13.3
Impact Factor
Theranostics 2017; 7(4):974-986. doi:10.7150/thno.17830 This issue Cite
Research Paper
Integration of Receptor Tyrosine Kinases Determines Sensitivity to PI3Kα-selective Inhibitors in Breast Cancer
1. Division of Anti-tumor Pharmacology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China.
2. University of Chinese Academy of Sciences, Beijing, China.
Abstract
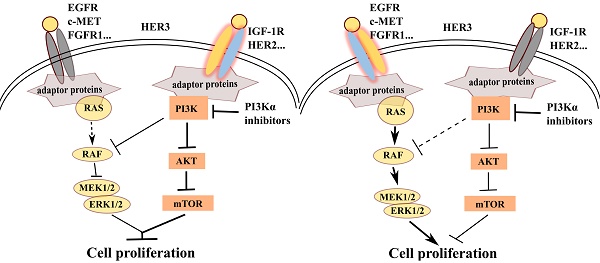
PI3Kα-selective inhibitor BYL719 is currently in phase II/III clinical trial for the treatment of breast cancer, but highly variable response has been observed among patients. We sought to discover predictive biomarker for the efficacy of BYL719 by dissecting the proliferative signaling pathway mediated by PI3K in breast cancer. BYL719 concurrently inhibited the phosphorylation of AKT and ERK in PIK3CA-mutated human breast cancer cells. PI3K-regulated ERK phosphorylation was independent of canonical PDK1/AKT/mTOR pathway, while it was associated with RAF/MEK. Hyper-activation of EGFR or RAS abrogated inhibition of ERK phosphorylation by BYL719. Furthermore, hyper-activation of receptor tyrosine kinases (RTKs) including EGFR, c-MET, FGFR and HER3 but not IGF-1R restored ERK phosphorylation and cell viability suppressed by BYL719, suggesting the discriminative functions of RTKs in cell signaling and proliferation. By profiling 22 breast cancer cell lines, we found that BYL719 was more potent in cell lines where phosphorylation of both AKT and ERK was attenuated than those where only AKT phosphorylation was inhibited. The potency of BYL719 was further found to be significantly correlated with the expression profile of RTKs in breast cancer cells. Specifically, overexpression of EGFR, c-MET and/or FGFR1 forecasted resistance, while overexpression of IGF-1R and/or HER2 predicted sensitivity to BYL719 in breast cancer cells. Similar correlation between BYL719 efficacy and expression profile of RTKs was found in patient-derived xenograft models of breast cancer. Thus, inhibition of ERK phosphorylation by PI3Kα inhibitor BYL719 contributes to its antitumor efficacy and is determined by the converged signaling from RTKs. The expression profile of RTKs in breast cancer tissue could be potentially developed as a predictive biomarker for the efficacy of PI3Kα inhibitors.
Keywords: BYL719, PI3K, ERK, receptor tyrosine kinase, breast cancer, predictive biomarker.
 Global reach, higher impact
Global reach, higher impact