13.3
Impact Factor
Theranostics 2017; 7(6):1524-1530. doi:10.7150/thno.19371 This issue Cite
Short Research Communication
Synthesis of 5-[18F]Fluoro-α-methyl Tryptophan: New Trp Based PET Agents
1. Department of Radiology and Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA;
2. Institute of Chemistry and BioMedical Sciences, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China.
* These authors contributed equally to the research work.
Received 2017-1-27; Accepted 2017-3-9; Published 2017-4-7
Abstract
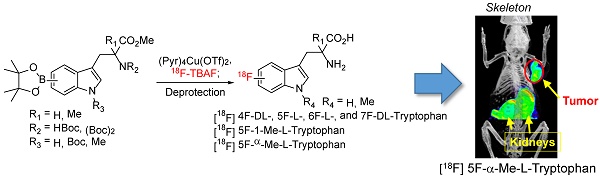
Indoleamine 2,3-dioxygenase (IDO1) plays a special role in the biology of various cancer types, because it breaks down the essential amino acid tryptophan for immune cell activation. Upregulation of IDO1 significantly correlates with the number of various T cell types in tumor tissues in melanoma and other cancers, suggesting that IDO expression is linked with effective and ineffective ('exhausted') immune response in cancer. Based on the reported IDO inhibitors (α-Methylated and indole-N-methylated tryptophan (Trp)), here we report the synthesis of potential IDO1 imaging agents through direct introduction of 18F into the tryptophan aromatic ring. Overall, the resulting PET agents could be obtained in high radiochemical purity (>97%) with labeling yield ranges from 4.2-14.9% decay corrected yield. Using Trp as the model compound, our results also demonstrate that 18F could be directly introduced to the Trp backbone at the 4, 5, 6, and 7 position. Moreover, our initial imaging study suggests that 5-[18F]F-L-α-methyl tryptophan (5-[18F]F-AMT) holds great potential for cancer imaging. The success of this approach will provide researchers easy access to a library of Trp/Trp-derivative based PET agents for biomedical research, including potential IDO1 targeted imaging.
Keywords: 18F radiolabeling, Positron emission tomography (PET), Tryptophan (Trp), Indoleamine 2, 3-dioxygenase (IDO1), Immunotherapy.
Introduction
Several PD-1/PD-L1 pathway inhibitors were recently approved by FDA for various solid tumor malignancies. However, not every patient will respond to these expensive, lifelong treatments [1]. In contrast to small molecule inhibitors, whose use is contingent upon a companion diagnostic, there are no reliable biomarkers for predicting/monitoring immunotherapy outcomes. Clearly, there is an urgent need to identify predictive biomarkers of response to these treatments. Indoleamine 2,3-dioxygenase (IDO1) plays a special role in the biology of various cancer types because it breaks down the essential amino acid tryptophan for immune cell activation.[2-5] Upregulation of IDO1 significantly correlates with the number of various T cell types in tumor tissues in melanoma and other cancers, suggesting that IDO expression is linked with effective and ineffective ('exhausted') immune response in cancer [2, 6-8].
As a powerful and highly sensitive imaging technology, positron emission tomography (PET) is becoming increasingly popular to non-invasively study metabolic processes and track radiolabeled biomolecules in vivo [9-13]. Among various PET radionuclides, 18F is one of the most widely used ones due to its clinically attractive half-life (t1/2 = 110.8 min, allowing synthesis and transportation), high positron efficiency (β+ = 97%, leading to high sensitivity), and relatively low positron energy (leading to better resolution compared with PET isotopes that have higher positron energy) [14]. To date, 18F-2-deoxy-2-fluoroglucose (FDG) is the most widely used PET agent in the clinic, however, it has limitations for a number of applications.[15, 16] Currently, there is a great need to develop novel PET probes that are more specific to the process of interest. In light of the great significance of IDO1 in immunotherapy, we have been actively developing novel 18F labeled PET agents to monitor its activity in vivo.
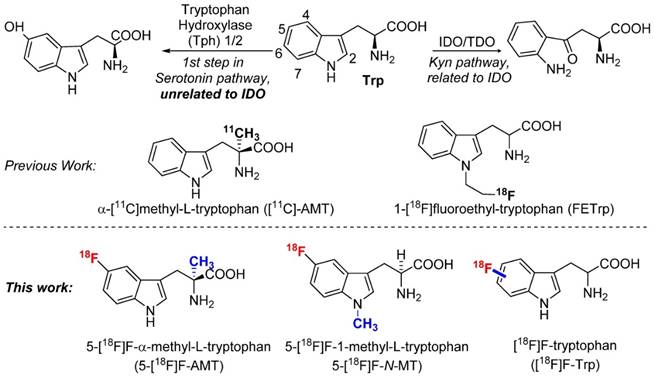
As an essential amino acid, tryptophan (Trp) and its metabolites are involved in various human diseases including cancer, neurodegenerative disorders, and diabetes [2, 17-19]. For example, in addition to participating in protein synthesis, tryptophan could also be metabolized to L‐kynurenine through the indoleamine 2,3-dioxygenase 1 (IDO1) pathway, which plays a key role in tumor growth and immune system suppression.[20] Alternatively, tryptophan could be metabolized to serotonin that regulates various neuro-activities and insulin secretion (Fig 1) [21]. Clearly, radiolabeled tryptophan and its derivatives could potentially offer a non-invasive method to study tryptophan metabolism in various diseases [22-33]. As mentioned above, we have been particularly interested in developing a PET agent to study IDO1 activity during cancer progression or treatment.
Previously, several 11C and 18F labeled tryptophan derivatives have been developed and tested as PET agents for IDO targeted imaging [25, 34-36]. In fact, α-[11C]methyl-L-tryptophan ([11C]-AMT), an analogue of tryptophan, is an inhibitor of IDO, but not a substrate of protein synthesis. [11C]-AMT has demonstrated great potential in cancer research studies. However, due to the short half-life of 11C, the application of [11C]-AMT is limited to a certain extent to facilities with an on-site cyclotron. Novel 18F‐labeled tryptophan derivatives may be able to address this limitation. Indeed, 18F‐labeled tryptophan derivatives have been synthesized by introducing additional functional groups (such as an aliphatic chain) for fluorination [25, 30]. Herein, we report the radiosynthesis of tryptophan and tryptophan analogs having 18F attached to their aromatic ring.
Results and Discussion
In our design (shown in Fig 1), we aimed to directly incorporate 18F into the tryptophan aromatic ring because the comparable size of F and H would not considerably change its biological activity (F is a bioisostere of H; the polarity would be different, which may change the clearance rate, binding potential, or metabolites). Moreover, because the competitive Trp metabolism pathway involves the enzyme tryptophan hydroxylase (Tph, introducing the OH group at Trp 5 position), replacing the C-H with C-F bond at 5 position may make the resulting PET agent more specific to IDO. To synthesize our desired compound, electrophilic fluorination could have been the easiest approach. Unfortunately, electrophilic [18F]-fluorination suffers from several well-recognized drawbacks from a radiochemistry point of view. Carrier added methods are generally used for the production of [18F]F2 gas that requires special equipment for its handling. It can react unselectively and lead to a mixture of products. This complication could lower the radiochemical yield (RCY) and make the final product difficult to purify. Therefore, it would be ideal if our lead compound could be made from [18F]F-, which is difficult to achieve on an electron-rich aromatic ring. However, with the recent development of metal catalyzed fluorinations, direct radiolabeling of the tryptophan aromatic ring using no-carrier added 18F becomes feasible [24, 37, 38].
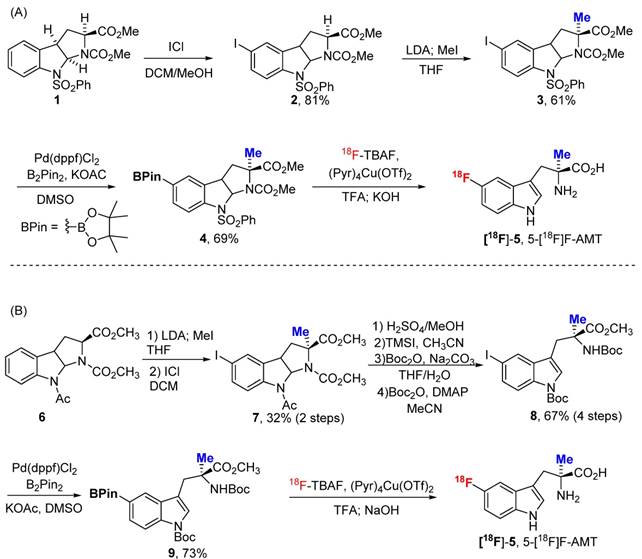
Adapting the copper-catalysed reaction, we first explored the synthesis of 5-[18F]F-α-methyl-L-tryptophan ([18F]-5, 5-[18F]F-AMT). As shown in Scheme 1, the 5-BPin-AMT (derivatives 4 and 9, precursors for 5F-AMT) could be synthesized by two approaches. The method shown in Scheme 1A has fewer synthetic steps to reach the boronic ester precursor. However, the elevated temperature of the deprotection conditions following radiofluorination are harsher. Conversely, the alternative method has more synthetic steps (Scheme 1B), but has the benefit of a milder deprotection step (lower temperatures) following radiofluorination. We were able to synthesize 5-[18F]F-AMT in 7.6% isolated yield with precursor 4 (10.9% decay corrected yield) and 10% isolated yield with precursor 9 (14.9% decay corrected yield). Both precursors yielded the product with a high radiochemical purity (see supporting material).
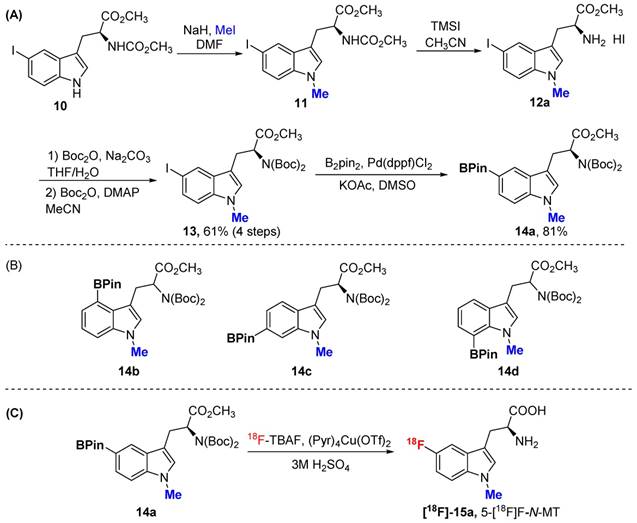
In addition to 5F-AMT, N-alkylated tryptophan derivatives have also been developed as substrates or inhibitors of enzymes (for example, 1-Me-D-Trp is a potent IDO inhibitor; 18F-FETrp is a potential PET agent for IDO). So we expanded the approach to include tryptophan derivatives with N-methyl substitution. We synthesized 1-methyl tryptophan derivatives 14a-d possessing the BPin prosthetic at position 4, 5, 6, or 7 of the indole ring in moderate to high yields, 31-81% (Schemes 2A and 2B and supporting information). With these agents in hand, we then selected 5-Bpin-1-Me-Trp derivative 14a as an example for a labeling test. 5-[18F]Fluoro-1-methyl-L-tryptophan ([18F]-15a, 5-[18F]F-N-MT) was obtained in 7.9% isolated yield (11.2% decay corrected yield) with a high radiochemical purity.
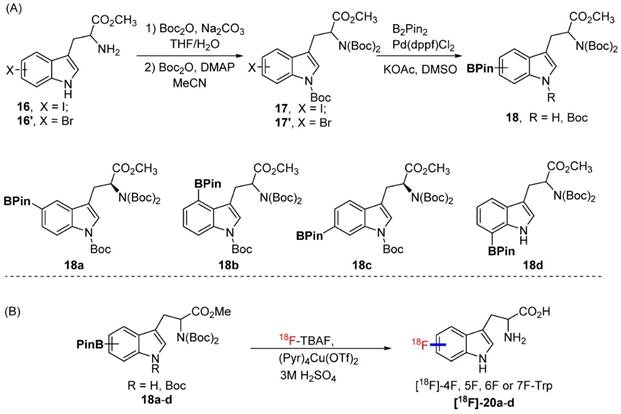
Although replacing the C-H bond with C-F bond at 5 position is preferred in our initial approach (with the assumption that it may block the metabolic pathway at 5 position similar to 18F-FDG vs glucose), it could also be valuable to generate Trp based PET agent that are fluorinated at other positions of indole ring. To explore this idea, we synthesized 18F-labeled derivatives of tryptophan devoid of a methyl substituent at the 1-position or the alpha position. In fact, 4-[18F]F-Trp, 5-[18F]F-Trp and 6-[18F]F-Trp have been reported before, but limited examples of radiofluorination at position 7 exist [24, 31-33, 37, 38]. As shown in Scheme 3A, starting from iodinated or brominated tryptophan derivatives, the boronic ester group was successfully introduced at positions 4, 5, 6, and 7 of the indole ring via Miyaura borylation (18a-d). The nitrogen atom on the indole ring was also protected by the Boc protecting group to prevent a decrease in radiolabeling yield due to the presence of the acidic N-H group.
We then explored the radiolabeling of 4-, 5-, 6-, and 7-BPin-Trp (Scheme 3B). Starting from dry 18F-tetrabutyl ammonium fluoride (18F-TBAF), the labelling was carried out at 110 degree for 20 min followed by one step deprotection yielding 4-, 5-, 6-, 7-[18F]F-Trp ([18F]-20a-d) in 2.8-10% isolated yield (4.2-14.9% with decay correction). Because the Cu reagent is base sensitive, the labeling yield was found to decrease when a larger volume of 18F-TBAF solution was used for the reaction. This has been attributed to the higher amounts of residual tetrabutylammonium bicarbonate phase transfer catalyst present in larger volumes of 18F-TBAF solution.[39] Protection of the acidic indole -NH- was employed to increase the yield of the acid sensitive 18F fluoride utilized in the labeling reaction. However, during the synthesis of 7-BPin-Trp derivative 18d, the Boc protecting group on the indole nitrogen atom was lost, and could not be reinstalled, likely due to steric crowding between the large BPin and the Boc protecting groups. Nonetheless, 7-[18F]F-Trp [18F]-20d could still be obtained in a reasonable yield (8.8% yield, decay corrected), indicating that protection of the indole -NH- group is not mandatory. In addition, for some of the agents (4-BPin and 7-BPin Trp), optically pure starting materials were not used, as our main focus was to validate that the chemistry and radiochemistry could be applied to these categories of compound. In future studies, we plan to separate the stereoisomers by chiral chromatography and evaluate the importance of optical purity [25]. Clearly, our work demonstrates that 18F-labeling using boronate precursors can be adapted for the synthesis of 4-, 5-, 6- and 7-[18F]fluoro tryptophan derivatives. The same approach may allow fluorination at similar position of N-methyl and α-methyl Trp as well.
Introducing 18F at the aromatic ring represents minimal structural change and holds the potential to block Tph pathway by replacing C-H bond with C-F bond.
Two approaches for the synthesis of 5-[18F]F-AMT. The deprotection steps include both acid (TFA) and base (KOH/NaOH).
(A) The procedure for the preparation of 5Bpin-N-MT. (B) The prepared Bpin-N-MT. (C) The procedure for the synthesis of 5-[18F]F-N-MT.
(A) The procedures for the preparation of Bpin-Trp. (B) The procedure for the synthesis of [18F]-4F, 5F, 6F or 7F-Trp.
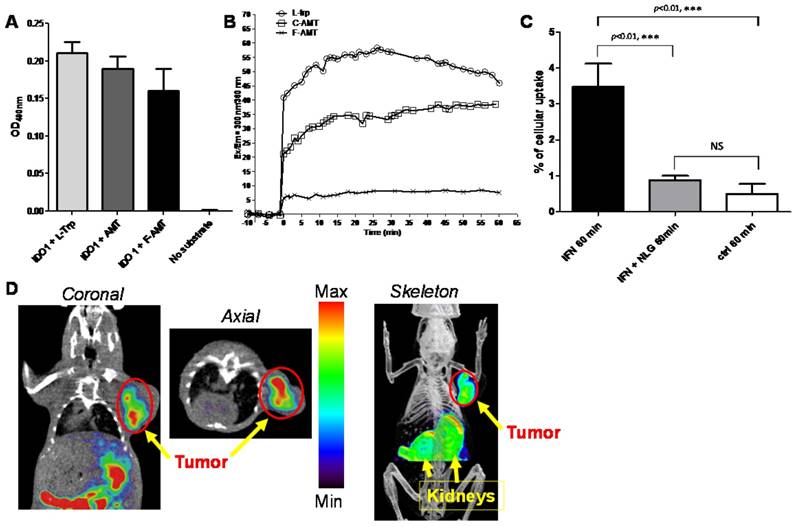
We would like to point out that initial biological evaluations of our newly synthesized probe has been performed in this report. Additional experiments are still needed to investigator whether the IDO expression correlates with PD1/PD-L1 expression in these models. Nonetheless, we do feel it is highly valuable to provide researchers an easy access to a library of Trp/Trp-derivative based PET agents using our reported approach. In order to ensure that 5-[18F]F-AMT could be used for IDO imaging, we performed cell and enzyme based assays. We first tested whether 5-[18F]F-AMT is a substrate for the IDO enzyme, and compared the result with that of the natural substrate Trp and a known IDO agent, α-methyl-L-tryptophan (AMT). As shown in Fig 2A, the absorbance at 490nm for the reaction containing L-Trp, AMT, or F-AMT was 0.21 ± 0.01, 0.19 ± 0.02, and 0.16 ± 0.03 (results from 3 independent assays), while the control reaction system had no absorbance. These results suggested that all three compounds are substrates for IDO1. It is known that IFNγ treatment can upregulate IDO1 expression [40]. We therefore performed a cell based assay to further evaluate our agents. The uptake of 5-[18F]F-AMT in IFNγ treated cells was 3.474 ± 0.6451 % compared with 0.50 ± 0.28 % in control cells after 1 h incubation (p<0.001). When the IFNγ -treated cells were incubated with NLG919, an IDO1 inhibitor, the uptake of 5-[18F]F-AMT decreased to 0.88 ± 0.12 %, which was comparable with control cells (p>0.05) but significantly lower than that in IFNγ -treated cells. These results indicated that the cell uptake of 5-[18F]F-AMT is associated with IDO1 expression levels (Figure 2B). The serotonin pathway is a competitive metabolic route for Trp and its derivatives. In the first step Tph adds a hydroxyl group to position 5 of the indole ring. We evaluated whether the introduction of fluorine at the 5-position would affect metabolism by Tph. As shown in Fig S1, immediately after adding L-Trp to the reaction system containing the tryptophan hydroxylase enzyme, the fluorescence signal increased from a baseline value of -0.266 to 40.885 and reached a peak value 58.350 at 26 min. Immediately after adding AMT to the reaction system, the fluorescence signal increased from a baseline value of -0.312 to 21.145 and reached a peak value of 36.607 at 34 min. However, adding 5-F-AMT to the reaction system only resulted in an increase to 5.635 (from -1.026), and the peak value was only 8.176 (at 25 min). These results demonstrate that, compared with Trp and AMT, 5-[18F]F-AMT is a worse substrate for Tph.
There are at least four variables in our tryptophan based probe design: 1) fluorination position on tryptophan aromatic ring; 2) D and L configuration; 3) N-substitution at position 1; 4) α-methylation. Combination of two or multiple variables would significantly expand the library of Trp/Trp-derivative based PET agents for investigators to evaluate further in biomedical research. Indeed, the initial small animal PET imaging using 5-[18F]F-AMT has revealed prominent tumor uptake in B16F10 tumor model (Fig 2C). The tumor is clearly visible. The kidney showed initial high uptake at 30 min, which could be cleared at late time point. Further evaluation is being pursued in our follow up study.
(A) IDO1 enzyme assay: L-Trp, AMT, and F-AMT are substrates to IDO1 enzyme. (B) Tph enzyme kinetics assay. (C) 5-[18F]F-AMT uptake in untreated HeLa cells and those treated with IFN-γ without/with IDO1 inhibitor NLG919. (D) Decay-corrected whole-body small PET-CT images of B16F10 melanoma after 30 min injection of 5-[18F]F-AMT and ROI derived biodistribution data. The images are coronal, axial, and skeleton view.
Conclusion
In conclusion, our results clearly demonstrated 18F could be directly introduced to the Trp backbone (including the 1-methyl or α-methyl Trp) at position 5. Using Trp as the model compound, fluorination at position 4, 5, 6, and 7 has also been validated. Moreover, the initial small animal PET imaging demonstrated the great potential of using 5-[18F]F-AMT for targeted cancer imaging. A detailed mechanism study is being pursued in our follow up study to further confirm its specificity in vivo.
Supplementary Material
Experimental procedures, characterization data for new compounds, HPLC traces, and cell biology, enzyme assay and imaging procedures.
Acknowledgements
This work was supported by National Natural Science Foundation of China (21472085, 21332005), Qin Lan Project, UNC LCCC pilot grant, NC TraCS Pilot Award (550KR131622) and UNC Radiology Department and Biomedical Research Imaging Center.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hettich M, Braun F, Bartholoma MD, Schirmbeck R, Niedermann G. High-Resolution PET Imaging with Therapeutic Antibody-based PD-1/PD-L1 Checkpoint Tracers. Theranostics. 2016;6:1629-40
2. van Baren N, Van den Eynde BJ. Tumoral Immune Resistance Mediated by Enzymes That Degrade Tryptophan. Cancer Immunol Res. 2015;3:978-85
3. Dounay AB, Tuttle JB, Verhoest PR. Challenges and Opportunities in the Discovery of New Therapeutics Targeting the Kynurenine Pathway. J Med Chem. 2015;58:8762-82
4. Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front Immunol. 2014;5:673
5. Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319-29
6. Heng B, Lim CK, Lovejoy DB, Bessede A, Gluch L, Guillemin GJ. Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget. 2016;7:6506-20
7. Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ. et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin Cancer Res. 2015;21:5427-33
8. Zhai L, Lauing KL, Chang AL, Dey M, Qian J, Cheng Y. et al. The role of IDO in brain tumor immunotherapy. J Neurooncol. 2015;123:395-403
9. Czernin J, Phelps ME. Positron emission tomography scanning: current and future applications. Annu Rev Med. 2002;53:89-112
10. Dimastromatteo J, Brentnall T, Kelly KA. Imaging in pancreatic disease. Nat Rev Gastroenterol Hepatol. 2017;14:97-109
11. Lindenberg L, Ahlman M, Turkbey B, Mena E, Choyke P. Advancement of MR and PET/MR in Prostate Cancer. Semin Nucl Med. 2016;46:536-43
12. Mesguich C, Zanotti-Fregonara P, Hindie E. New Perspectives Offered by Nuclear Medicine for the Imaging and Therapy of Multiple Myeloma. Theranostics. 2016;6:287-90
13. Lutje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SA, Rosenbaum-Krumme S. et al. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics. 2015;5:1388-401
14. Li Z, Conti PS. Radiopharmaceutical chemistry for positron emission tomography. Adv Drug Deliv Rev. 2010;62:1031-51
15. Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154-61
16. Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T. et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979;44:127-37
17. Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K. et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373-388
18. Herrera R, Manjarrez G, Hernandez J. Inhibition and kinetic changes of brain tryptophan-5-hydroxylase during insulin-dependent diabetes mellitus in the rat. Nutr Neurosci. 2005;8:57-62
19. Fernstrom MH, Fernstrom JD. Large changes in serum free tryptophan levels do not alter brain tryptophan levels: studies in streptozotocin-diabetic rats. Life Sci. 1993;52:907-16
20. Routy JP, Routy B, Graziani GM, Mehraj V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int J Tryptophan Res. 2016;9:67-77
21. Oh CM, Park S, Kim H. Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metab J. 2016;40:89-98
22. Chugani DC, Muzik O. Alpha[11C]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2-9
23. Xie L, Maeda J, Kumata K, Yui J, Zhang Y, Hatori A. et al. Development of 1-N-[11C]-Methyl-L- and -D-Tryptophan for pharmacokinetic imaging of the immune checkpoint inhibitor 1-Methyl-Tryptophan. Sci Rep. 2015;5:16417
24. Schäfer D, Weiß P, Ermert J, Castillo Meleán J, Zarrad F, Neumaier B. Preparation of No-Carrier-Added 6-[18F]Fluoro-l-tryptophan via Cu-Mediated Radiofluorination. European Journal of Organic Chemistry. 2016;2016:4621-8
25. Xin Y, Cai H. Improved Radiosynthesis and Biological Evaluations of L- and D-1-[18F]Fluoroethyl-Tryptophan for PET Imaging of IDO-Mediated Kynurenine Pathway of Tryptophan Metabolism. Mol Imaging Biol. 2016 doi:10.1007/s11307-016-1024-z
26. Kramer SD, Mu L, Muller A, Keller C, Kuznetsova OF, Schweinsberg C. et al. 5-(2-18F-fluoroethoxy)-L-tryptophan as a substrate of system L transport for tumor imaging by PET. J Nucl Med. 2012;53:434-42
27. Chiotellis A, Mu L, Muller A, Selivanova SV, Keller C, Schibli R. et al. Synthesis and biological evaluation of 18F-labeled fluoropropyl tryptophan analogs as potential PET probes for tumor imaging. Eur J Med Chem. 2013;70:768-80
28. Shih IH, Duan XD, Kong FL, Williams MD, Yang K, Zhang YH. et al. Automated synthesis of 18F-fluoropropoxytryptophan for amino acid transporter system imaging. Biomed Res Int. 2014;2014:492545
29. Li R, Wu SC, Wang SC, Fu Z, Dang Y, Huo L. Synthesis and evaluation of l-5-(2-[18F]fluoroethoxy)tryptophan as a new PET tracer. Appl Radiat Isot. 2010;68:303-8
30. Michelhaugh SK, Muzik O, Guastella AR, Klinger NV, Polin LA, Cai H. et al. Assessment of tryptophan uptake and kinetics using 1-(2-[18F]fluoroethyl)-L-tryptophan and alpha-[11C]-methyl-L-tryptophan PET imaging in mice implanted with patient-derived brain tumor xenografts. J Nucl Med. 2017;58:208-213
31. Atkins HL, Christman DR, Fowler JS, Hauser W, Hoyte RM, Klopper JF. et al. Organic radiopharmaceuticals labeled with isotopes of short half-life. V. 18F-labeled 5- and 6-fluorotryptophan. J Nucl Med. 1972;13:713-9
32. Chirakal R, Sayer B.G, Firnau G, Garnett E.S. Synthesis of 18F labelled fluoro-melatonins and 5-hydroxy-fluoro-tryptophans. J Labelled Compd Radiopharm. 1988;25:63-71
33. Weiss PS, Ermert J, Castillo Melean J, Schafer D, Coenen HH. Radiosynthesis of 4-[18F]fluoro-L-tryptophan by isotopic exchange on carbonyl-activated precursors. Bioorg Med Chem. 2015;23:5856-69
34. Henrottin J, Lemaire C, Egrise D, Zervosen A, Van den Eynde B, Plenevaux A. et al. Fully automated radiosynthesis of N(1)-[18F]fluoroethyl-tryptophan and study of its biological activity as a new potential substrate for indoleamine 2,3-dioxygenase PET imaging. Nucl Med Biol. 2016;43:379-89
35. Michelhaugh SK, Muzik O, Guastella AR, Klinger NV, Polin LA, Cai H. et al. Assessment of Tryptophan Uptake and Kinetics Using 1-(2-18F-Fluoroethyl)-l-Tryptophan and alpha-11C-Methyl-l-Tryptophan PET Imaging in Mice Implanted with Patient-Derived Brain Tumor Xenografts. J Nucl Med. 2017;58:208-13
36. Chaly T, Diksic M. Synthesis of "no-carrier-added" alpha-[11C]methyl-L-tryptophan. J Nucl Med. 1988;29:370-4
37. Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J. et al. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew Chem Int Ed Engl. 2014;53:7751-5
38. Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS. et al. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org Lett. 2015;17:5780-3
39. Zlatopolskiy BD, Zischler J, Krapf P, Zarrad F, Urusova EA, Kordys E. et al. Copper-mediated aromatic radiofluorination revisited: efficient production of PET tracers on a preparative scale. Chemistry. 2015;21:5972-9
40. Sarkar SA, Wong R, Hackl SI, Moua O, Gill RG, Wiseman A. et al. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes. 2007;56:72-9
Author contact
![]() Corresponding authors: hongjianluedu.cn; ziboliunc.edu
Corresponding authors: hongjianluedu.cn; ziboliunc.edu
 Global reach, higher impact
Global reach, higher impact