13.3
Impact Factor
Theranostics 2024; 14(5):2190-2209. doi:10.7150/thno.92909 This issue Cite
Research Paper
G protein subunit alpha i2's pivotal role in angiogenesis
1. Department of Orthopedics, Clinical Research Center of Neurological Disease, The Second Affiliated Hospital of Soochow University, Institution of Neuroscience, Soochow University, Suzhou, China.
2. Department of Joint Surgery and Geriatric Orthopedics, Affiliated Hospital of YouJiang Medical University for Nationalities, Guangxi Key Laboratory of Basic and Translational Research of Bone and Joint Degenerative Diseases, Guangxi Biomedical Materials Engineering Research Center for Bone and Joint Degenerative Diseases, Baise City, China.
3. Department of Neuroscience, Case Western Reserve University, Cleveland, USA.
4. The Fourth Medical School, Eye hospital, Nanjing Medical University, Nanjing, China.
# Equal contributors.
Abstract
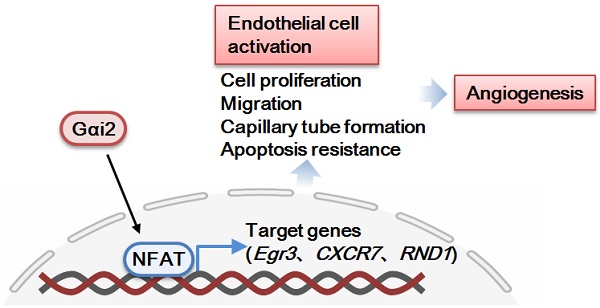
Here we explored the potential role of Gαi2 (G protein subunit alpha i2) in endothelial cell function and angiogenesis.
Methods: Genetic methodologies such as shRNA, CRISPR/Cas9, dominant negative mutation, and overexpression were utilized to modify Gαi2 expression or regulate its function. Their effects on endothelial cell functions were assessed in vitro. In vivo, the endothelial-specific Gαi2 shRNA adeno-associated virus (AAV) was utilized to silence Gαi2 expression. The impact of this suppression on retinal angiogenesis in control mice and streptozotocin (STZ)-induced diabetic retinopathy (DR) mice was analyzed.
Results: Analysis of single-cell RNA sequencing data revealed Gαi2 (GNAI2) was predominantly expressed in retinal endothelial cells and expression was increased in retinal endothelial cells following oxygen-induced retinopathy (OIR) in mice. Moreover, transcriptome analysis linking Gαi2 to angiogenesis-related processes/pathways, supported by increased Gαi2 expression in experimental OIR mouse retinas, highlighted its possible role in angiogenesis. In various endothelial cell types, shRNA-induced silencing and CRISPR/Cas9-mediated knockout (KO) of Gαi2 resulted in substantial reductions in cell proliferation, migration, invasion, and capillary tube formation. Conversely, Gαi2 over-expression in endothelial cells induced pro-angiogenic activities, enhancing cell proliferation, migration, invasion, and capillary tube formation. Furthermore, our investigation revealed a crucial role of Gαi2 in NFAT (nuclear factor of activated T cells) activation, as evidenced by the down-regulation of NFAT-luciferase reporter activity and pro-angiogenesis NFAT-targeted genes (Egr3, CXCR7, and RND1) in Gαi2-silenced or -KO HUVECs, which were up-regulated in Gαi2-overexpressing endothelial cells. Expression of a dominant negative Gαi2 mutation (S48C) also down-regulated NFAT-targeted genes, slowing proliferation, migration, invasion, and capillary tube formation in HUVECs. Importantly, in vivo experiments revealed that endothelial Gαi2 knockdown inhibited retinal angiogenesis in mice, with a concomitant down-regulation of NFAT-targeted genes in mouse retinal tissue. In contrast, Gαi2 over-expression in endothelial cells enhanced retinal angiogenesis in mice. Single-cell RNA sequencing data confirmed increased levels of Gαi2 specifically in retinal endothelial cells of mice with streptozotocin (STZ)-induced diabetic retinopathy (DR). Importantly, endothelial Gαi2 silencing ameliorated retinal pathological angiogenesis in DR mice.
Conclusion: Our study highlights a critical role for Gαi2 in NFAT activation, endothelial cell activation and angiogenesis, offering valuable insights into potential therapeutic strategies for modulating these processes.
 Global reach, higher impact
Global reach, higher impact