13.3
Impact Factor
Theranostics 2018; 8(13):3643-3653. doi:10.7150/thno.26021 This issue Cite
Research Paper
Phosphorylation of IRS4 by CK1γ2 promotes its degradation by CHIP through the ubiquitin/lysosome pathway
1. State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China.
2. Department of Pathology, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China.
3. Department of Medical Oncology, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, China.
#These authors contributed equally to this work.
Abstract
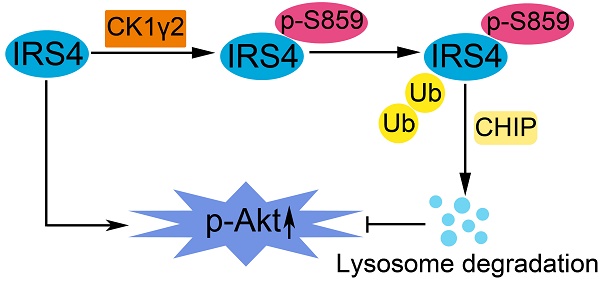
IRS4, a member of the insulin receptor substrate protein family, can induce constitutive PI3K/AKT hyperactivation and cell proliferation even in the absence of insulin or growth factors and promote tumorigenesis, but its regulation has only been explored at the transcriptional level.
Methods: Scansite was used to predict the potential protein kinases that may regulate the functions of IRS4, and mass spectrometry was used to identify the E3 ligase for IRS4. The protein interaction was carried out by immunoprecipitation, and protein stability was measured by cycloheximide treatment. In vitro kinase assay was used to determine the phosphorylation of IRS4 by casein kinase 1γ2 (CK1γ2). Colony formation assay and xenograft-bearing mice were employed to assess the cancer cell growth in vitro and in vivo, respectively. Immunohistochemistry was performed to examine protein levels of both IRS4 and CK1γ2 in osteosarcoma specimens and their relationship was evaluated by χ2 test. Two-tailed Student's t-test or the Mann-Whitney U test were used to compare the differences between subgroups.
Results: IRS4 was phosphorylated at Ser859 by CK1γ2 in vitro and in vivo, which promoted the polyubiquitination and degradation of IRS4 through the ubiquitin/lysosome pathway by the carboxyl terminus of Hsc70-interacting protein(CHIP). Using osteosarcoma cell lines, the ectopic nonphosphorylated mutant of IRS4 by CK1γ2 triggered higher level of p-Akt and displayed faster cell proliferation and cancer growth in vitro and in nude mice. In addition, a negative correlation in protein levels between CK1γ2 and IRS4 was observed in osteosarcoma cell lines and tissue samples.
Conclusions: IRS4, as a new substrate of CHIP, is negatively regulated by CK1γ2 at the posttranslational level, and specific CK1γ2 agonists may be a potentially effective strategy for treating patients with osteosarcoma.
Keywords: IRS4, CK1γ2, CHIP, ubiquitin/lysosome, p-Akt.
 Global reach, higher impact
Global reach, higher impact