13.3
Impact Factor
Theranostics 2016; 6(6):795-807. doi:10.7150/thno.13725 This issue Cite
Research Paper
Histone Deacetylase Inhibitors Delivery using Nanoparticles with Intrinsic Passive Tumor Targeting Properties for Tumor Therapy
1. Institut de Chimie des Milieux et Matériaux de Poitiers, University of Poitiers, CNRS UMR 7285, 4 rue Michel Brunet TSA 51106, B27, Poitiers. F-86073 Poitiers cedex 9, France
2. Centre de Recherche contre le Cancer Nantes et Angers, University of Nantes, CNRS UMR 6299, Inserm, U892, 8 quai Moncousu, F-44000 Nantes cedex 1, France
3. Laboratoire de Chimie des Polymères Organiques, University of Bordeaux, CNRS UMR 5629, 16 Avenue Pey-Berland, F-33607 Pessac, France
4. Réseau Epigénétique du Cancéropôle Grand Ouest.
# These authors have equally contributed to this work.
* These authors have equally contributed to this work.
Abstract
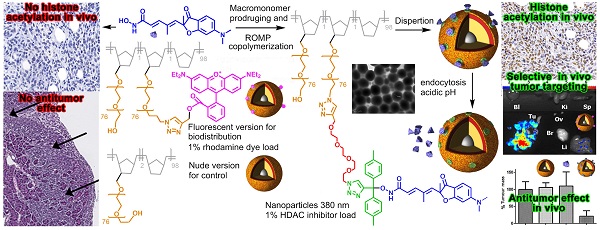
Fast clearance, metabolism and systemic toxicity are major limits for the clinical use of anti-cancer drugs. Histone deacetylase inhibitors (HDACi) present these defects despite displaying promising anti-tumor properties on tumor cells in vitro and in in vivo model of cancers. Specific delivery of anti-cancer drugs into the tumor should improve their clinical benefit by limiting systemic toxicity and by increasing the anti-tumor effect. In this work, we describe a simple and flexible polymeric nanoparticle platform highly targeting the tumor in vivo and triggering impressive tumor weight reduction when functionalized with HDACi. Our nanoparticles were produced by Ring-Opening Metathesis Polymerization of azido-polyethylene oxide-norbornene macromonomers and functionalized using click chemistry. Using an orthotopic model of peritoneal invasive cancer, a highly selective accumulation of the particles in the tumor was obtained. A combination of epigenetic drugs involving a pH-responsive histone deacetylase inhibitor (HDACi) polymer conjugated to these particles gave 80% reduction of tumor weight without toxicity whereas the free HDACi has no effect. Our work demonstrates that the use of a nanovector with theranostic properties leads to an optimized delivery of potent HDACi in tumor and then, to an improvement of their anti-tumor properties in vivo.
Keywords: polymeric nanoparticle, epigenetic, HDAC, cancer, theranostics, peritoneal, mesothelioma.
 Global reach, higher impact
Global reach, higher impact