13.3
Impact Factor
Theranostics 2024; 14(3):1168-1180. doi:10.7150/thno.87130 This issue Cite
Research Paper
Midline-1 regulates effector T cell motility in experimental autoimmune encephalomyelitis via mTOR/microtubule pathway
1. Department of Rheumatology and Immunology, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
2. Cardiovascular Research Institute, Case Western Reserve University, Cleveland, Ohio 44106, USA.
3. Wexner Medical Center, The Ohio State University, Columbus, Ohio 43210, USA.
4. Sinopharm Dongfeng General Hospital, Hubei University of Medicine, Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine), Shiyan, Hubei 442008, China.
5. Department of Immunology, School of Medicine, Yangtze University, Jingzhou, Hubei 434023, China.
6. Department of Gastrointestinal Surgery, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
7. Institute of Allergy and Clinical Immunology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
8. Department of Otolaryngology-Head and Neck Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China.
9. Key Laboratory of Vascular Aging (HUST), Ministry of Education, Wuhan, Hubei 430030, China.
Abstract
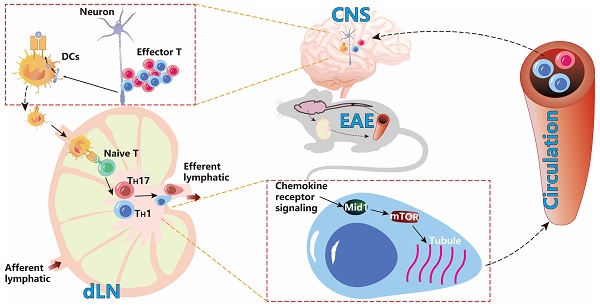
Background: Effector T cell activation, migration, and proinflammatory cytokine production are crucial steps in autoimmune disorders such as multiple sclerosis (MS). While several therapeutic approaches targeting T cell activation and proinflammatory cytokines have been developed for the treatment of autoimmune diseases, there are no therapeutic agents targeting the migration of effector T cells, largely due to our limited understanding of regulatory mechanisms of T cell migration in autoimmune disease. Here we reported that midline-1 (Mid1) is a key regulator of effector T cell migration in experimental autoimmune encephalomyelitis (EAE), a widely used animal model of MS.
Methods: Mid1-/- mice were generated by Crispr-Cas9 technology. T cell-specific Mid1 knockout chimeric mice were generated by adoptive transfer of Mid1-/- T cells into lymphocyte deficient Rag2-/- mice. Mice were either immunized with MOG35-55 (active EAE) or received adoptive transfer of pathogenic T cells (passive EAE) to induce EAE. In vitro Transwell® assay or in vivo footpad injection were used to assess the migration of T cells.
Results: Mid1 was significantly increased in the spinal cord of wild-type (Wt) EAE mice and disruption of Mid1 in T cells markedly suppressed the development of both active and passive EAE. Transcriptomic and flow cytometric analyses revealed a marked reduction in effector T cell number in the central nervous system of Mid1-/- mice after EAE induction. Conversely, an increase in the number of T cells was observed in the draining lymph nodes of Mid1-/- mice. Mice that were adoptively transferred with pathogenic Mid1-/- T cells also exhibited milder symptoms of EAE, along with a lower T cell count in the spinal cord. Additionally, disruption of Mid1 significantly inhibited T-cell migration both in vivo and in vitro. RNA sequencing suggests a suppression in multiple inflammatory pathways in Mid1-/- mice, including mTOR signaling that plays a critical role in cell migration. Subsequent experiments confirmed the interaction between Mid1 and mTOR. Suppression of mTOR with rapamycin or microtubule spindle formation with colcemid blunted the regulatory effect of Mid1 on T cell migration. In addition, mTOR agonists MHY1485 and 3BDO restored the migratory deficit caused by Mid1 depletion.
Conclusion: Our data suggests that Mid1 regulates effector T cell migration to the central nervous system via mTOR/microtubule pathway in EAE, and thus may serve as a potential therapeutic target for the treatment of MS.
Keywords: Mid1, Experimental autoimmune encephalomyelitis, T cell migration, mTOR, Motility.
 Global reach, higher impact
Global reach, higher impact