13.3
Impact Factor
Theranostics 2015; 5(6):643-655. doi:10.7150/thno.11372 This issue Cite
Research Paper
3D Porous Calcium-Alginate Scaffolds Cell Culture System Improved Human Osteoblast Cell Clusters for Cell Therapy
1. Institute of Biomedical Engineering, College of Medicine and College of Engineering, National Taiwan University, Taipei, Taiwan (R.O.C.).
2. Biomaterials Translational Research Center, China Medical University Hospital, Taichung, Taiwan (R.O.C.).
3. Department of Biomedical Electronics Translational Research Center and Biomimetic Systems Research Center, National Chiao Tung University, Hsinchu, Taiwan (R.O.C.).
4. Department of Orthopedics, College of Medicine, National Taiwan University, Taipei, Taiwan (R.O.C.).
5. Department of Orthopedic Surgery, National Taiwan University Hospital, Taipei, Taiwan (R.O.C.).
6. Department of Orthopedic Surgery, National Taiwan University Hospital, Hsin-Chu Branch, Hsinchu, Taiwan (R.O.C.).
7. Institute of Biomedical Engineering and Nanomedicine (I-BEN), National Health Research Institutes, Miaoli, Taiwan (R.O.C.).
* These authors contributed equally to this work.
Abstract
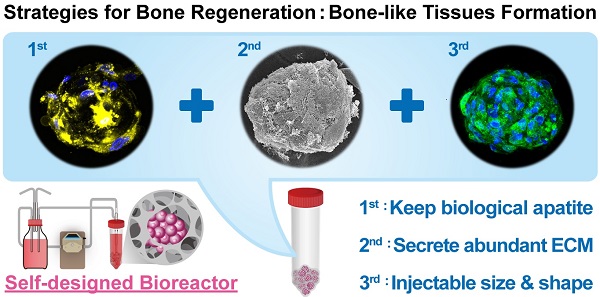
Age-related orthopedic disorders and bone defects have become a critical public health issue, and cell-based therapy is potentially a novel solution for issues surrounding bone tissue engineering and regenerative medicine. Long-term cultures of primary bone cells exhibit phenotypic and functional degeneration; therefore, culturing cells or tissues suitable for clinical use remain a challenge. A platform consisting of human osteoblasts (hOBs), calcium-alginate (Ca-Alginate) scaffolds, and a self-made bioreactor system was established for autologous transplantation of human osteoblast cell clusters. The Ca-Alginate scaffold facilitated the growth and differentiation of human bone cell clusters, and the functionally-closed process bioreactor system supplied the soluble nutrients and osteogenic signals required to maintain the cell viability. This system preserved the proliferative ability of cells and cell viability and up-regulated bone-related gene expression and biological apatite crystals formation. The bone-like tissue generated could be extracted by removal of calcium ions via ethylenediaminetetraacetic acid (EDTA) chelation, and exhibited a size suitable for injection. The described strategy could be used in therapeutic application and opens new avenues for surgical interventions to correct skeletal defects.
Keywords: Three-dimensional culture, Preformed scaffolds, Bone-like tissues, Perfusion, Autografts
 Global reach, higher impact
Global reach, higher impact