13.3
Impact Factor
Theranostics 2015; 5(10):1098-1114. doi:10.7150/thno.11679 This issue Cite
Research Paper
Direct Imaging of Cerebral Thromboemboli Using Computed Tomography and Fibrin-targeted Gold Nanoparticles
1. Molecular Imaging and Neurovascular Research Laboratory, Dongguk University College of Medicine, Goyang, South Korea;
2. Biomedical Research Institute, Korea Institute of Science and Technology, Seoul, Republic of Korea;
3. Departments of Radiology and Experimental Diagnostic Imaging, University of Texas M. D. Anderson Cancer Center, Houston, TX;
4. Center for Systems Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA;
5. Research Institute of Advanced Materials, Department of Materials Science and Engineering, Seoul National University, Seoul, Republic of Korea.
*These authors contributed equally to this work.
Abstract
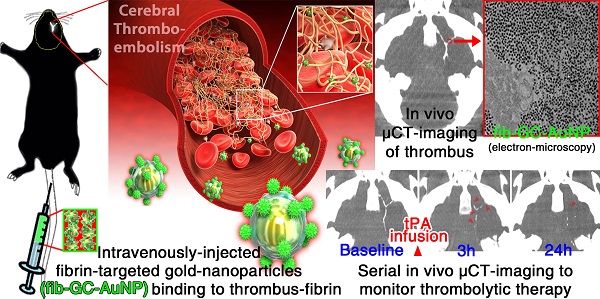
Computed tomography (CT) is the current standard for time-critical decision-making in stroke patients, informing decisions on thrombolytic therapy with tissue plasminogen activator (tPA), which has a narrow therapeutic index. We aimed to develop a CT-based method to directly visualize cerebrovascular thrombi and guide thrombolytic therapy. Glycol-chitosan-coated gold nanoparticles (GC-AuNPs) were synthesized and conjugated to fibrin-targeting peptides, forming fib-GC-AuNP. This targeted imaging agent and non-targeted control agent were characterized in vitro and in vivo in C57Bl/6 mice (n = 107) with FeCl3-induced carotid thrombosis and/or embolic ischemic stroke. Fibrin-binding capacity was superior with fib-GC-AuNPs compared to GC-AuNPs, with thrombi visualized as high density on microCT (mCT). mCT imaging using fib-GC-AuNP allowed the prompt detection and quantification of cerebral thrombi, and monitoring of tPA-mediated thrombolytic effect, which reflected histological stroke outcome. Furthermore, recurrent thrombosis could be diagnosed by mCT without further nanoparticle administration for up to 3 weeks. fib-GC-AuNP-based direct cerebral thrombus imaging greatly enhance the value and information obtainable by regular CT, has multiple uses in basic / translational vascular research, and will likely allow personalized thrombolytic therapy in clinic by a) optimizing tPA-dosing to match thrombus burden, b) enabling the rational triage of patients to more radical therapies such as endovascular clot-retrieval, and c) potentially serving as a theranostic platform for targeted delivery of concurrent thrombolysis.
Keywords: direct thrombus imaging, gold nanoparticles, computed tomography, cerebral infarction, molecular imaging
 Global reach, higher impact
Global reach, higher impact