13.3
Impact Factor
Theranostics 2015; 5(8):905-918. doi:10.7150/thno.11304 This issue Cite
Research Paper
Erythropoietin Improves the Accumulation and Therapeutic Effects of Carboplatin by Enhancing Tumor Vascularization and Perfusion
1. Experimental Molecular Imaging, Medical Faculty, RWTH Aachen University, Aachen, Germany.
2. Department of Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany.
3. Division of Systems Biology of Signal Transduction, DKFZ-ZMBH Alliance, German Cancer Research Center (DKFZ), Heidelberg, Germany.
4. Translational Lung Research Center (TLRC), Member of the German Center for Lung Research (DZL), Heidelberg, Germany.
5. Roche Pharmaceutical Research and Early Development, Pharmaceutical Sciences, Roche Innovation Center, Penzberg, Germany.
Abstract
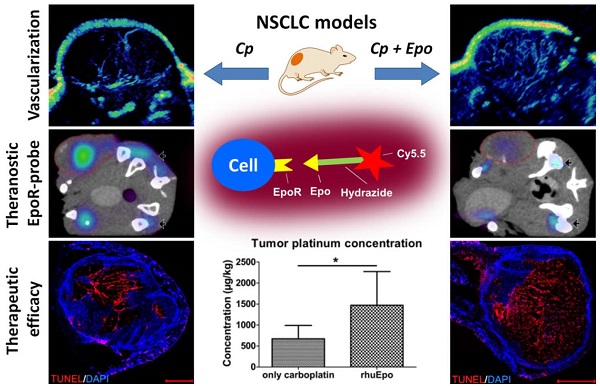
Recombinant human erythropoietin (rhuEpo) is currently under debate for the treatment of chemotherapy-induced anemia due to clinical trials showing adverse effects in Epo-treated patients and the discovery of the erythropoietin-receptor (EpoR) in tumor and endothelial cells. Here, using Epo-Cy5.5 as theranostic near-infrared fluorescent probe we analyzed the effects of rhuEpo as co-medication to carboplatin in non-small-cell-lung-cancer (NSCLC)-xenografts with different tumor cell EpoR-expression (H838 ~8-fold higher than A549). Nude mice bearing subcutaneous A549 and H838 NSCLC-xenografts received either only carboplatin or carboplatin and co-medication of rhuEpo in two different doses. Tumor sizes and relative blood volumes (rBV) were longitudinally measured by 3D-contrast-enhanced ultrasound (3D-US). Tumoral EpoR-levels were determined by combined fluorescence molecular tomography (FMT)/ micro computed tomography (µCT) hybrid imaging. We found that rhuEpo predominantly acted on the tumor endothelium. In both xenografts, rhuEpo co-medication significantly increased vessel densities, diameters and the amount of perfused vessels. Accordingly, rhuEpo induced EpoR-phoshorylation and stimulated proliferation of endothelial cells. However, compared with solely carboplatin-treated tumors, tumor growth was significantly slower in the groups co-medicated with rhuEpo. This is explained by the Epo-mediated vascular remodeling leading to improved drug delivery as obvious by a more than 2-fold higher carboplatin accumulation and significantly enhanced tumor apoptosis. In addition, co-medication of rhuEpo reduced tumor hypoxia and diminished intratumoral EpoR-levels which continuously increased during carboplatin (Cp) -treatment. These findings suggest that co-medication of rhuEpo in well balanced doses can be used to improve the accumulation of anticancer drugs. Doses and indications may be personalized and refined using theranostic EpoR-probes.
Keywords: Angiogenesis, Lung cancer, Chemotherapy, Non-Invasive Imaging, Theranostic Agent, Fluorescence Molecular Tomography
 Global reach, higher impact
Global reach, higher impact