13.3
Impact Factor
Theranostics 2024; 14(6):2573-2588. doi:10.7150/thno.88864 This issue Cite
Research Paper
Hypofractionated radiotherapy combined with lenalidomide improves systemic antitumor activity in mouse solid tumor models
1. Department of Radiation Oncology, Faculty of Medicine, University of Freiburg, Freiburg, Germany.
2. Faculty of Biology, University of Freiburg, Freiburg, Germany.
3. Laboratory of Biosynthesis of Nucleic Acids, Institute of Molecular Biology and Genetics of NASU, Kyiv, Ukraine.
4. German Cancer Consortium (DKTK), Partner Site Freiburg, Freiburg, Germany.
5. German Cancer Research Center (DKFZ), Heidelberg, Germany.
6. Division of Thoracic Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
7. Department of Radiation Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China.
†KO and RL contributed equally.
Abstract
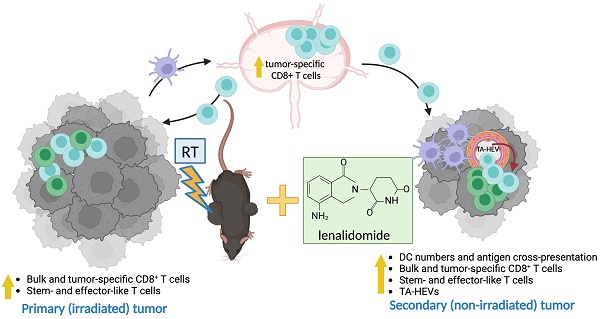
Background: Hypofractionated radiotherapy (hRT) can induce a T cell-mediated abscopal effect on non-irradiated tumor lesions, especially in combination with immune checkpoint blockade (ICB). However, clinically, this effect is still rare, and ICB-mediated adverse events are common. Lenalidomide (lena) is an anti-angiogenic and immunomodulatory drug used in the treatment of hematologic malignancies. We here investigated in solid tumor models whether lena can enhance the abscopal effect in double combination with hRT.
Methods: In two syngeneic bilateral tumor models (B16-CD133 melanoma and MC38 colon carcinoma), the primary tumor was treated with hRT. Lena was given daily for 3 weeks. Besides tumor size and survival, the dependence of the antitumor effects on CD8+ cells, type-I IFN signaling, and T cell costimulation was determined with depleting or blocking antibodies. Tumor-specific CD8+ T cells were quantified, and their differentiation and effector status were characterized by multicolor flow cytometry using MHC-I tetramers and various antibodies. In addition, dendritic cell (DC)-mediated tumor antigen cross-presentation in vitro and directly ex vivo and the composition of tumor-associated vascular endothelial cells were investigated.
Results: In both tumor models, the hRT/lena double combination induced a significant abscopal effect. Control of the non-irradiated secondary tumor and survival were considerably better than with the respective monotherapies. The abscopal effect was strongly dependent on CD8+ cells and associated with an increase in tumor-specific CD8+ T cells in the non-irradiated tumor and its draining lymph nodes. Additionally, we found more tumor-specific T cells with a stem-like (TCF1+ TIM3- PD1+) and a transitory (TCF1- TIM3+ CD101- PD1+) exhausted phenotype and more expressing effector molecules such as GzmB, IFNγ, and TNFα. Moreover, in the non-irradiated tumor, hRT/lena treatment also increased DCs cross-presenting a tumor model antigen. Blocking type-I IFN signaling, which is essential for cross-presentation, completely abrogated the abscopal effect. A gene expression analysis of bone marrow-derived DCs revealed that lena augmented the expression of IFN response genes and genes associated with differentiation, maturation (including CD70, CD83, and CD86), migration to lymph nodes, and T cell activation. Flow cytometry confirmed an increase in CD70+ CD83+ CD86+ DCs in both irradiated and abscopal tumors. Moreover, the hRT/lena-induced abscopal effect was diminished when these costimulatory molecules were blocked simultaneously using antibodies. In line with the enhanced infiltration by DCs and tumor-specific CD8+ T cells, including more stem-like cells, hRT/lena also increased tumor-associated high endothelial cells (TA-HECs) in the non-irradiated tumor.
Conclusions: We demonstrate that lena can augment the hRT-induced abscopal effect in mouse solid tumor models in a CD8 T cell- and IFN-I-dependent manner, correlating with enhanced anti-tumor CD8 T cell immunity, DC cross-presentation, and TA-HEC numbers. Our findings may be helpful for the planning of clinical trials in (oligo)metastatic patients.
Keywords: radiotherapy, abscopal effect, lenalidomide, dendritic cell cross-presentation
 Global reach, higher impact
Global reach, higher impact