13.3
Impact Factor
Theranostics 2023; 13(12):4102-4120. doi:10.7150/thno.79898 This issue Cite
Research Paper
Focused ultrasound mitigates pathology and improves spatial memory in Alzheimer's mice and patients
1. Department of Biomedical Engineering, Columbia University, New York, USA.
2. Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University Irving Medical Center, New York, USA.
3. Department of Neurology, Columbia University Irving Medical Center, New York, USA.
4. UK Dementia Research Institute, University College London, London, UK.
5. Department of Radiology, Columbia University Irving Medical Center, New York, USA.
Abstract
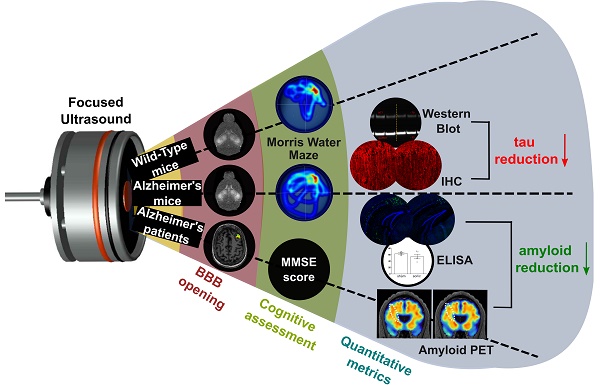
Rationale: Bilateral sonication with focused ultrasound (FUS) in conjunction with microbubbles has been shown to separately reduce amyloid plaques and hyperphosphorylated tau protein in the hippocampal formation and the entorhinal cortex in different mouse models of Alzheimer's disease (AD) without any therapeutic agents. However, the two pathologies are expressed concurrently in human disease. Therefore, the objective of this study is to investigate the effects of repeated bilateral sonications in the presence of both pathologies.
Methods: Herein, we investigate its functional and morphological outcomes on brains bearing both pathologies simultaneously. Eleven transgenic mice of the 3xTg-AD line (14 months old) expressing human amyloid beta and human tau and eleven age-matched wild-type littermates received four weekly bilateral sonications covering the hippocampus followed by working memory testing. Afterwards, immunohistochemistry and immunoassays (western blot and ELISA) were employed to assess any changes in amyloid beta and human tau. Furthermore, we present preliminary data from our clinical trial using a neuronavigation-guided FUS system for sonications in AD patients (NCT04118764).
Results: Interestingly, both wild-type and transgenic animals that received FUS experienced improved working memory and spent significantly more time in the escape platform-quadrant, with wild-type animals spending 43.2% (sham: 37.7%) and transgenic animals spending 35.3% (sham: 31.0%) of the trial in the target quadrant. Furthermore, this behavioral amelioration in the transgenic animals correlated with a 58.3% decrease in the neuronal length affected by tau and a 27.2% reduction in total tau levels. Amyloid plaque population, volume and overall load were also reduced overall. Consistently, preliminary data from a clinical trial involving AD patients showed a 1.8% decrease of amyloid PET signal 3-weeks after treatment in the treated hemisphere compared to baseline.
Conclusion: For the first time, it is shown that bilateral FUS-induced BBB opening significantly and simultaneously ameliorates both coexistent pathologies, which translated to improvements in spatial memory of transgenic animals with complex AD, the human mimicking phenotype. The level of cognitive improvement was significantly correlated with the volume of BBB opening. Non-transgenic animals were also shown to exhibit similar memory amelioration for the first time, indicating that BBB opening results into benefits in the neuronal function regardless of the existence of AD pathology. A potential mechanism of action for the reduction of the both pathologies investigated was the cholesterol metabolism, specifically the LRP1b receptor, which exhibited increased expression levels in transgenic mice following FUS-induced BBB opening. Initial clinical evidence supported that the beta amyloid reduction shown in rodents could be translatable to humans with significant amyloid reduction shown in the treated hemisphere.
Keywords: Alzheimer's Disease, Focused Ultrasound, Blood-Brain Barrier Opening, Amyloid beta and Tau, Drug-free therapy
 Global reach, higher impact
Global reach, higher impact