13.3
Impact Factor
Theranostics 2017; 7(2):377-389. doi:10.7150/thno.16627 This issue Cite
Research Paper
Anti-angiogenic Nanotherapy Inhibits Airway Remodeling and Hyper-responsiveness of Dust Mite Triggered Asthma in the Brown Norway Rat
1. Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA
2. Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
3. Philips Research Laboratories, Hamburg, Germany
Abstract
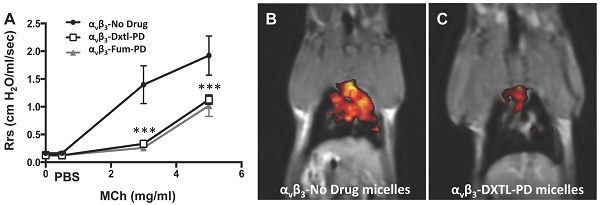
Although angiogenesis is a hallmark feature of asthmatic inflammatory responses, therapeutic anti-angiogenesis interventions have received little attention. Objective: Assess the effectiveness of anti-angiogenic Sn2 lipase-labile prodrugs delivered via αvβ3-micellar nanotherapy to suppress microvascular expansion, bronchial remodeling, and airway hyper-responsiveness in Brown Norway rats exposed to serial house dust mite (HDM) inhalation challenges. Results: Anti-neovascular effectiveness of αvβ3-mixed micelles incorporating docetaxel-prodrug (Dxtl-PD) or fumagillin-prodrug (Fum-PD) were shown to robustly suppress neovascular expansion (p<0.01) in the upper airways/bronchi of HDM rats using simultaneous 19F/1H MR neovascular imaging, which was corroborated by adjunctive fluorescent microscopy. Micelles without a drug payload (αvβ3-No-Drug) served as a carrier-only control. Morphometric measurements of HDM rat airway size (perimeter) and vessel number at 21d revealed classic vascular expansion in control rats but less vascularity (p<0.001) after the anti-angiogenic nanotherapies. CD31 RNA expression independently corroborated the decrease in airway microvasculature. Methacholine (MCh) induced respiratory system resistance (Rrs) was high in the HDM rats receiving αvβ3-No-Drug micelles while αvβ3-Dxtl-PD or αvβ3-Fum-PD micelles markedly and equivalently attenuated airway hyper-responsiveness and improved airway compliance. Total inflammatory BAL cells among HDM challenged rats did not differ with treatment, but αvβ3+ macrophages/monocytes were significantly reduced by both nanotherapies (p<0.001), most notably by the αvβ3-Dxtl-PD micelles. Additionally, αvβ3-Dxtl-PD decreased BAL eosinophil and αvβ3+ CD45+ leukocytes relative to αvβ3-No-Drug micelles, whereas αvβ3-Fum-PD micelles did not. Conclusion: These results demonstrate the potential of targeted anti-angiogenesis nanotherapy to ameliorate the inflammatory hallmarks of asthma in a clinically relevant rodent model.
Keywords: Asthma, Nanomedicine, Prodrug, Angiogenesis, Fluorine MRI, Respiratory function
 Global reach, higher impact
Global reach, higher impact