13.3
Impact Factor
Theranostics 2024; 14(5):1873-1885. doi:10.7150/thno.90627 This issue Cite
Research Paper
Cancer-associated fibroblast spatial heterogeneity and EMILIN1 expression in the tumor microenvironment modulate TGF-β activity and CD8+ T-cell infiltration in breast cancer
1. Department of General Surgical Science, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan.
2. Department of Breast Surgery, International University of Health and Welfare, Chiba, Japan.
3. Institut régional du Cancer de Montpellier (ICM)-Val d'Aurelle, Montpellier, France.
4. Tumor Microenvironment and Resistance to Treatment Lab, INSERM U1194, Montpellier, France.
5. Université de Montpellier, Montpellier, France.
6. Department of Pathology and Diagnostics, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan.
7. Cancer Bioinformatics and Systems Biology Team, INSERM U1194, Montpellier, France.
8. Division of Integrated Oncology Research, Gunma University, Initiative for Advanced Research (GIAR), Maebashi, Gunma, Japan.
Abstract
Rationale: The tumor microenvironment (TME) and its multifaceted interactions with cancer cells are major targets for cancer treatment. Single-cell technologies have brought major insights into the TME, but the resulting complexity often precludes conclusions on function.
Methods: We combined single-cell RNA sequencing and spatial transcriptomic data to explore the relationship between different cancer-associated fibroblast (CAF) populations and immune cell exclusion in breast tumors. The significance of the findings was then evaluated in a cohort of tumors (N=75) from breast cancer patients using immunohistochemistry analysis.
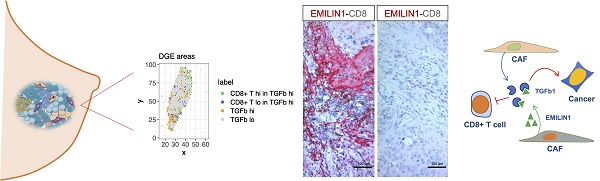
Results: Our data show for the first time the degree of spatial organization of different CAF populations in breast cancer. We found that IL-iCAFs, Detox-iCAFs, and IFNγ-iCAFs tended to cluster together, while Wound-myCAFs, TGFβ-myCAFs, and ECM-myCAFs formed another group that overlapped with elevated TGF-β signaling. Differential gene expression analysis of areas with CD8+ T-cell infiltration/exclusion within the TGF-β signaling-rich zones identified elastin microfibrillar interface protein 1 (EMILIN1) as a top modulated gene. EMILIN1, a TGF-β inhibitor, was upregulated in IFNγ-iCAFs directly modulating TGFβ immunosuppressive function. Histological analysis of 75 breast cancer samples confirmed that high EMILIN1 expression in the tumor margins was related to high CD8+ T-cell infiltration, consistent with our spatial gene expression analysis. High EMILIN1 expression was also associated with better prognosis of patients with breast cancer, underscoring its functional significance for the recruitment of cytotoxic T cells into the tumor area.
Conclusion: Our data show that correlating TGF-β signaling to a CAF subpopulation is not enough because proteins with TGF-β-modulating activity originating from other CAF subpopulations can alter its activity. Therefore, therapeutic targeting should remain focused on biological processes rather than on specific CAF subtypes.
Keywords: spatial transcriptomics, cancer invasion, tumor immunity, CAF subpopulations, patient outcome
 Global reach, higher impact
Global reach, higher impact