13.3
Impact Factor
Theranostics 2016; 6(8):1145-1159. doi:10.7150/thno.15257 This issue Cite
Research Paper
Synthesis and Preclinical Evaluation of Sulfonamido-based [11C-Carbonyl]-Carbamates and Ureas for Imaging Monoacylglycerol Lipase
1. Gordon Center of Medical Imaging, Division of Nuclear Medicine and Molecular Imaging, Massachusetts General Hospital & Department of Radiology, Harvard Medical School, Boston, MA, 02114, USA.
2. Molecular Imaging Center, National Institute of Radiological Sciences, Chiba, 263-8555, Japan.
3. School of Pharmaceutical Science and Technology, Tianjin University, Tianjin, 300072, China.
*These authors contributed equally to the work.
Abstract
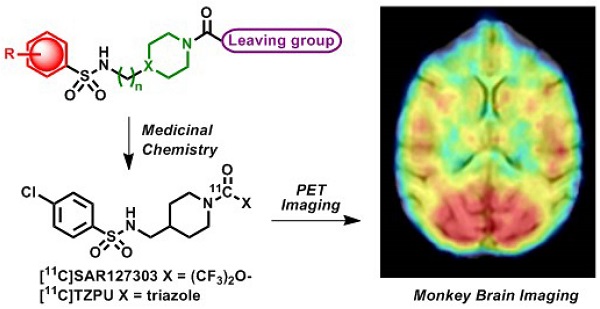
Monoacylglycerol lipase (MAGL) is a 33 kDa member of the serine hydrolase superfamily that preferentially degrades 2-arachidonoylglycerol (2-AG) to arachidonic acid in the endocannabinoid system. Inhibition of MAGL is not only of interest for probing the cannabinoid pathway but also as a therapeutic and diagnostic target for neuroinflammation. Limited attempts have been made to image MAGL in vivo and a suitable PET ligand for this target has yet to be identified and is urgently sought to guide small molecule drug development in this pathway. Herein we synthesized and evaluated the physiochemical properties of an array of eleven sulfonamido-based carbamates and ureas with a series of terminal aryl moieties, linkers and leaving groups. The most potent compounds were a novel MAGL inhibitor, N-((1-(1H-1,2,4-triazole-1-carbonyl)piperidin-4-yl) methyl)-4-chlorobenzenesulfonamide (TZPU; IC50 = 35.9 nM), and the known inhibitor 1,1,1,3,3,3-hexafluoropropan-2-yl 4-(((4-chlorophenyl)sulfonamido) methyl)piperidine-1-carboxylate (SAR127303; IC50 = 39.3 nM), which were also shown to be selective for MAGL over fatty acid amide hydrolase (FAAH), and cannabinoid receptors (CB1 & CB2). Both of these compounds were radiolabeled with carbon-11 via [11C]COCl2, followed by comprehensive ex vivo biodistribution and in vivo PET imaging studies in normal rats to determine their brain permeability, specificity, clearance and metabolism. Whereas TZPU did not show adequate specificity to warrant further evaluation, [11C]SAR127303 was advanced for preliminary PET neuroimaging studies in nonhuman primate. The tracer showed good brain permeability (ca. 1 SUV) and heterogeneous regional brain distribution which is consistent with the distribution of MAGL.
Keywords: positron emission tomography, monoacylglycerol lipase, MAGL, carbon-11, nonhuman primate, SAR127303.
 Global reach, higher impact
Global reach, higher impact