13.3
Impact Factor
Theranostics 2016; 6(5):698-709. doi:10.7150/thno.14338 This issue Cite
Research Paper
Preliminary Therapy Evaluation of 225Ac-DOTA-c(RGDyK) Demonstrates that Cerenkov Radiation Derived from 225Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumor Visualization
1. Department of Cancer Biology, Wake Forest School of Medicine, Winston-Salem, NC 27157 USA;
2. Department of Biochemistry, Wake Forest School of Medicine, Winston-Salem, NC 27157 USA;
3. Small Animal Imaging Laboratory, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL 33612 USA;
4. Department of Pathology-Section on Comparative Medicine, Wake Forest School of Medicine, Winston-Salem, NC 27157 USA;
5. Department of Cancer Imaging and Metabolism, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL 33612 USA;
6. Department of Neurosurgery, Wake Forest School of Medicine, Winston-Salem, NC 27157 USA;
7. Department of Radiology, Wake Forest School of Medicine, Winston-Salem, NC 27157 USA.
Abstract
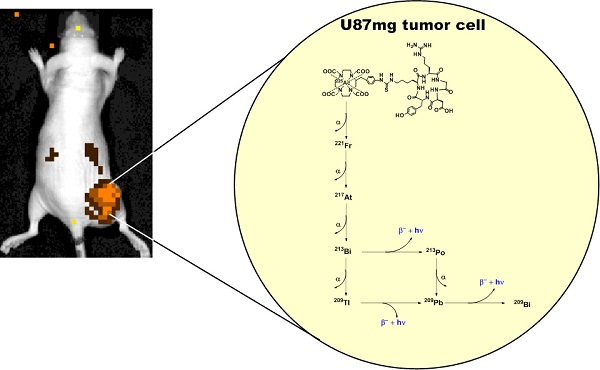
The theranostic potential of 225Ac-based radiopharmaceuticals continues to increase as researchers seek innovative ways to harness the nuclear decay of this radioisotope for therapeutic and imaging applications. This communication describes the evaluation of 225Ac-DOTA-c(RGDyK) in both biodistribution and Cerenkov luminescence imaging (CLI) studies. Initially, La-DOTA-c(RGDyK) was prepared as a non-radioactive surrogate to evaluate methodologies that would contribute to an optimized radiochemical synthetic strategy and estimate the radioactive conjugate's affinity for αvβ3, using surface plasmon resonance spectroscopy. Surface plasmon resonance spectroscopy studies revealed the IC50 and Ki of La-DOTA-c(RGDyK) to be 33 ± 13 nM and 26 ± 11 nM, respectively, and suggest that the complexation of the La3+ ion to the conjugate did not significantly alter integrin binding. Furthermore, use of this surrogate allowed optimization of radiochemical synthesis strategies to prepare 225Ac-DOTA-c(RGDyK) with high radiochemical purity and specific activity similar to other 225Ac-based radiopharmaceuticals. This radiopharmaceutical was highly stable in vitro. In vivo biodistribution studies confirmed the radiotracer's ability to target αvβ3 integrin with specificity; specificity was detected in tumor-bearing animals using Cerenkov luminescence imaging. Furthermore, tumor growth control was achieved using non-toxic doses of the radiopharmaceutical in U87mg tumor-bearing nude mice. To our knowledge, this is the first report to describe the CLI of αvβ3+ tumors in live animals using the daughter products derived from 225Ac decay in situ. This concept holds promise to further enhance development of targeted alpha particle therapy.
Keywords: Actinium-225, Targeted Alpha Particle Therapy, Cerenkov Luminescence Imaging, αvβ3 integrin.
 Global reach, higher impact
Global reach, higher impact