13.3
Impact Factor
Theranostics 2015; 5(6):609-617. doi:10.7150/thno.11222 This issue Cite
Research Paper
Charge and Hydrophobicity Effects of NIR Fluorophores on Bone-Specific Imaging
1. Division of Hematology/Oncology, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215;
2. Key Laboratory of Structure-based Drug Design and Discovery, Ministry of Education, Shenyang Pharmaceutical University, Shenyang, China, 110016;
3. Advanced Imaging Research Center, The University of Texas Southwestern Medical Center, Dallas, TX 75390;
4. Department of Biomedical Engineering, OHSU Center for Spatial Systems Biology, Knight Cancer Institute, Oregon Health & Science University, Portland, OR 97201.
*These authors contributed equally to this work.
Abstract
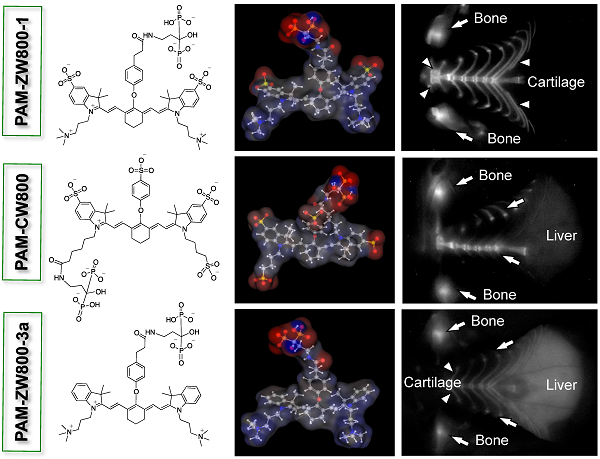
Recent advances in near-infrared (NIR) fluorescence imaging enabled real-time intraoperative detection of bone metastases, bone growth, and tissue microcalcification. Pamidronate (PAM) has been widely used for this purpose because of its high binding affinity toward bone and remarkable therapeutic effects. Herein we describe the development of a series of PAM-conjugated NIR fluorophores that varied in net charges and hydrophobicity, and compared their bone targeting efficiency, biodistribution, and blood clearance. Since the targeting moiety, PAM, is highly negatively charged but small, the overall in vivo bone targeting and biodistribution were mediated by the physicochemical properties of conjugated fluorophores.
Keywords: Near-infrared fluorophores, Bone targeting, Charge and hydrophobicity, Microcalcification, Zwitterionic fluorophores.
 Global reach, higher impact
Global reach, higher impact