13.3
Impact Factor
Theranostics 2024; 14(5):2036-2057. doi:10.7150/thno.91084 This issue Cite
Research Paper
Depletion of ApoA5 aggravates spontaneous and diet-induced nonalcoholic fatty liver disease by reducing hepatic NR1D1 in hamsters
1. Institute of Cardiovascular Sciences, State Key Laboratory of Vascular Homeostasis and Remodeling, School of Basic Medical Sciences, Peking University, Beijing, China.
2. Beijing Key Laboratory of Cardiovascular Receptors Research, Peking University Third Hospital, Beijing, China.
3. Department of Urology, China-Japan Friendship Hospital, Beijing, China.
*These authors contributed equally to this work.
#Lead contact.
Abstract
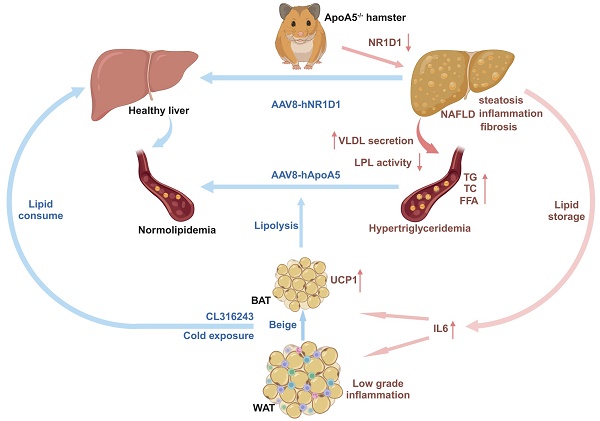
Background: ApoA5 mainly synthesized and secreted by liver is a key modulator of lipoprotein lipase (LPL) activity and triglyceride-rich lipoproteins (TRLs). Although the role of ApoA5 in extrahepatic triglyceride (TG) metabolism in circulation has been well documented, the relationship between ApoA5 and nonalcoholic fatty liver disease (NAFLD) remains incompletely understood and the underlying molecular mechanism still needs to be elucidated.
Methods: We used CRISPR/Cas9 gene editing to delete Apoa5 gene from Syrian golden hamster, a small rodent model replicating human metabolic features. Then, the ApoA5-deficient (ApoA5-/-) hamsters were used to investigate NAFLD with or without challenging a high fat diet (HFD).
Results: ApoA5-/- hamsters exhibited hypertriglyceridemia (HTG) with markedly elevated TG levels at 2300 mg/dL and hepatic steatosis on a regular chow diet, accompanied with an increase in the expression levels of genes regulating lipolysis and small adipocytes in the adipose tissue. An HFD challenge predisposed ApoA5-/- hamsters to severe HTG (sHTG) and nonalcoholic steatohepatitis (NASH). Mechanistic studies in vitro and in vivo revealed that targeting ApoA5 disrupted NR1D1 mRNA stability in the HepG2 cells and the liver to reduce both mRNA and protein levels of NR1D1, respectively. Overexpression of human NR1D1 by adeno-associated virus 8 (AAV8) in the livers of ApoA5-/- hamsters significantly ameliorated fatty liver without affecting plasma lipid levels. Moreover, restoration of hepatic ApoA5 or activation of UCP1 in brown adipose tissue (BAT) by cold exposure or CL316243 administration could significantly correct sHTG and hepatic steatosis in ApoA5-/- hamsters.
Conclusions: Our data demonstrate that HTG caused by ApoA5 deficiency in hamsters is sufficient to elicit hepatic steatosis and HFD aggravates NAFLD by reducing hepatic NR1D1 mRNA and protein levels, which provides a mechanistic link between ApoA5 and NAFLD and suggests the new insights into the potential therapeutic approaches for the treatment of HTG and the related disorders due to ApoA5 deficiency in the clinical trials in future.
Keywords: Apolipoprotein A5, Syrian golden hamster, hypertriglyceridemia, nonalcoholic fatty liver disease, NR1D1
 Global reach, higher impact
Global reach, higher impact