13.3
Impact Factor
Theranostics 2024; 14(3):940-953. doi:10.7150/thno.91268 This issue Cite
Review
Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: status 2023
1. Department of Nuclear Medicine, Medical University of Innsbruck, Innsbruck, Austria.
2. Department of Experimental and Clinical Medicine, “Magna Graecia” University of Catanzaro, Catanzaro, Italy.
Abstract
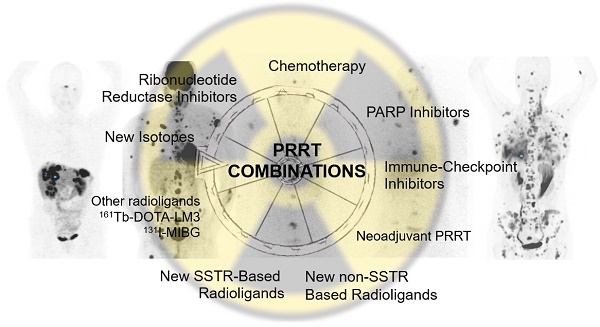
A growing body of literature reports on the combined use of peptide receptor radionuclide therapy (PRRT) with other anti-tumuor therapies in order to anticipate synergistic effects with perhaps increased safety issues. Combination treatments to enhance PRRT outcome are based on improved tumour perfusion, upregulation of somatostatin receptors (SSTR), radiosensitization with DNA damaging agents or targeted therapies. Several Phase 1 or 2 trials are currently recruiting patients in combined regimens. The combination of PRRT with cytotoxic chemotherapy, capecitabine and temozolomide (CAPTEM), seems to become clinically useful especially in pancreatic neuroendocrine tumours (pNETs) with acceptable safety profile. Neoadjuvant PRRT prior to surgery, PRRT combinations of intravenous and intraarterial routes of application, combinations of PRRT with differently radiolabelled (alpha, beta, Auger) SSTR-targeting agonists and antagonists, inhibitors of immune checkpoints (ICIs), poly (ADP-ribose) polymerase-1 (PARP1i), tyrosine kinase (TKI), DNA-dependent protein kinase, ribonucleotide reductase or DNA methyltransferase (DMNT) are tested in currently ongoing clinical trials. The combination with [131I]I-MIBG in rare NETs (such as paraganglioma, pheochromocytoma) and new non-SSTR-targeting radioligands are used in the personalization process of treatment. The present review will provide an overview of the current status of ongoing PRRT combination treatments.
Keywords: PRRT, combination treatments, chemotherapy, neuroendocrine tumours, SSTR
 Global reach, higher impact
Global reach, higher impact