13.3
Impact Factor
Theranostics 2023; 13(12):4016-4029. doi:10.7150/thno.83495 This issue Cite
Research Paper
OX40L-Armed Oncolytic Virus Boosts T-cell Response and Remodels Tumor Microenvironment for Pancreatic Cancer Treatment
1. State Key Laboratory of Medicinal Chemical Biology and College of life science, Nankai University, Tianjin, China.
2. CNBG-Nankai University Joint Research and Development Center, Tianjin, China.
3. Shanghai Institute for Advanced Immunochemical Studies, ShanghaiTech University, Shanghai, China.
4. Department of Hematology, Tianjin First Central Hospital, Tianjin 300192, China.
Abstract
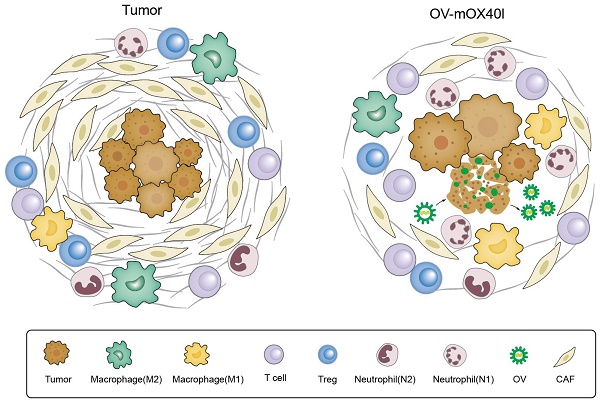
Rationale: The resistance of pancreatic ductal adenocarcinoma (PDAC) to immunotherapies is caused by the immunosuppressive tumor microenvironment (TME) and dense extracellular matrix. Currently, the efficacy of an isolated strategy targeting stromal desmoplasia or immune cells has been met with limited success in the treatment of pancreatic cancer. Oncolytic virus (OV) therapy can remodel the TME and damage tumor cells either by directly killing them or by enhancing the anti-tumor immune response, which holds promise for the treatment of PDAC. This study aimed to investigate the therapeutic effect of OX40L-armed OV on PDAC and to elucidate the underlying mechanisms.
Methods: Murine OX40L was inserted into herpes simplex virus-1 (HSV-1) to construct OV-mOX40L. Its expression and function were assessed using reporter cells, cytopathic effect, and immunogenic cell death assays. The efficacy of OV-mOX40L was then evaluated in a KPC syngeneic mouse model. Tumor-infiltrating immune and stromal cells were analyzed using flow cytometry and single-cell RNA sequencing to gain insight into the mechanisms of oncolytic virotherapy.
Results: OV-mOX40L treatment delayed tumor growth in KPC tumor-bearing C57BL/6 mice. It also boosted the tumor-infiltrating CD4+ T cell response, mitigated cytotoxic T lymphocyte (CTL) exhaustion, and reduced the number of regulatory T cells. The treatment of OV-mOX40L reprogrammed macrophages and neutrophils to a more pro-inflammatory anti-tumor state. In addition, the number of myofibroblastic cancer-associated fibroblasts (CAF) was reduced after treatment. Based on single-cell sequencing analysis, OV-mOX40L, in combination with anti-IL6 and anti-PD-1, significantly extended the lifespan of PDAC mice.
Conclusion: OV-mOX40L converted the immunosuppressive tumor immune microenvironment to a more activated state, remodeled the stromal matrix, and enhanced T cell response. OV-mOX40L significantly prolonged the survival of PDAC mice, either as a monotherapy or in combination with synergistic antibodies. Thus, this study provides a multimodal therapeutic strategy for pancreatic cancer treatment.
Keywords: oncolytic virus, pancreatic cancer, OX40L, tumor immune microenvironment, T cell
 Global reach, higher impact
Global reach, higher impact