13.3
Impact Factor
Theranostics 2015; 5(3):218-226. doi:10.7150/thno.10636 This issue Cite
Research Paper
Quantitative Hepatitis B Core Antibody Level Is a New Predictor for Treatment Response In HBeAg-positive Chronic Hepatitis B Patients Receiving Peginterferon
1. Department of Infectious Diseases, Center for Liver Diseases, Peking University First Hospital, Beijing, China;
2. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, China;
3. Department of Infectious Diseases, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China;
4. Department of Infectious Diseases, the Third Affiliated Hospital of Sun Yet-Sen University, Guangzhou, China;
5. Department of Infectious Diseases, Henan Provincial People's Hospital, Zhengzhou, China;
6. Department of Infectious Diseases, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China;
7. Department of Infectious Diseases, Tang Du Hospital, the Fourth Military Medical University, Xi'an, China;
8. Department of Infectious Diseases, the First Affiliated Hospital of Anhui Medical University, Hefei, China;
9. Department of Infectious Diseases, PLA 81st Hospital, Nanjing, China;
10. Department of Infectious Diseases, the Second Military Medical University Changhai Hospital, Shanghai, China;
11. Liver Research Center, Beijing Friendship Hospital, Capital Medical University, Beijing, China;
12. Department of Infectious Diseases, the First Affiliated Hospital of Guangxi Medical University, Nanning, China;
13. Center of Infectious Diseases, West China Hospital, Sichuan University, Chengdu, China;
14. Department of Infectious Diseases, the Second Affiliated Hospital of Harbin Medical University, Harbin, China;
15. Center for Liver Diseases, the First Affiliated Hospital of Changchun University, Changchun, China;
16. Xiamen Amoytop Biotech Co. Ltd, Xiamen, China.
*These authors contributed equally to this work.
Abstract
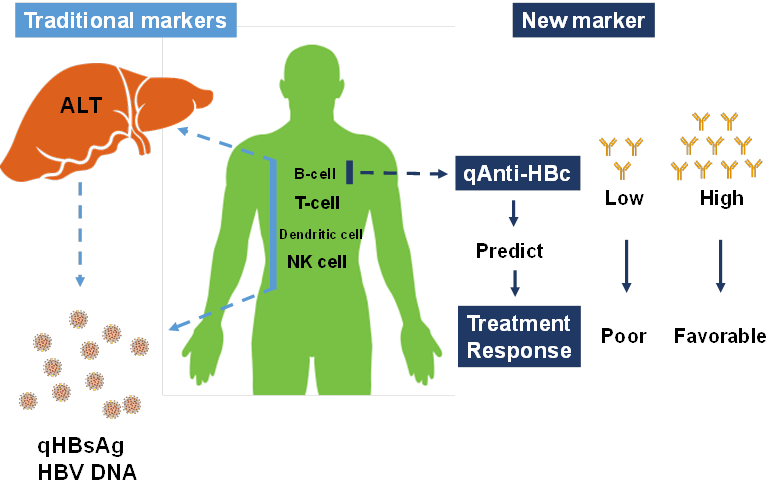
A recent study revealed that quantitative hepatitis B core antibody (qAnti-HBc) level could serve as a novel marker for predicting treatment response. In the present study, we further investigated the predictive value of qAnti-HBc level in HBeAg-positive patients undergoing PEG-IFN therapy. A total of 140 HBeAg-positive patients who underwent PEG-IFN therapy for 48 weeks and follow-up for 24 weeks were enrolled in this study. Serum samples were taken every 12 weeks post-treatment. The predictive value of the baseline qAnti-HBc level for treatment response was evaluated. Patients were further divided into 2 groups according to the baseline qAnti-HBc level, and the response rate was compared. Additionally, the kinetics of the virological and biochemical parameters were analyzed. Patients who achieved response had a significantly higher baseline qAnti-HBc level (serological response [SR], 4.52±0.36 vs. 4.19±0.58, p=0.001; virological response [VR], 4.53±0.35 vs. 4.22±0.57, p=0.005; combined response [CR], 4.50±0.36 vs. 4.22±0.58, p=0.009)). Baseline qAnti-HBc was the only parameter that was independently correlated with SR (p=0.008), VR (p=0.010) and CR(p=0.019). Patients with baseline qAnti-HBc levels ≥30,000 IU/mL had significantly higher response rates, more HBV DNA suppression, and better hepatitis control in PEG-IFN treatment. In conclusion, qAnti-HBc level may be a novel biomarker for predicting treatment response in HBeAg-positive patients receiving PEG-IFN therapy.
Keywords: quantitative anti-HBc, chronic hepatitis B, PEG-IFN treatment, treatment response prediction, pretreatment biomarker.
 Global reach, higher impact
Global reach, higher impact