emission tomography; PFS: progression-free survival; PLGA: poly (lactic-co-glycolic acid); RCC: renal cell carcinoma; SP: side population; TEM1: tumor endothelial marker 1; TME: tumor microenvironment; Treg: regulatory T cells; UCB: urothelial carcinoma of the bladder.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82220108004; 82173204; 82203633), the Innovation Capability Support Program of Shaanxi (2023-CX-TD-72; 2021TD-39; 2020PT-021), the Natural Science Basic Research Program of Shaanxi (2022JZ-62), and the Fundamental Research Funds for the Central Universities (G2021KY05102).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48
3. Bejarano L, Jordao MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11:933-59
4. Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M. et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866-82
5. Grout JA, Sirven P, Leader AM, Maskey S, Hector E, Puisieux I. et al. Spatial Positioning and Matrix Programs of Cancer-Associated Fibroblasts Promote T-cell Exclusion in Human Lung Tumors. Cancer Discov. 2022;12:2606-25
6. Jungwirth U, van Weverwijk A, Evans RJ, Jenkins L, Vicente D, Alexander J. et al. Impairment of a distinct cancer-associated fibroblast population limits tumour growth and metastasis. Nat Commun. 2021;12:3516
7. Castillo-Sanchez R, Churruca-Schuind A, Martinez-Ival M, Salazar EP. Cancer-associated Fibroblasts Communicate with Breast Tumor Cells Through Extracellular Vesicles in Tumor Development. Technol Cancer Res Treat. 2022;21:15330338221131647
8. Peng L, Wang D, Han Y, Huang T, He X, Wang J. et al. Emerging Role of Cancer-Associated Fibroblasts-Derived Exosomes in Tumorigenesis. Front Immunol. 2021;12:795372
9. Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y. et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282-301
10. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131
11. Khan KA, McMurray JL, Mohammed F, Bicknell R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019;286:3299-332
12. Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci U S A. 1992;89:10832-6
13. Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi HP, Garin-Chesa P. et al. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276:7408-14
14. St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E. et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197-202
15. Valdez Y, Maia M, Conway EM. CD248: reviewing its role in health and disease. Curr Drug Targets. 2012;13:432-9
16. Rouleau C, Curiel M, Weber W, Smale R, Kurtzberg L, Mascarello J. et al. Endosialin protein expression and therapeutic target potential in human solid tumors: sarcoma versus carcinoma. Clin Cancer Res. 2008;14:7223-36
17. MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR. et al. Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett. 2005;579:2569-75
18. Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D. et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172:486-94
19. Kiyohara E, Donovan N, Takeshima L, Huang S, Wilmott JS, Scolyer RA. et al. Endosialin Expression in Metastatic Melanoma Tumor Microenvironment Vasculature: Potential Therapeutic Implications. Cancer Microenviron. 2015;8:111-8
20. Huber MA, Kraut N, Schweifer N, Dolznig H, Peter RU, Schubert RD. et al. Expression of stromal cell markers in distinct compartments of human skin cancers. J Cutan Pathol. 2006;33:145-55
21. Carson-Walter EB, Winans BN, Whiteman MC, Liu Y, Jarvela S, Haapasalo H. et al. Characterization of TEM1/endosialin in human and murine brain tumors. BMC Cancer. 2009;9:417
22. Brady J, Neal J, Sadakar N, Gasque P. Human endosialin (tumor endothelial marker 1) is abundantly expressed in highly malignant and invasive brain tumors. J Neuropathol Exp Neurol. 2004;63:1274-83
23. Simonavicius N, Robertson D, Bax DA, Jones C, Huijbers IJ, Isacke CM. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod Pathol. 2008;21:308-15
24. Rouleau C, Smale R, Sancho J, Fu YS, Kurtzberg L, Weber W. et al. Endosialin: a novel malignant cell therapeutic target for neuroblastoma. Int J Oncol. 2011;39:841-51
25. Fujii S, Fujihara A, Natori K, Abe A, Kuboki Y, Higuchi Y. et al. TEM1 expression in cancer-associated fibroblasts is correlated with a poor prognosis in patients with gastric cancer. Cancer Med. 2015;4:1667-78
26. O'Shannessy DJ, Somers EB, Chandrasekaran LK, Nicolaides NC, Bordeaux J, Gustavson MD. Influence of tumor microenvironment on prognosis in colorectal cancer: Tissue architecture-dependent signature of endosialin (TEM-1) and associated proteins. Oncotarget. 2014;5:3983-95
27. Davies G, Cunnick GH, Mansel RE, Mason MD, Jiang WG. Levels of expression of endothelial markers specific to tumour-associated endothelial cells and their correlation with prognosis in patients with breast cancer. Clin Exp Metastasis. 2004;21:31-7
28. Kuk C, Kulasingam V, Gunawardana CG, Smith CR, Batruch I, Diamandis EP. Mining the ovarian cancer ascites proteome for potential ovarian cancer biomarkers. Mol Cell Proteomics. 2009;8:661-9
29. Xu C, Zhang K, Yang F, Zhou X, Liu S, Li Y. et al. CD248+ Cancer-Associated Fibroblasts: A Novel Prognostic and Therapeutic Target for Renal Cell Carcinoma. Front Oncol. 2021;11:773063
30. Pietrzyk Ł, Wdowiak P. Endosialin (TEM1) as a Diagnostic, Progression, and Prognostic Serum Marker for Patients With Colorectal Cancer-A Preliminary Study. Cancer Control. 2020;27:1073274820903351
31. O'Shannessy DJ, Smith MF, Somers EB, Jackson SM, Albone E, Tomkowicz B. et al. Novel antibody probes for the characterization of endosialin/TEM-1. Oncotarget. 2016;7:69420-35
32. Guo Y, Hu J, Wang Y, Peng X, Min J, Wang J. et al. Tumour endothelial marker 1/endosialin-mediated targeting of human sarcoma. Eur J Cancer. 2018;90:111-21
33. O'Shannessy DJ, Dai H, Mitchell M, Huntsman S, Brantley S, Fenstermacher D. et al. Endosialin and Associated Protein Expression in Soft Tissue Sarcomas: A Potential Target for Anti-Endosialin Therapeutic Strategies. Sarcoma. 2016;2016:5213628
34. Thway K, Robertson D, Jones RL, Selfe J, Shipley J, Fisher C. et al. Endosialin expression in soft tissue sarcoma as a potential marker of undifferentiated mesenchymal cells. Br J Cancer. 2016;115:473-9
35. de Gooyer JM, Versleijen-Jonkers YMH, Hillebrandt-Roeffen MHS, Frielink C, Desar IME, de Wilt JHW. et al. Immunohistochemical selection of biomarkers for tumor-targeted image-guided surgery of myxofibrosarcoma. Sci Rep. 2020;10:2915
36. Kondo Y, Honoki K, Kishi S, Mori S, Fujiwara-Tani R, Tsukamoto S. et al. Endosialin/CD248 may be a potential therapeutic target to prevent the invasion and metastasis in osteosarcoma. Oncol Lett. 2022;23:42
37. Rouleau C, Smale R, Fu YS, Hui G, Wang F, Hutto E. et al. Endosialin is expressed in high grade and advanced sarcomas: evidence from clinical specimens and preclinical modeling. Int J Oncol. 2011;39:73-89
38. Rouleau C, Sancho J, Campos-Rivera J, Teicher BA. Endosialin expression in side populations in human sarcoma cell lines. Oncol Lett. 2012;3:325-9
39. Sun DX, Liao GJ, Liu KG, Jian H. Endosialin-expressing bone sarcoma stem-like cells are highly tumor-initiating and invasive. Mol Med Rep. 2015;12:5665-70
40. Teicher BA. CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget. 2019;10:993-1009
41. Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C. et al. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci U S A. 2006;103:3351-6
42. Maia M, DeVriese A, Janssens T, Moons M, Lories RJ, Tavernier J. et al. CD248 facilitates tumor growth via its cytoplasmic domain. BMC Cancer. 2011;11:162
43. Becker R, Lenter MC, Vollkommer T, Boos AM, Pfaff D, Augustin HG. et al. Tumor stroma marker endosialin (Tem1) is a binding partner of metastasis-related protein Mac-2 BP/90K. Faseb j. 2008;22:3059-67
44. Viski C, König C, Kijewska M, Mogler C, Isacke CM, Augustin HG. Endosialin-Expressing Pericytes Promote Metastatic Dissemination. Cancer Res. 2016;76:5313-25
45. Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E. et al. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci U S A. 2007;104:17965-70
46. Park GB, Jeong JY, Kim D. Modified TLR-mediated downregulation of miR-125b-5p enhances CD248 (endosialin)-induced metastasis and drug resistance in colorectal cancer cells. Mol Carcinog. 2020;59:154-67
47. Huang R, Li Z, Zhang J, Zeng Z, Zhang J, Li M. et al. Construction of Bone Metastasis-Specific Regulation Network Based on Prognostic Stemness-Related Signatures in Breast Invasive Carcinoma. Front Oncol. 2020;10:613333
48. Lax S, Hardie DL, Wilson A, Douglas MR, Anderson G, Huso D. et al. The pericyte and stromal cell marker CD248 (endosialin) is required for efficient lymph node expansion. Eur J Immunol. 2010;40:1884-9
49. Lu S, Lu T, Zhang J, Gan L, Wu X, Han D. et al. CD248 promotes migration and metastasis of osteosarcoma through ITGB1-mediated FAK-paxillin pathway activation. BMC Cancer. 2023;23:290
50. Di Benedetto P, Ruscitti P, Liakouli V, Del Galdo F, Giacomelli R, Cipriani P. Linking myofibroblast generation and microvascular alteration: The role of CD248 from pathogenesis to therapeutic target (Review). Mol Med Rep. 2019;20:1488-98
51. Bagley RG, Honma N, Weber W, Boutin P, Rouleau C, Shankara S. et al. Endosialin/TEM 1/CD248 is a pericyte marker of embryonic and tumor neovascularization. Microvasc Res. 2008;76:180-8
52. Bagley RG, Rouleau C, St Martin T, Boutin P, Weber W, Ruzek M. et al. Human endothelial precursor cells express tumor endothelial marker 1/endosialin/CD248. Mol Cancer Ther. 2008;7:2536-46
53. Hong CL, Yu IS, Pai CH, Chen JS, Hsieh MS, Wu HL. et al. CD248 Regulates Wnt Signaling in Pericytes to Promote Angiogenesis and Tumor Growth in Lung Cancer. Cancer Res. 2022;82:3734-50
54. Rybinski K, Imtiyaz HZ, Mittica B, Drozdowski B, Fulmer J, Furuuchi K. et al. Targeting endosialin/CD248 through antibody-mediated internalization results in impaired pericyte maturation and dysfunctional tumor microvasculature. Oncotarget. 2015;6:25429-40
55. Liu S, Xu C, Zhang K, Han D, Yang F, Li Y. et al. CD248 as a bridge between angiogenesis and immunosuppression: a promising prognostic and therapeutic target for renal cell carcinoma. Ann Transl Med. 2021;9:1741
56. Li Y, Zhang K, Yang F, Jiao D, Li M, Zhao X. et al. Prognostic Value of Vascular-Expressed PSMA and CD248 in Urothelial Carcinoma of the Bladder. Front Oncol. 2021;11:771036
57. Li C, Chacko AM, Hu J, Hasegawa K, Swails J, Grasso L. et al. Antibody-based tumor vascular theranostics targeting endosialin/TEM1 in a new mouse tumor vascular model. Cancer Biol Ther. 2014;15:443-51
58. Khan KA, Naylor AJ, Khan A, Noy PJ, Mambretti M, Lodhia P. et al. Multimerin-2 is a ligand for group 14 family C-type lectins CLEC14A, CD93 and CD248 spanning the endothelial pericyte interface. Oncogene. 2017;36:6097-108
59. Tomkowicz B, Rybinski K, Sebeck D, Sass P, Nicolaides NC, Grasso L. et al. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol Ther. 2010;9:908-15
60. Hong YK, Lee YC, Cheng TL, Lai CH, Hsu CK, Kuo CH. et al. Tumor Endothelial Marker 1 (TEM1/Endosialin/CD248) Enhances Wound Healing by Interacting with Platelet-Derived Growth Factor Receptors. J Invest Dermatol. 2019;139:2204-14.e7
61. Brett E, Zielins ER, Chin M, Januszyk M, Blackshear CP, Findlay M. et al. Isolation of CD248-expressing stromal vascular fraction for targeted improvement of wound healing. Wound Repair Regen. 2017;25:414-22
62. Kontsekova S, Polcicova K, Takacova M, Pastorekova S. Endosialin: molecular and functional links to tumor angiogenesis. Neoplasma. 2016;63:183-92
63. Teicher BA. Newer vascular targets: endosialin (review). Int J Oncol. 2007;30:305-12
64. Yang F, Wei Y, Han D, Li Y, Shi S, Jiao D. et al. Interaction with CD68 and Regulation of GAS6 Expression by Endosialin in Fibroblasts Drives Recruitment and Polarization of Macrophages in Hepatocellular Carcinoma. Cancer Res. 2020;80:3892-905
65. Wu J, Liu X, Wu J, Lou C, Zhang Q, Chen H. et al. CXCL12 derived from CD248-expressing cancer-associated fibroblasts mediates M2-polarized macrophages to promote nonsmall cell lung cancer progression. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166521
66. Zhang K, Xu C, Liu S, Jiang Y, Zhao X, Ma S. et al. The Diagnostic and Immunotherapeutic Value of CD248 in Renal Cell Carcinoma. Front Oncol. 2021;11:644612
67. Lu T, Zhang J, Lu S, Yang F, Gan L, Wu X. et al. Endosialin-positive tumor-derived pericytes promote tumor progression through impeding the infiltration of CD8(+) T cells in clear cell renal cell carcinoma. Cancer Immunol Immunother. 2023;72:1739-50
68. Ochs K, Sahm F, Opitz CA, Lanz TV, Oezen I, Couraud PO. et al. Immature mesenchymal stem cell-like pericytes as mediators of immunosuppression in human malignant glioma. J Neuroimmunol. 2013;265:106-16
69. Diaz LA Jr, Coughlin CM, Weil SC, Fishel J, Gounder MM, Lawrence S. et al. A first-in-human phase I study of MORAb-004, a monoclonal antibody to endosialin in patients with advanced solid tumors. Clin Cancer Res. 2015;21:1281-8
70. Norris RE, Fox E, Reid JM, Ralya A, Liu XW, Minard C. et al. Phase 1 trial of ontuxizumab (MORAb-004) in children with relapsed or refractory solid tumors: A report from the Children's Oncology Group Phase 1 Pilot Consortium (ADVL1213). Pediatr Blood Cancer. 2018;65:e26944
71. Doi T, Aramaki T, Yasui H, Muro K, Ikeda M, Okusaka T. et al. A phase I study of ontuxizumab, a humanized monoclonal antibody targeting endosialin, in Japanese patients with solid tumors. Invest New Drugs. 2019;37:1061-74
72. D'Angelo SP, Hamid OA, Tarhini A, Schadendorf D, Chmielowski B, Collichio FA. et al. A phase 2 study of ontuxizumab, a monoclonal antibody targeting endosialin, in metastatic melanoma. Invest New Drugs. 2018;36:103-13
73. Grothey A, Strosberg JR, Renfro LA, Hurwitz HI, Marshall JL, Safran H. et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of the Efficacy and Safety of Monotherapy Ontuxizumab (MORAb-004) Plus Best Supportive Care in Patients with Chemorefractory Metastatic Colorectal Cancer. Clin Cancer Res. 2018;24:316-25
74. Jones RL, Chawla SP, Attia S, Schöffski P, Gelderblom H, Chmielowski B. et al. A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas. Cancer. 2019;125:2445-54
75. Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R. et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25:2764-9
76. Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T. et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190-6
77. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-42
78. Zhao A, Nunez-Cruz S, Li C, Coukos G, Siegel DL, Scholler N. Rapid isolation of high-affinity human antibodies against the tumor vascular marker Endosialin/TEM1, using a paired yeast-display/secretory scFv library platform. J Immunol Methods. 2011;363:221-32
79. Fierle JK, Brioschi M, de Tiani M, Wetterwald L, Atsaves V, Abram-Saliba J. et al. Soluble trivalent engagers redirect cytolytic T cell activity toward tumor endothelial marker 1. Cell Rep Med. 2021;2:100362
80. D'Onofrio A, Gano L, Melo R, Mendes F, Oliveira MC, Denoël T. et al. Biological evaluation of new TEM1 targeting recombinant antibodies for radioimmunotherapy: In vitro, in vivo and in silico studies. Eur J Pharm Biopharm. 2021;158:233-44
81. Delage JA, Faivre-Chauvet A, Fierle JK, Gnesin S, Schaefer N, Coukos G. et al. (177)Lu radiolabeling and preclinical theranostic study of 1C1m-Fc: an anti-TEM-1 scFv-Fc fusion protein in soft tissue sarcoma. EJNMMI Res. 2020;10:98
82. Delage JA, Faivre-Chauvet A, Barbet J, Fierle JK, Schaefer N, Coukos G. et al. Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [(177)Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics. 2021;13:96
83. Capone E, Piccolo E, Fichera I, Ciufici P, Barcaroli D, Sala A. et al. Generation of a novel Antibody-Drug Conjugate targeting endosialin: potent and durable antitumor response in sarcoma. Oncotarget. 2017;8:60368-77
84. Rouleau C, Gianolio DA, Smale R, Roth SD, Krumbholz R, Harper J. et al. Anti-Endosialin Antibody-Drug Conjugate: Potential in Sarcoma and Other Malignancies. Mol Cancer Ther. 2015;14:2081-9
85. Marty C, Langer-Machova Z, Sigrist S, Schott H, Schwendener RA, Ballmer-Hofer K. Isolation and characterization of a scFv antibody specific for tumor endothelial marker 1 (TEM1), a new reagent for targeted tumor therapy. Cancer Lett. 2006;235:298-308
86. Matthaiou EI, Guo Y, Barar J, Sandaltzopoulos R, Kandalaft LE, Li C. et al. TEM1-targeting PEGylated PLGA shikonin nanoformulation for immunomodulation and eradication of ovarian cancer. Bioimpacts. 2022;12:65-86
87. Facciponte JG, Ugel S, De Sanctis F, Li C, Wang L, Nair G. et al. Tumor endothelial marker 1-specific DNA vaccination targets tumor vasculature. J Clin Invest. 2014;124:1497-511
88. Ugel S, Facciponte JG, De Sanctis F, Facciabene A. Targeting tumor vasculature: expanding the potential of DNA cancer vaccines. Cancer Immunol Immunother. 2015;64:1339-48
89. Yuan X, Yang M, Chen X, Zhang X, Sukhadia S, Musolino N. et al. Characterization of the first fully human anti-TEM1 scFv in models of solid tumor imaging and immunotoxin-based therapy. Cancer Immunol Immunother. 2017;66:367-78
90. Li C, Wang J, Hu J, Feng Y, Hasegawa K, Peng X. et al. Development, optimization, and validation of novel anti-TEM1/CD248 affinity agent for optical imaging in cancer. Oncotarget. 2014;5:6994-7012
91. Cicone F, Denoël T, Gnesin S, Riggi N, Irving M, Jakka G. et al. Preclinical Evaluation and Dosimetry of [(111)In]CHX-DTPA-scFv78-Fc Targeting Endosialin/Tumor Endothelial Marker 1 (TEM1). Mol Imaging Biol. 2020;22:979-91
92. Chacko AM, Li C, Nayak M, Mikitsh JL, Hu J, Hou C. et al. Development of 124I immuno-PET targeting tumor vascular TEM1/endosialin. J Nucl Med. 2014;55:500-7
93. Ohradanova A, Gradin K, Barathova M, Zatovicova M, Holotnakova T, Kopacek J. et al. Hypoxia upregulates expression of human endosialin gene via hypoxia-inducible factor 2. Br J Cancer. 2008;99:1348-56
94. Opavsky R, Haviernik P, Jurkovicova D, Garin MT, Copeland NG, Gilbert DJ. et al. Molecular characterization of the mouse Tem1/endosialin gene regulated by cell density in vitro and expressed in normal tissues in vivo. J Biol Chem. 2001;276:38795-807
Author contact
![]() Corresponding authors: Weihong Wen, Institute of Medical Research, Northwestern Polytechnical University, Xi'an, China; Email: weihongwenedu.cn; Weijun Qin, Department of Urology, Xijing Hospital, Fourth Military Medical University, Xi'an, China; Email: qinwjedu.cn; Fa Yang, Department of Urology, Xijing Hospital, Fourth Military Medical University, Xi'an, China; Email: yangfaedu.cn.
Corresponding authors: Weihong Wen, Institute of Medical Research, Northwestern Polytechnical University, Xi'an, China; Email: weihongwenedu.cn; Weijun Qin, Department of Urology, Xijing Hospital, Fourth Military Medical University, Xi'an, China; Email: qinwjedu.cn; Fa Yang, Department of Urology, Xijing Hospital, Fourth Military Medical University, Xi'an, China; Email: yangfaedu.cn.
 Global reach, higher impact
Global reach, higher impact