13.3
Impact Factor
Theranostics 2017; 7(2):295-307. doi:10.7150/thno.16192 This issue Cite
Research Paper
Targeting of Magnetic Nanoparticle-coated Microbubbles to the Vascular Wall Empowers Site-specific Lentiviral Gene Delivery in vivo
1. Walter Brendel Centre of Experimental Medicine, BMC, Ludwig-Maximilians-University, Großhaderner Str. 9, 82152 Planegg, Germany.
2. Institute of Pharmacology and Toxicology, Biomedical Center University of Bonn, Sigmund-Freud-Strasse 25, 53105 Bonn, Germany.
3. Central Institute of Medical Engineering (IMETUM), Technical University Munich, Boltzmannstrasse 11, 85748 Garching, Germany.
4. Institute of Experimental Oncology and Therapy Research, Technical University Munich, Ismaninger Strasse 22, 81675 Munich, Germany.
5. Physikalisch-Technische Bundesanstalt, Abbestrasse 2-12, 10587 Berlin, Germany.
6. Institute of Physiology I, Life&Brain Center, University Clinic Bonn, 53127 Bonn.
7. Medizinische Klinik und Poliklinik I, Klinikum der Universität München, Marchioninistrasse 15, 81377 Munich, Germany.
8. Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Ziemssenstrasse 1, 80336 Munich, Germany.
9. Invasive Cardiology, Starnberg Community Hospital, Osswaldstrasse 1, 82319 Starnberg, Germany.
10. DZHK (German Center for Cardiovascular Research) partner site Munich Heart Alliance, Munich.
11. Munich cluster for systems neurology, (SyNergy) Munich.
Abstract
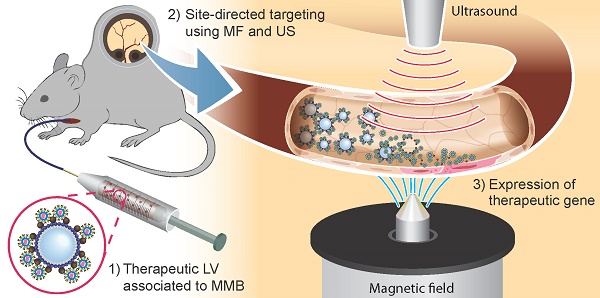
In the field of vascular gene therapy, targeting systems are promising advancements to improve site-specificity of gene delivery. Here, we studied whether incorporation of magnetic nanoparticles (MNP) with different magnetic properties into ultrasound sensitive microbubbles may represent an efficient way to enable gene targeting in the vascular system after systemic application. Thus, we associated novel silicon oxide-coated magnetic nanoparticle containing microbubbles (SO-Mag MMB) with lentiviral particles carrying therapeutic genes and determined their physico-chemical as well as biological properties compared to MMB coated with polyethylenimine-coated magnetic nanoparticles (PEI-Mag MMB). While there were no differences between both MMB types concerning size and lentivirus binding, SO-Mag MMB exhibited superior characteristics regarding magnetic moment, magnetizability as well as transduction efficiency under static and flow conditions in vitro. Focal disruption of lentiviral SO-Mag MMB by ultrasound within isolated vessels exposed to an external magnetic field decisively improved localized VEGF expression in aortic endothelium ex vivo and enhanced the angiogenic response. Using the same system in vivo, we achieved a highly effective, site-specific lentiviral transgene expression in microvessels of the mouse dorsal skin after arterial injection. Thus, we established a novel lentiviral MMB technique, which has great potential towards site-directed vascular gene therapy.
Keywords: lentiviral gene delivery, magnetic targeting, ultrasound, magnetic microbubbles, VEGF, endothelial cells.
 Global reach, higher impact
Global reach, higher impact