13.3
Impact Factor
Theranostics 2017; 7(5):1266-1276. doi:10.7150/thno.18304 This issue Cite
Research Paper
Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcεRI-positive cells in the skin
1. Department of Dermatology and Allergy, Charité - Universitätsmedizin, Berlin, Germany;
2. Department of Dermatology, University Medicine Mainz, Germany;
3. Department of Dermatology, University Allergy Center, University Hospital Carl Gustav Carus, Technical University Dresden, Germany;
4. Department of Dermatology, University Hospital Muenster, Germany;
5. Translational Medicine, Novartis Pharma AG, Basel, Switzerland
* Current address: Novartis Pharma AG, Basel, Switzerland
† Current address: Clovis Oncology, Cambridge UK.
Received 2016-11-11; Accepted 2017-1-16; Published 2017-3-6
Abstract
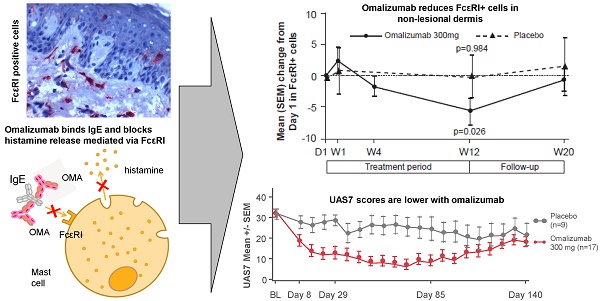
Background. Treatment with omalizumab, a humanized recombinant monoclonal anti-IgE antibody, results in clinical efficacy in patients with Chronic Spontaneous Urticaria (CSU). The mechanism of action of omalizumab in CSU has not been elucidated in detail.
Objectives. To determine the effects of omalizumab on levels of high affinity IgE receptor-positive (FcεRI+) and IgE-positive (IgE+) dermal cells and blood basophils. Treatment efficacy and safety were also assessed.
Study design. In a double-blind study, CSU patients aged 18‑75 years were randomized to receive 300 mg omalizumab (n=20) or placebo (n=10) subcutaneously every 4 weeks for 12 weeks. Changes in disease activity were assessed by use of the weekly Urticaria Activity Score (UAS7). Circulating IgE levels, basophil numbers and levels of expression of FcεRI+ and IgE+ cells in the skin and in blood basophils were determined.
Results. Patients receiving omalizumab showed a significantly greater decrease in UAS7 compared with patients receiving placebo. At Week 12 the mean difference in UAS7 between treatment groups was -14.82 (p=0.0027), consistent with previous studies.
Total IgE levels in serum were increased after omalizumab treatment and remained elevated up to Week 12. Free IgE levels decreased after omalizumab treatment.
Mean levels of FcεRI+ skin cells in patients treated with omalizumab 300 mg were decreased at Week 12 compared with baseline in the dermis of both non-lesional and lesional skin, reaching levels comparable with those seen in healthy volunteers (HVs). There were no statistically significant changes in mean FcɛRI+ cell levels in the placebo group. Similar results were seen for changes in IgE+ cells, although the changes were not statistically significant.
The level of peripheral blood basophils increased immediately after treatment start and returned to Baseline values after the follow-up period. The levels of FcεRI and IgE expression on peripheral blood basophils were rapidly reduced by omalizumab treatment up to Week 12.
Conclusions. Treatment with omalizumab resulted in rapid clinical benefits in patients with CSU. Treatment with omalizumab was associated with reduction in FcɛRI+ and IgE+ basophils and intradermal cells.
Keywords: Omalizumab, Chronic Spontaneous urticaria, mode of action
Introduction
Omalizumab (Xolair®) is a recombinant humanized monoclonal antibody that binds to IgE at its binding site to the high-affinity IgE receptor (FcεRI) [1]. Free IgE levels fall between 89 to 98% over 16 to 24 weeks of therapy. Associated with the decrease in free IgE levels is a down-regulation in the expression of FcεRI receptors on basophils and mast cells [2-4]. In addition, recent data show that omalizumab is able to dissociate pre-bound IgE from mast cells and basophils [5, 6].
Omalizumab is effective in the treatment of chronic spontaneous / idiopathic urticaria (CSU / CIU) [7-13] including in patients who have relapsed after stopping treatment with omalizumab [14]. Omalizumab is approved for the treatment of CSU (in the EU) and CIU (in the USA) patients who remain symptomatic despite H1-antihistamine treatment [15, 16].
Urticaria is characterized by the development of hives, angioedema, or both [17]. Pathogenesis of CSU is not fully understood [17]. It appears to be a mast cell- and basophil-mediated hypersensitivity reaction [18-22]. Histamine and other mediators are released on activation of basophils and mast cells, and these mediators ultimately give rise to the clinical signs and symptoms of CSU. The pathology of CSU lesions resembles that of allergen-mediated late-phase skin reactions, suggesting involvement of FcεRI activation of mast cells and basophils [23]. Antigen cross-linking of IgE bound to FcεRI is a known mediator of basophil and mast cell activation [24]. Also, approximately 45% of CSU patients have histamine-releasing autoantibodies against either FcεRI or IgE [25, 26]; the clinical significance is unclear, but as cross-linking of IgE or FcεRI on mast cell or basophil surface may result in activation and release of inflammatory mediators [27-29], these antibodies may be involved in disease pathogenesis [30, 31]. It has also been suggested that basophils in CSU patients may have distinct alterations in FcεRIα mediated degranulation, independent of any role of autoantibodies [32-34].
Little is known about the effects of omalizumab on basophil numbers and function in CSU. A previous skin biopsy study in patients with allergic rhinitis [4] showed that omalizumab treatment was associated with a decrease in the number of FcεRI+ mast cells in the skin and basophils in the blood, and that this was associated with a reduction in the size of allergen-induced hives in intradermal skin tests. Treatment with omalizumab reduced serum IgE and IgE+ cells in the airway mucosa of patients with allergic asthma [35]. The effects of omalizumab on basophils from the circulation of CSU patients has been described before [36] but little is known about local effects of omalizumab treatment in the skin. Therefore, the current study took biopsies from lesional and non-lesional skin of CSU patients, to evaluate the effects of treatment with omalizumab on basophil numbers and levels of FcεRI+ and IgE+ skin cells in CSU patients compared with that of skin from healthy volunteers.
Methods
This was an exploratory, double-blind, parallel group, randomized, placebo-controlled Phase II study (NCT01599637, EudraCT no. 2011-004216-31; Figure 1). Patients were enrolled at 4 centers in Germany. The study was conducted in accordance with applicable local regulations (including European Directive 2001/20/EC, US Code of Federal Regulations Title 21, and Japanese Ministry of Health, Labor, and Welfare), the International Conference on Harmonization E6 Guideline for Good Clinical Practice, and the ethical principles of the Declaration of Helsinki. The study protocol and all amendments were reviewed by the Independent Ethics Committee (IEC) or Institutional Review Board (IRB) for each center and written informed consent was obtained from study participants before enrollment.
Study design
For CSU patients, the study consisted of a 10-day screening period, during which patient eligibility for study entry was assessed, followed by a 3-day washout period and a 14-day baseline period (Figure 1). During this period, urticaria activity was assessed. In patients who had taken rescue medication due to high disease activity (a short-acting, non-sedating antihistamine), the following day was not counted in order to exclude any carry-over effect. However, if the urticaria activity score (UAS) for that day was 6, the day was counted irrespective of medication use on the previous day. For the calculation of the baseline UAS7 seven individual days were calculated counting backwards, beginning with the last day before randomization. The treatment period was 12 weeks; on Day 1 of the treatment period, patients were randomized in a 2:1 ratio to omalizumab 300 mg or placebo (3 subcutaneous doses at 4-weekly intervals, i.e. at Day 1, Week 4 and Week 8). After stopping the treatment, patients were followed for an additional 8 weeks (follow-up). The total duration of the study per patient was approximately 23 weeks. Skin biopsies were taken from CSU patients at baseline, Week 1, Week 4 (optional), Week 12, and Week 20 (after follow-up) from non-lesional and lesional skin (as available). The optional biopsy at Week 4 was taken from patients who showed response to treatment at that time-point (defined as UAS7 ≤6), while no response (UAS7 ≥16) was seen at Week 1. On dosing days (Day 1 and Day 29), biopsies were taken pre-dose and blood samples were taken for measurement of basophils. The eligibility of the healthy volunteers (HVs) was assessed at a screening visit, then at Day 1 a single skin biopsy was collected and study completion assessments performed.
Study population
The study included patients, aged 18 to 75 years, diagnosed with CSU who remained symptomatic despite H1-antihistamine treatment at approved doses, defined as presence of itch and hives for over 6 weeks prior to baseline, with UAS7 score of at least 16 and itch component of UAS7 of at least 8 during the 14 days prior to randomization. Patients had to have had a CSU diagnosis for at least 6 months, and be on an approved dose of an H1-antihistamine for CSU prior to entering the study.
Exclusion criteria included weight >130 kg or <40 kg at screening; heavy smoking; chronic urticaria other than CSU; diseases with symptoms of urticaria or angioedema (e.g. urticarial vasculitis, urticaria pigmentosa, erythema multiforme, mastocytosis, hereditary or acquired angioedema, lymphoma, leukemia, or generalized cancer); history or presence of atopic dermatitis, bullous pemphigoid, dermatitis herpetiformis, senile pruritus or other skin disease associated with itch; evidence of parasitic infection; any clinically relevant major systemic disease that could potentially complicate interpretation of study results; and inability to comply with study and follow-up procedures. All subjects (both CSU patients and controls) with asthma and atopic dermatitis were excluded. History of clinically significant hypersensitivity to omalizumab or similar drugs, or to local anesthetics, and history of anaphylactic shock were also exclusion criteria.
Patients were not allowed to be using other investigational drugs at the time of randomization (Day 1), or within 30 days or 5 half-lives of enrollment, whichever was longer; could not have had previous treatment with omalizumab; could not be receiving routine doses of systemic or topical corticosteroids, hydroxychloroquine, methotrexate, cyclosporine, cyclophosphamide or any other immunomodulating drug (e.g. interferons) that could alter the response to omalizumab treatment, or IV immunoglobulin G, or plasmapheresis within 30 days prior to ‑2 weeks. Regular oral doxepin use within 6 weeks prior to ‑2 weeks, use of any H2 antihistamine or any leukotriene receptor antagonists (LTRAs) within 7 days prior to ‑2 weeks, or use of any H1 antihistamines at greater than approved doses within 3 days prior to ‑2 weeks were also not permitted. Patients needed to be on stable doses for 4 weeks prior to randomization (Day 1). Loratadine (a short-acting, non-sedating antihistamine), was allowed to be used as a rescue medication on an as needed basis up to 4 times the recommended dose of 10 mg, for angioedema or other reasons specified by the patient. The use of loratadine was documented by the patient in a diary.
Study design.
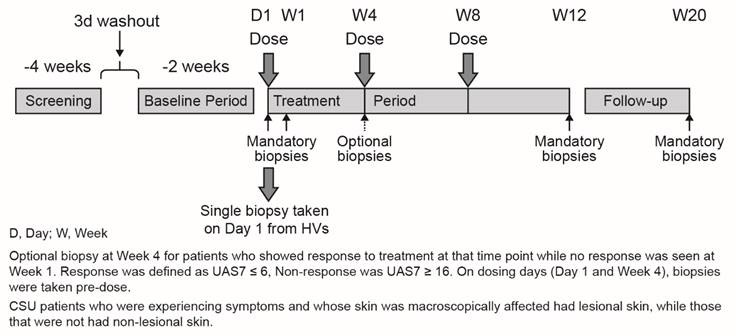
Age and gender-matched healthy volunteers participated in the study to the CSU patients, and were in good health. Exclusion criteria for healthy volunteers were similar to those for CSU patients (except for those criteria specifically related to urticaria and its treatment), with the addition of the use of any prescription drug or over-the-counter medication within 2 weeks prior to Day 1 (paracetamol was acceptable, but its use had to be documented).
Study endpoints
The primary variable was the relative change from baseline to Week 12 in the FcεRI+ and/or IgE+ skin cells, based on skin biopsies collected from CSU patients. Skin biopsy data from healthy volunteers were used as a reference for data from CSU patients.
Assessments
Clinical efficacy and quality of life were assessed using UAS7, patient's and investigator's global assessment of symptoms using a graded Likert scale scoring of symptoms (urticaria lesions [hives] and itch), angioedema, Dermatology Life Quality Index (DLQI), Skindex-29, and Chronic Urticaria Quality of Life Questionnaire (CU-Q2OL) [37].
Patient symptoms of itch and hives were recorded via electronic diary (eDiary) each morning and evening on a daily basis to assess UAS. The composite eDiary-recorded score with numeric severity intensity ratings on a scale of 0-3 (0 = none to 3 = intense/severe) for the number of hives and the intensity of itch. Daily UAS was calculated as the average of the morning and evening scores; UAS7 is the sum of the daily UAS scores over 7 days. Response to treatment was defined as a 90% reduction from baseline in UAS7, a cut-off previously used to illustrate complete response in a retreatment study [14].
Safety was assessed in terms of the frequency and severity of adverse events (AEs), vital signs, ECG evaluations, and clinical laboratory measurements. Serum omalizumab concentrations were evaluated using a validated ELISA method with an anticipated Lower Limit of Quantification (LLOQ) of 28 ng/mL.
Measurement of basophils
Blood samples were taken and incubated with antibodies (Lineage cocktail-FITC [Becton Dickinson], CD203c/PerCPCy5.5 [Biolegend], IgE-PE [Biolegend] and FcεR1-APC [eBioscience]) at RT, fixed with 1% paraformaldehyde in phosphate buffered saline (PBS) and the samples stored frozen at -80°C before shipment on dry ice to a central processing laboratory. Thawed samples were processed as follows: after centrifugation for 7 min at 500g at RT, the pellets were washed and aspirated twice, before being resuspended in 200μl 1% paraformaldehyde in PBS. They were then analyzed within 4 hours by fluorescent antibody cell sorting (FACS) performed on a FACS-Canto II (Becton Dickinson, CA, USA) using the FACSDiva software after adjustment with antibodies bound to compensation particles. Mean values and the coefficient of variation (CV) were calculated and a CV <30% was acceptable.
Analysis of IgE
Blood samples were collected into additive free tubes and allowed to clot over a minimum of 30 min at room temperature (RT). Serum was collected after centrifugation at approximately 3500 rpm for 10 min. Serum samples were split into 2 aliquots and stored at -20°C until analysis at the central laboratory. Serum levels of free IgE and total IgE (i.e. the sum of free and bound IgE) were determined by specific immunoassays, as described previously [38].
Biopsies
Patients provided biopsies from non-lesional and lesional skin (as available). An optional biopsy could be performed at Week 4; this was targeted at slower responding patients and therefore the numbers of biopsies collected at this time-point depended on response to treatment. Skin biopsies with a minimum diameter of 6 mm were taken at each scheduled time-point. Baseline biopsies were taken from one location with lesional skin and from one with non-lesional skin. Following treatment, biopsies were taken at locations close to the locations chosen at baseline. Biopsy samples were stored at ‑80°C until sectioned.
Histopathology procedures
Biopsy samples were examined using immunohistochemistry techniques. Samples from skin biopsies were sectioned (5 µm) with a cryostat at approximately -21°C and placed on glass slides. After fixing with acetone (-20°C for 10 min), sections were dried and kept at -20°C. Staining (IHC, Giemsa, or H&E) was carried out on thawed samples.
Immunohistochemical staining with alkaline phosphatase (AP) was performed to analyze FcεRI and IgE. Samples were thawed and blocked sequentially with biotin and avidin for 15 mins at RT, followed by 2% fetal calf serum (FCS) in Tris-buffered saline (TBS) for 20 mins at RT. Samples were then incubated with specific primary antibodies, diluted in TBS + 1% FCS for 1 hour at RT: mouse anti-CD117 (BD 555713), diluted 1:400; mouse anti-CD11c (BD 550375) diluted 1:400; mouse anti-CD8 (DakoM7103) diluted 1:150; mouse anti CD4 (Dako M7310) diluted 1:200; mouse anti-CD3 (Dako (M7254) diluted 1:400; mouse anti FCεR1 (eBioscience 14-5899) diluted 1:400; mouse anti-tryptase (Dako M7052) diluted 1:500; or rabbit anti-IgE (Dako A0094) diluted 1:5000. Negative control staining was carried out using the same concentration of IgG1 (Dako X0931), IgG2bk (eBioscience 14-4732) or rabbit IgG (Dako X0936).
Samples were then incubated with a biotinylated secondary antibody (Link Antibody ready to use, Dako REAL Detection System K50050) for 15 mins at RT, then with streptavidin-alkaline phosphatase (Dako REAL Detection System K5005) for 15 mins at RT, then with levamisole for 15 mins at RT before being counterstained with hematoxylin for 5 seconds at RT and mounted in aqueous solution.
Hematoxylin and eosin staining was carried out on cryosections using Papanicolaous Solution 1a Harris' hematoxylin solution (Merck # 1.09253.2500) and Eosin-Phloxin Solution (Hollborn, 1 L, # 601017). Samples were mounted in Clarion environmentally safe permanent mounting media.
Alkaline Giemsa-Staining was carried out on cryosections using Giemsa Azur Eosin-Methylenblau 1:10 with 2% sodium borate solution before mounting with Clarion environmentally safe permanent mounting media.
Skin biopsy samples were scored on an average number of positive cells from 5 visual fields.
Data analyses
Analyses of cellular data were based on the pharmacodynamic (PD) analysis population, which included all subjects who received at least one dose of study drug and had evaluable PD data. The primary variable was the relative change from baseline in FcεRI+ and/or IgE+ skin cells, based on skin biopsies collected from patients with CSU after 12 weeks of treatment relative to placebo. A two-tailed two-sample t-test was used to compare groups that employed a 5% significance level.
Correlation between UAS7 and the FcεRI- and IgE-positive skin cells for baseline values and for the change from baseline to Day 85 was assessed using Spearman's and Pearson's correlation analysis. All results are given as two-tailed p-values, and p<0.05 was considered statistically significant.
All efficacy analyses were performed on the intent-to-treat (ITT) population which included all randomized patients who received at least one dose of study drug and had post-randomization efficacy data. The treatment groups were based on the treatment assigned at randomization. For efficacy endpoints, descriptive statistics were provided by treatment group and time-point. UAS7 was also modeled using a repeated measures ANCOVA model with baseline UAS7 score as a covariate, and treatment, time, and treatment by time interaction as fixed factors.
All subjects who received at least one dose of study drug were included in the safety analysis.
Results
Disposition of participants
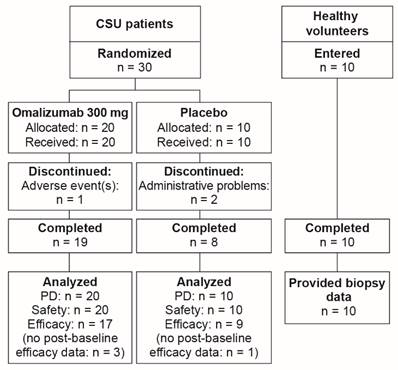
Thirty patients were randomized to treatment, 20 in the omalizumab group and 10 in the placebo group (Figure 2). One patient receiving omalizumab discontinued prematurely (due to an AE), as did two patients in the placebo group (due to administrative problems). Data were analyzed from 17 omalizumab-treated patients and 9 patients in the placebo group. All 10 healthy volunteers who entered the study completed their single study visit.
Participant flow.
Participant demographics and baseline characteristics
The omalizumab and placebo groups of CSU patients were generally well matched for demographic characteristics. All patients were Caucasian and 87% were female. The mean age was 38.7 years, with a range of 23 to 63 years. The mean BMI was 27.7 kg/m2. There were four subjects with reported allergies in the omalizumab-treated group and one subject in the placebo-treated group.
Of the 10 healthy control subjects, all were Caucasian and female, with a mean age of 36.4 years (range 24 to 51 years) and mean BMI of 24.2 kg/m2.
UAS7 scores at baseline were comparable between the groups. Mean (SD) baseline serum total IgE concentrations were 1037 (2474) ng/mL in the omalizumab 300 mg group and 442 (581) ng/mL in the placebo group (Table 1).
Omalizumab reduces CSU activity
Decreases in UAS7 after omalizumab treatment initiation were rapid (with separation of the curves at Day 3) and sustained (Figure 3). At Week 12, the LS mean difference in UAS7 between treatment groups was -14.82 (p=0.0027).
More than 50% of patients in the omalizumab group were UAS7 responders at Week 12 (Figure 4). About half of these had become responders by the end of Week 2. In contrast, <15% of patients receiving placebo had improved by Week 12, without any change occurring by Week 2.
The DLQI overall score, Skindex-29 score and CU-Q2OL overall score all showed larger decreases from baseline in the omalizumab group than the placebo group. Mean (SD) scores at Week 12 are shown in Table 2. In addition, physicians' and patients' global assessment scores, itch, hives scores as well as loratadine use showed statistically significant differences between omalizumab and placebo-treated patients at Week 12 (Table 2).
Demographic summary.
| Omalizumab 300 mg | Placebo | Healthy volunteers | |
|---|---|---|---|
| N=20 | N=10 | N=10 | |
| Age (years) | 37.5 (11.0) | 41.1 (8.0) | 36.4 (9.7) |
| Height (cm) | 171.4 (7.3) | 172.1 (10.8) | 166.4 (6.3) |
| Weight (kg) | 78.5 (16.0) | 87.4 (21.6) | 66.9 (13.4) |
| Female - n (%) | 18 (90) | 8 (80) | 10 (100) |
| Race Caucasian - n (%) | 20 (100) | 10 (100) | 10 (100) |
| BMI (kg/m2) | 26.8 (5.8) | 29.3 (5.6) | 24.2 (5.3) |
| Baseline IgE (ng/ml) | 1037 (2474) | 442 (581) | N/A |
| Baseline UAS7 | 32.2 (8.0) | 31.6 (7.7) | N/A |
Data are provided as mean (Standard deviation) unless otherwise indicated
Age was calculated from date of screening and date of birth
Weight and height were taken from screening vital signs evaluations
BMI, Body Mass Index , UAS, Urticaria Activity Score , UAS7, the sum of the daily UAS scores over 7 days, IgE, Immunoglobulin E
Longitudinal UAS7 modulation by treatment. Mean (SEM) UAS7 by treatment and week over the treatment and follow-up period (A) or by UAS per day during the first week of the treatment period (B).
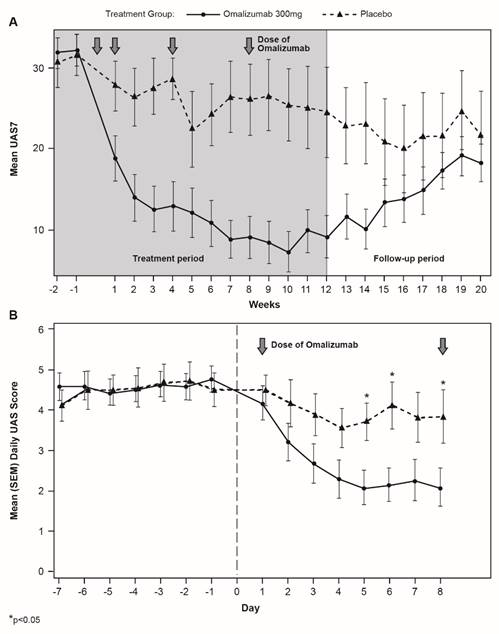
Responders to treatment. Proportion of patients with a defined first occurrence of UAS7 response, defined as a greater than 90% reduction from baseline in UAS7 score (UAS7 is the sum of the daily UAS scores over 7 consecutive days).
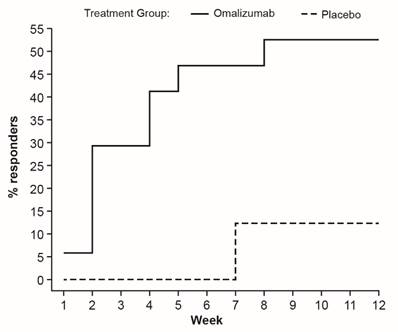
Omalizumab increases total serum IgE levels
Total serum IgE levels are known to increase 3- to 5-fold after omalizumab administration due to formation of IgE-anti-IgE complexes and altered rates of clearance. In this study, total IgE serum levels increased after the first treatment and remained elevated up to Week 12. The increase largely occurred by Day 8. No change was observed in placebo-treated subjects and in these subjects total IgE equals free IgE (Figure 5A).
In omalizumab-treated subjects, the free IgE levels decreased after the first treatment and remained suppressed until Week 12 (Figure 5B). Virtually all of the decrease occurred during the first 48 hours following treatment.
Clinical efficacy data at Week 12.
| Efficacy analysis set | Mean (SD) change from baseline at Week 12 | ||
|---|---|---|---|
| Omalizumab (n=17) | Placebo (n=8) | P-value | |
| Itch assessment score | -11.4 (6.53) | -3.8 (6.63) | 0.0127 |
| Hives assessment score | -11.6 (7.29) | -3.8 (7.72) | 0.0223 |
| Loratadine use (days/week) | -1.5 (1.91) | 1.3 (3.41) | 0.0143 |
| Mean (SD) at Week 12 | |||
| Omalizumab | Placebo (n=8) | P-value | |
| DLQI overall score | 3.8 (6.59), n=16 | 14.6 (10.77) | 0.0058 |
| Skindex-29 score | 6.17 (7.12), n=16 | 22.63 (10.28) | 0.0001 |
| CU-Q2OL overall score | 14.51 (22.32), n=17 | 53.53 (29.82) | 0.0013 |
| Physician's Global Assessment | 0.8 (1.01), n=17 | 2.0 (1.31) | 0.0191 |
| Patient's Global Assessment | 0.9 (1.05), n=17 | 1.9 (0.99) | 0.0336 |
| DLQI, Disability Life Quality Index; CU-Q2OL, Chronic Urticaria Quality of Life Questionnaire | |||
Total IgE (A) and Free IgE (B) serum levels shown as mean (SEM). For serum free IgE at Day 1 (baseline), total IgE data was used. D, day; W, week
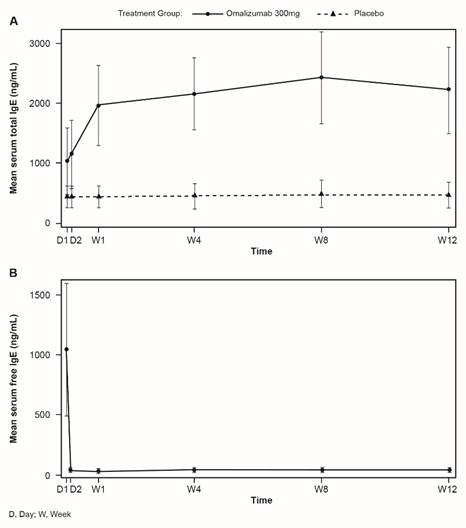
Omalizumab increases peripheral blood basophils and reduces basophil FcεRI and IgE expression
Mean levels of basophils are low in untreated CSU patients. In patients treated with omalizumab there was a non-statistically significant increase in peripheral blood basophils over the treatment period compared with placebo, mostly occurring by Week 2, but basophil levels returned to patients' baseline values after the follow-up period (Figure 6A).
Omalizumab treatment reduced FcεRI and IgE expression on peripheral blood basophils early in the treatment period (by Week 1) and the reduction was sustained through to Week 12 (Figure 6B and C). These values started to return towards baseline, but did not reach baseline values during the follow-up period.
Changes in peripheral blood basophils during omalizumab treatment (mean, SEM). A: Peripheral blood basophils. B: FcεRI expression. C: IgE expression. (* p<0.05, **p<0.01, and ***p<0.001; NS, not significant).). D, day; W, week
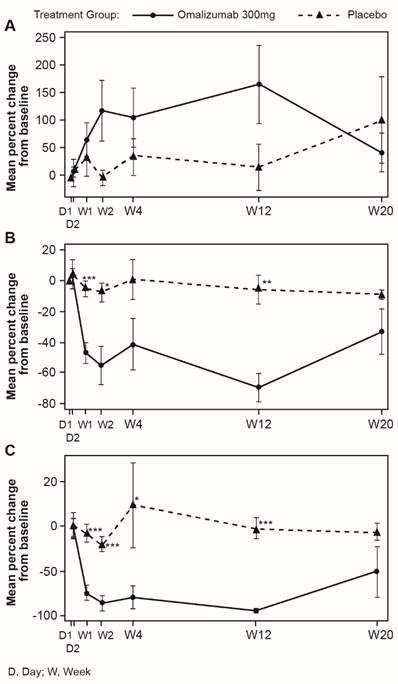
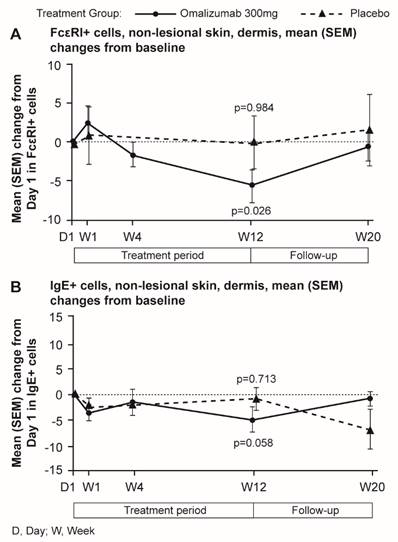
Omalizumab treatment normalized levels of FcεRI+ and IgE+ skin cells
Mean levels of FcεRI and IgE expressing cells were higher in untreated CSU patients compared with HVs. Omalizumab, but not placebo, reduced these levels to those seen in HVs. In patients treated with omalizumab, mean levels of FcɛRI+ cells were statistically significantly decreased at Week 12 compared with baseline (Day 1) in non-lesional skin (14.7 vs. 14.2 in HVs per visual field). Similar changes were observed for lesional skin (data not shown). No statistically significant changes in mean FcɛRI+ cell levels occurred in the placebo group (Figure 7).
Similarly, mean levels of IgE+ cells in the dermis of the omalizumab-treated patients were reduced to levels comparable with those of HVs at baseline. Decreases from baseline to Week 12 in mean levels of IgE+ cells were observed in patients treated with omalizumab in dermis of non-lesional skin (9.80 vs. 8.76 in HV per visual field), (Figure 7). Little difference was observed for lesional skin (data not shown).
There was a good correlation of baseline UAS7 scores and FcεRI-positive skin cells at baseline in lesional and non-lesional skin and the changes from baseline for these two parameters correlated for the non-lesional skin. No correlations were observed between UAS7 and IgE-positive skin cells at baseline but changes in UAS7 correlated with changes in IgE-positive skin cells after treatment in non-lesional skin as shown by Spearman correlation coefficient (Table 3).
Correlation between UAS7 and FcεRI-positive skin cells at baseline.
| Skin sample | N | Pearson Correlation (p-value) | Spearman Correlation (p-value) |
|---|---|---|---|
| Correlation between UAS7 at baseline and FcεRI-positive skin cells at baseline. | |||
| Lesional skin | 16 | 0.56 (0.02) | 0.60 (0.01) |
| Non-lesional skin | 23 | 0.42 (0.04) | 0.41 (0.05) |
| Correlation between change from baseline to Day 85 for UAS7 and change from baseline to Day 85 in FcεRI-positive skin cells | |||
| Lesional skin | 16 | 0.33 (0.2) | 0.24 (0.4) |
| Non-lesional skin | 23 | 0.44 (0.04) | 0.38 (0.07) |
| Correlation between UAS7 at baseline and IgE-positive skin cells at baseline | |||
| Lesional skin | 16 | 0.33 (0.2) | 0.22 (0.4) |
| Non-lesional skin | 23 | 0.39 (0.07) | 0.32 (0.1) |
| Correlation between change from baseline to Day 85 for UAS7 and change from baseline to Day 85 in IgE-positive skin cells | |||
| Lesional skin | 16 | 0.19 (0.5) | 0.15 (0.6) |
| Non-lesional skin | 23 | 0.38 (0.08) | 0.45 (0.03) |
Mean (SEM) changes from baseline in FcεRI+ and IgE+ cells per visual field in biopsy samples of dermis from CSU patients. A FcεRI+ cells, non-lesional skin. Baseline values, mean (SD): omalizumab 18.91 (9.42); placebo 20.48 (5.54). B IgE+ cells, non-lesional skin. Baseline values, mean (SD): omalizumab 13.82 (10.21); placebo 16.59 (9.35). D, day; W, week
Omalizumab is well tolerated
Omalizumab was generally well-tolerated in this study. The overall incidence of AEs was slightly higher in the omalizumab group than in the placebo group (85% vs. 70%) and one person in the omalizumab group discontinued due to urticaria, which was considered mild and not considered to be related to study drug. The most frequently reported AEs overall was nasopharyngitis, which occurred at a similar rate in the omalizumab 300 mg and placebo groups. Influenza and urticaria were the next most frequent AEs overall and were reported at the same frequency in each treatment group. The AE profile was not unexpected in the patient population being studied.
Discussion
Omalizumab has been shown to be effective in the treatment of CSU, but its mechanism of action is not fully understood. Omalizumab decreases circulating levels of free IgE in the bloodstream, but how this pharmacodynamic effect leads to the alleviation of the clinical signs and symptoms is not completely clear. In this study, treatment of CSU patients with omalizumab 300 mg for 12 weeks was associated with reduced free IgE, consistent with the effect seen in asthma patients.[39] A statistically significant reduction in levels of FcɛRI+ cells in the dermis of non-lesional and lesional skin was observed and these results were temporally associated with the plateau of the improvement in clinical response. Reduction in FcɛRI+ cells was also observed in a previous study of skin biopsies from patients with allergic rhinitis [4], and would be expected as FcεRI+ cell density is dependent on free IgE, which decreases during omalizumab treatment. IgE+ cells in the dermis of non-lesional and lesional skin also decreased following omalizumab treatment although this decrease was not statistically significant. The decrease in IgE+ cells was also expected, as omalizumab binds to free IgE and prevents it binding to FcεRI. Subsequently, FcεRI are down-regulated [5]. Levels of FcεRI+ and IgE+ cells in omalizumab-treated patients at Week 12 had reduced to levels of those seen in healthy subjects at baseline. Overall, these results demonstrate that the systemic effects of omalizumab lead to local changes in the skin and there seems to be no difference in FceRI+ and IgE levels between affected (lesional) and unaffected (non-lesional) skin.
While the mode of action of omalizumab in CSU patients is not fully understood, there are a few key hypotheses. One is that the density of IgE receptors at the surface of mast cells and basophils is proportional to the plasma-free IgE level [40]. Omalizumab binds to the same region on IgE as FcεRI [41], reducing free IgE to near undetectable levels. This should therefore down-regulate the FcεRI receptors so that e.g. potentially relevant IgG autoantibodies cannot cross-link FcεRI [42]. Cell activation would be suppressed, as would all the subsequent inflammatory processes. As a consequence, the frequency and severity of symptoms of CSU should be markedly diminished. This would account for a therapeutic effect of omalizumab in the approximately 30% of CSU patients with such autoantibodies. However, in patients without detectable autoantibodies, IgE and FcεRI seem to play an important role as well, as demonstrated by the efficacy of omalizumab in this population [43].
It has also been suggested that down-regulation of FcεRI may be accompanied by an increase in the threshold above which degranulation of mast cells is triggered [44, 45]. This may be an independent mechanism of action, unrelated to prevention of cross-linkage of surface receptors. For degranulation to occur, a threshold number of receptors would need to be activated. The fall in free IgE after omalizumab treatment (both in the circulation and in tissue) and the reduction in the receptor density might therefore increase the threshold for degranulation to a much higher set point. Therefore, these two mechanisms may complement each other.
The efficacy of omalizumab in this study was similar to that observed in Phase III studies [9, 10]. Statistically significant and clinically meaningful reductions in UAS7 scores were observed over 12 weeks of treatment, with a mean decrease relative to placebo of -14.82 at Week 12. UAS7 decreased rapidly, with changes evident by one week after the first dose. Defining response to treatment as a 90% reduction in UAS7 from baseline, more than half of patients responded during the study, in line with that reported in key clinical studies of omalizumab [46]. Other clinical endpoints also showed improvements, including the physicians' and patients' global assessment scores, itch and hives assessment scores, percentage of angioedema-free days and use of rescue medication. Quality of life assessments (CU-Q2OL overall score, DLQI overall score and Skindex-29 score) also showed improvements. The observed correlations of FcεRI-positive skin cells with UAS7 scores point to the relevance of these cells in CSU and further supports the observation that reducing these cells is linked to the mode of action and clinical efficacy of omalizumab.
Omalizumab decreases serum free IgE levels, and is associated with decreases in FcεRI+ cells and IgE+ cells in lesional and non-lesional skin (dermis) of patients with CSU. These results support the hypothesis that one mode of action of omalizumab in CSU involves reduction of free IgE, resulting in a down-regulation of surface IgE receptors, and subsequent reduction in downstream signaling via the FcεRI pathway [42]. However, the clinical response to omalizumab defined e.g. by UAS7 change seems to occur much faster than the histological changes in the skin. Therefore, further mechanisms might be involved in the omalizumab mode of action. Interestingly, the reduction of FcεRI and IgE positive peripheral basophils was much quicker and more closely reflected by the clinical response. This might be the result of an exchange of cells between different compartments (e.g. skin and blood). Importantly, changes in basophil parameters after omalizumab treatment were quick in onset like the decrease of free IgE but they were not indicative of clinical response as there was no correlation between basophil parameters and UAS7 (data not shown). Further investigations are required to determine the role and observed changes of peripheral basophils in CSU.
In conclusion, omalizumab was effective and well-tolerated in the treatment of CSU patients in this study, consistent with the results of earlier Phase II and III studies. While the results of this exploratory study do require verification and extension in a larger and appropriately powered investigation, the data suggest that the decrease in free IgE, resulting from omalizumab treatment, may lead to a reduction in FcεRI+ cells and IgE+ cells in the dermis (to levels seen in healthy subjects). In addition, the changes observed in omalizumab-treated patients in peripheral blood basophils, in terms of basophil numbers, FcεRI expression and responsiveness of basophils to activating stimulation by anti-IgE, may also be involved in the therapeutic effect of omalizumab. This is the first report demonstrating that the clinical effect of omalizumab correlates with histopathological markers.
Acknowledgements
Additional analyses of the data were performed by Fan Yang and Yaritza Ruiz (Novartis Pharma AG). The authors thank Southdown and Tina Patrick, Novartis Ireland Ltd. for providing medical writing and editorial support, which was supported by Novartis Pharma AG, Basel, Switzerland in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Competing interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf. Marcus Maurer and Martin Metz have received a research grant to their institution from Novartis; Marcus Maurer has been a paid consultant for Novartis and Genentech, received grants from Novartis, and been paid by Novartis for lectures and development of educational presentations; Andrea Bauer has been paid by Novartis for educational lectures and has been a member of the Novartis urticaria board; Randolf Brehler has received a research grant to the institution and has been paid for lectures and consultancy; Petra Staubach has been a paid consultant for Novartis: she has received travel support and received payment to her institution as the study centre, research grants, and payments for lectures and expert testimony; Janine Gericke is currently employed by Novartis Pharma GmbH; her contribution to the work in this publication was performed while she was employed by Charité - Universitätsmedizin Berlin, where she received research grants from Novartis; Panayiotis Georgiou contributed to this work while employed at Novartis; he is now employed by Clovis Oncology, in which he holds stock options, and he also holds shares in Glaxo SmithKline; Rainer Hillenbrand, Joanna Ashton-Chess, Philip Jarvis, Veit Erpenbeck and Michael Kangas have received no support from any organisation for the submitted work, have had no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years and are employees of Novartis.
References
1. Omalizumab. anti-IgE monoclonal antibody E25, E25, humanised anti-IgE MAb, IGE 025, monoclonal antibody E25, Olizumab, Xolair, rhuMAb-E25. BioDrugs. 2002;16:380-6
2. Hill DA, Siracusa MC, Ruymann KR, Tait Wojno ED, Artis D, Spergel JM. Omalizumab therapy is associated with reduced circulating basophil populations in asthmatic children. Allergy. 2014;69:674-7
3. Lin H, Boesel KM, Griffith DT, Prussin C, Foster B, Romero FA. et al. Omalizumab rapidly decreases nasal allergic response and FcεRI on basophils. J Allergy Clin Immunol. 2004;113:297-302
4. Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;114:527-30
5. Serrano-Candelas E, Martinez-Aranguren R, Valero A, Bartra J, Gastaminza G, Goikoetxea MJ. et al. Comparable actions of omalizumab on mast cells and basophils. Clin Exp Allergy. 2016;46:92-102
6. Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P. et al. Accelerated dissociation of IgE-FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol. 2014;133:1709-19.e8
7. Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A. et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567-73.e1
8. Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S. et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202-9 e5
9. Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A. et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924-35
10. Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E. et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101-9
11. Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bulbul Baskan E, Bradley MS. et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol. 2015;135:67-75
12. Staubach P, Metz M, Chapman-Rothe N, Sieder C, Brautigam M, Canvin J. et al. Effect of omalizumab on angioedema in H -antihistamine resistant chronic spontaneous urticaria patients: results from X-ACT, a randomised controlled trial. Allergy. 2016;71:1135-44
13. Zhao ZT, Ji CM, Yu WJ, Meng L, Hawro T, Wei JF. et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137(6):1742-1750
14. Metz M, Ohanyan T, Church MK, Maurer M. Retreatment with omalizumab results in rapid remission in chronic spontaneous and inducible urticaria. JAMA Dermatol. 2014;150:288-90
15. Omalizumab Summary of Product Characteristics. 2015 European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000606/WC500057298.pdf
16. XOLAIR Prescribing Information. 2014 Genentech USA I. https://www.gene.com/download/pdf/xolair_prescribing.pdf
17. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW. et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868-87
18. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Gimenez-Arnau A. et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417-26
19. Elias J, Boss E, Kaplan AP. Studies of the cellular infiltrate of chronic idiopathic urticaria: prominence of T-lymphocytes, monocytes, and mast cells. J Allergy Clin Immunol. 1986;78:914-8
20. Natbony SF, Phillips ME, Elias JM, Godfrey HP, Kaplan AP. Histologic studies of chronic idiopathic urticaria. J Allergy Clin Immunol. 1983;71:177-83
21. Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM. et al. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: comparison of patients with and without anti-FcεRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103:484-93
22. Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109:694-700
23. Grattan CE, Boon AP, Eady RA, Winkelmann RK. The pathology of the autologous serum skin test response in chronic urticaria resembles IgE-mediated late-phase reactions. Int Arch Allergy Appl Immunol. 1990;93:198-204
24. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73-80
25. Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U. et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36
26. Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599-604
27. Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777-87
28. Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-72
29. Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-11
30. Kikuchi Y, Kaplan AP. Mechanisms of autoimmune activation of basophils in chronic urticaria. J Allergy Clin Immunol. 2001;107:1056-62
31. Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813-9
32. Vonakis BM, Saini SS. New concepts in chronic urticaria. Curr Opin Immunol. 2008;20:709-16
33. Vonakis BM, Vasagar K, Gibbons SP Jr, Gober L, Sterba PM, Chang H. et al. Basophil FcεRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol. 2007;119:441-8
34. Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956-63
35. Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF. et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583-93
36. Saavedra MC, Sur S. Down regulation of the high-affinity IgE receptor associated with successful treatment of chronic idiopathic urticaria with omalizumab. Clin Mol Allergy. 2011;9:2
37. Mlynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80
38. Zielen S, Lieb A, De La Motte S, Wagner F, de Monchy J, Fuhr R. et al. Omalizumab protects against allergen- induced bronchoconstriction in allergic (immunoglobulin E-mediated) asthma. Int Arch Allergy Immunol. 2013;160:102-10
39. Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al.Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med. 1999;341:1966-73
40. MacGlashan DW Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J. et al. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438-45
41. Hochhaus G, Brookman L, Fox H, Johnson C, Matthews J, Ren S. et al. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin. 2003;19:491-8
42. Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122:569-73
43. Zhao ZT, Ji CM, Yu WJ, Meng L, Hawro T, Wei JF. et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137:1742-50 e4
44. Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135:337-42
45. Asero R, Riboldi P, Tedeschi A, Cugno M, Meroni P. Chronic urticaria: a disease at a crossroad between autoimmunity and coagulation. Autoimmun Rev. 2007;7:71-6
46. Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K. et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol. 2016;137:474-81
Author contact
![]() Corresponding author: Marcus Maurer, MD, Professor of Dermatology and Allergy, Director of Research, Dpt. of Dermatology and Allergy, Allergie-Centrum-Charité, Charité - Universitätsmedizin Berlin, Charitéplatz 1 * 10117 Berlin * Germany. Tel +49 30 450 518 043; Email: Marcus.Maurerde
Corresponding author: Marcus Maurer, MD, Professor of Dermatology and Allergy, Director of Research, Dpt. of Dermatology and Allergy, Allergie-Centrum-Charité, Charité - Universitätsmedizin Berlin, Charitéplatz 1 * 10117 Berlin * Germany. Tel +49 30 450 518 043; Email: Marcus.Maurerde
 Global reach, higher impact
Global reach, higher impact