13.3
Impact Factor
Theranostics 2017; 7(6):1705-1718. doi:10.7150/thno.18301 This issue Cite
Research Paper
Peptide vaccination in the presence of adjuvants in patients after hematopoietic stem cell transplantation with CD4+ T cell reconstitution elicits consistent CD8+ T cell responses
1. Dept. of Internal Medicine V, Heidelberg University Hospital, 69120 Heidelberg, Germany;
2. German Cancer Consortium/Deutsches Konsortium für Translationale Krebsforschung (DKTK), 69120 Heidelberg, Germany;
3. Institute of Clinical Transfusion Medicine and Immunogenetics Ulm, German Red Cross Blood Transfusion Service Baden-Württemberg-Hessen and Institute of Transfusion Medicine, University of Ulm, 89081 Ulm, Germany;
4. Institute of Virology, Ulm University Medical Center, 89081 Ulm, Germany;
5. Dept. of Hematology & Laboratory of Translational Immunology, University Medical Center, 3584 CX Utrecht, Netherlands;
6. Dept. of Internal Medicine III, University of Ulm, 89081 Ulm, Germany;
7. Dept. of Virology, University of Heidelberg, 69120 Heidelberg, Germany;
8. Dept. of Internal Medicine, Diakonie-Hospital Stuttgart, 70176 Stuttgart, Germany.
Received 2016-11-11; Accepted 2017-3-6; Published 2017-4-10
Abstract
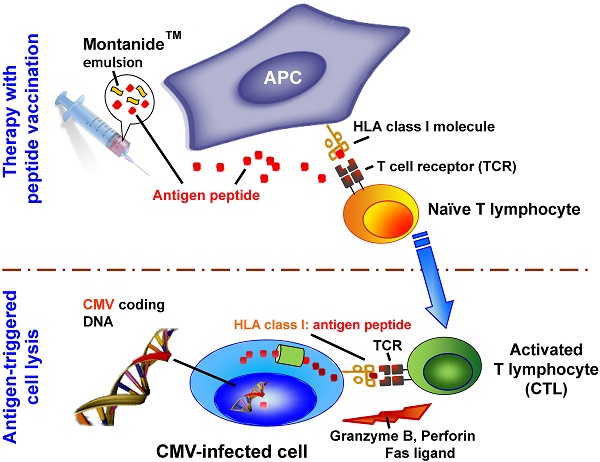
Rationale: Patients receiving an allogeneic stem cell graft from cytomegalovirus (CMV) seronegative donors are particularly prone to CMV reactivation with a high risk of disease and mortality. Therefore we developed and manufactured a novel vaccine and initiated a clinical phase I trial with a CMV phosphoprotein 65 (CMVpp65)-derived peptide.
Methods: Ten patients after allogeneic stem cell transplantation received four vaccinations at a biweekly interval. All patients were monitored for CMVpp65 antigenemia. Flow cytometry for CMV-specific CD8+ and γδ T cells as well as neutralizing anti-CMV antibodies were correlated to clinical parameters.
Results: The vaccination was well tolerated. Seven of nine patients cleared CMVpp65 antigenemia after four vaccinations and are still free from antigenemia to this day. Two patients with CMV reactivation showed persisting CMV antigenemia. One patient received prophylactic vaccination and did not develop antigenemia. An increase of up to six-fold in frequency of both CMV-specific CD8+ T cells and/or Vδ2negative γδ T cells was detected. Titers of neutralizing antibodies increased up to the tenfold. Humoral and cellular immune responses correlated with clearance of CMV.
Conclusion: In summary, CMVpp65 peptide vaccination for patients after allogeneic stem cell transplantation at high risk for CMV reactivation was safe, well tolerated and clinically encouraging. A study in solid-organ transplant patients is ongoing.
Keywords: CMVpp65, CMV
Introduction
Reactivation of cytomegalovirus (CMV) after allogeneic hematopoietic stem cell transplantation (HSCT) occurs in more than 60% of CMV-seropositive patients and remains a major cause of morbidity despite improved antiviral drug therapy [1-3]. Besides ganciclovir and foscarnet [4] novel antiviral drugs like maribavir [5] and letermovir [6] are under investigation in phase III clinical trials to reduce the incidence of active CMV infection and disease. Although CMV disease is rare under current pre-emptive antiviral treatment, CMV reactivation still has a remarkably unfavorable impact on the outcome after HSCT [1, 2].
Prolonged antiviral therapy, however, can cause pronounced side effects, particularly myelosuppression and nephrotoxicity. Therefore prophylaxis against CMV is no longer in use [7, 8]. Moreover early preemptive antiviral therapy may lead to an increase of CMV disease after day 100 post transplantation [9]. In the immunocompetent host, sufficient immune effectors prevent active infection after reactivation of herpes viruses including CMV. Beyond humoral immune response, cell-mediated immune response is essential for the control of CMV infection and disease [10, 11]. Studies have demonstrated that patients are protected against CMV disease once a detectable T cell response against CMV has been mounted [12].
Both phosphoproteins (pp)65 and pp150 contain highly immunogenic CMV antigens that are recognized by CD8+ T cells[13-15]. Induction of a CMV-specific CD8+ cytotoxic T cell response plays a crucial role in the control of viral replication of CMV [16]. Protective immunity against CMV can at least temporarily be achieved by infusions of CMV-specific CD8+ cytotoxic T cell clones of the donor [17, 18]. CMVpp65 peptide-specific T cells have been selected by streptamers [19] and successfully administered to patients resulting in the enduring clearance of CMV load [20]. Vaccination with dendritic cells loaded with a CMVpp65-derived peptide resulted in both immunological and clinical responses [21, 22]. Moreover, phase I and II trails were successfully conducted by Nakamura et al. and Kharfan-Dabaja et al., demonstrating a clinical response to the chosen vaccine formulation [23, 24].
The hypothesis of this study is that by vaccination with this liposomal system, CMV specific immune responses can also be enhanced in CMV-seropositive patients - even in those with a CMV-seronegative donor - by vaccination with CMVpp65-derived peptides without administration of T cells.
Materials and Methods
Samples from patients before/after allogeneic hematopoietic stem cell transplantation
All samples were taken from patients treated in this clinical study (EudraCT number: 2010-018884-40). Peripheral blood mononuclear cells (PBMC) from patients were prepared by Ficoll (Biochrom, Berlin, Germany) separation and tested freshly or after cryopreservation in FCS serum (PAN Biotech, Aidenbach, Germany) containing 10% DMSO (Sigma Aldrich, Steinheim, Germany) and stored in liquid nitrogen. Samples were collected from all patients before the 1st (T1), after each vaccination (T2 to T5), and approximately one (T6) or three months (T7) after the 4th vaccination whenever possible.
Study design
This clinical study entitled UL-CMV-1 (EudraCT no.: 2010-018884-40) was approved by the local ethical committee (IRB no.: 15/06) as well as by the Federal Regulatory Authority, the Paul-Ehrlich-Institute, Langen, Germany (PEI registration no. 1232/01) to be performed at the University Clinics of Ulm and Heidelberg. Informed consent was obtained from all patients. 300 µg of CMVpp65-derived peptide (495-NLVPMVATV-503, Bachem Distribution Services, Weil am Rhein, Germany) was emulsified with incomplete Freund's adjuvant ISA-51, Montanide® (Seppic, Paris, France) and administered subcutaneously four times in the proximal upper leg. A dose of 75 µg GM-CSF (Leukine®, Berlex, Richmond, CA) was administered subcutaneously in the vicinity of the peptide vaccine on the day of vaccination, as well as two days before and two days after peptide vaccination, i.e. five times per peptide administration. The primary aim of this phase I clinical trial was to test the safety and feasibility of this peptide vaccination. Secondary aims were the evaluation of cellular and humoral immune responses to the virus and the assessment of the CMV antigenemia status before and after peptide vaccination.
Patient inclusion and exclusion criteria
The inclusion criteria for patients were as follows: status after allogeneic hematopoietic stem cell transplantation, HLA-A2 expression positivity, patient CMV seropositive, CD4+ T cell number >50 cells/µl, Karnofsky Index ≥70% or ECOG-Status 0-II, expected survival time at least six months, liver and kidney function tests below the twofold of the normal upper values, no active infection except CMV, and intake of steroids less than 30 mg prednisolone per day. Patients with florid GvHD grade 2-4 were excluded from participation in this study. We have screened all of our patients for eligibility into our CMV vaccination study, thus with this respect they were consecutive patients.
Vaccine preparation
All 40 doses of the CMVpp65 peptide vaccine were produced under sterile conditions in clean rooms grade A in B in full compliance with European Community Good Manufacturing Practice (GMP) requirements. The vaccine was manufactured in compliance with standard operation procedures (SOPs) entirely developed by our team. The entire manufacturing process was developed and validated by our group. Manufacture licenses to the GMP production sites in Ulm and Heidelberg were issued by the control center drug monitoring at the local federal regulatory authority. Briefly, the CMVpp65495-503NLVPMVATV peptide (N9V; HLA-A2) lysate was resolubilized in DMSO and further dissolved in phosphate buffered saline with ethylene-diamine-tetraacetic acid (PBS /EDTA). Thereafter the mixture was drawn into a syringe with a total volume of 2,800 µl. An equal volume of ISA 51/Montanide™, was drawn into a second syringe, then both syringes were connected and a water-in-oil emulsion was produced by mixing the components slowly followed by fast mixing. The quality of the vaccine was determined by an optical control under the microscope and a control of the correct viscosity by dripping one drop of the mixture on sterile PBS. Thereafter the emulsified vaccine was filled into a fresh syringe for the patient and controls were taken for sterility, determination of peptide content and retention samples. Aliquots of the vaccine were sent to external laboratories for the assessment of sterility (L+S AG, Bad Bocklet, Germany) and the content of peptide in emulsion (C.A.T. GmbH, Tübingen, Germany).
All release criteria like weight and volume, visual control, drop test for consistency, microscopy for homogeneity of micellular structure were fulfilled. In validated post vaccination tests all vaccines tested sterile according to Ph. Eur. 2.6.1, and the content of peptide in emulsion was in the range of 300 µg +/- 20% per injection as measured by gas chromatography followed by mass spectrometry using the enantiomer labeling method.
Detection of CMV antigenemia and titers of CMV-specific antibodies
For the quantitative CMVpp65 antigenemia assay 500,000 leukocytes were carefully spun on a slide using a cytospin centrifuge. Cells were fixed and stained with an anti-CMVpp65 mouse monoclonal antibody, then washed and further incubated with an anti-mouse IgG FITC-labeled antibody. Slides were analyzed using immunofluorescence microscopy and CMVpp65 antigen-positive cells were counted. Generally, CMVpp65 antigenemia results in immunofluorescence testing (IFT) correlate well with results for CMV-DNA in qPCR [25].
Besides CMVpp65 antigenemia, CMV-specific antibodies, i.e. the CMV immunoglobulin G index was assessed by standard assays (Enzygnost anti-CMV IgG/IgM, Siemens, Eschborn, Germany). In addition to commercially available assays sera were also employed in a cell-based assay for neutralizing antibodies to evaluate the inhibition of CMV infection of fibroblasts as described earlier [26]. The amount of CMV positive cells after 72 hrs was counted by using an immunofluorescence microscope.
Antiviral therapy
Peripheral blood was monitored by immunofluorescence test (IFT) for CMVpp65 on a weekly basis for out-patients and twice weekly for in-patients. Preemptive antiviral therapy with valganciclovir orally or ganciclovir intravenously was started when CMV antigenemia was > 1/500,000 counted white blood cells (WBCs) in IFT as described above. In case of thrombocytopenia < 30 G/L foscavir was given intravenously until the viral load was cleared.
When CMV was absent in two subsequent IFT tests, (val)ganciclovir or foscavir was discontinued and replaced by acyclovir prophylaxis.
Assessment of toxicity of CMVpp65 peptide vaccination
Side effects were documented according to Common Terminology Criteria for Adverse Events v3.0 (CTCAE; http://ctep.cancer.gov). Before and three weeks after the fourth vaccination physical examination, body weight, ECOG performance score, laboratory tests (WBC count, kidney and liver function tests, electrophoresis, electrolytes, CRP, LDH, and coagulation tests), chest x-ray, electrocardiography, urine analysis and ultrasound examination of the abdomen was performed. Before each vaccination physical examination, laboratory tests (WBC, differential blood count, kidney and liver function tests, electrolytes, CRP, LDH, coagulation tests and urine analysis) were performed.
Tetramer staining
The frequency of CMV-specific CD8+ T lymphocytes was determined by staining with anti-CD8* APC antibody (BD, Heidelberg, Germany) and HLA-A2/CMV-tetramer*PE as described earlier [27].
A positive immunological response of CMV-specific CD8+ T cells was defined in case of an increase of specific CMV-tetramer+/CD8+ T cells of more than 100% during or after vaccination. Cells were analyzed on a FACS ARIA or BD LSR II™ flow cytometer. Data were analyzed using the FACS Diva™ software.
Interferon γ ELISPOT assays
ELISPOT assays to measure the release of interferon γ (IFNγ) were performed as previously described[28, 29] to determine reactivity of CMVpp65 peptide pulsed cells according to the manufacturer's instructions (BD, San Diego, USA) as described earlier.
γδ T cell analysis
Antibodies used for flow cytometry included: γδTCR*APC (allophycocyanin, clone B1, BD), γδTCR*PE (phycoerythrin, clone IMMU510, Beckman Coulter), γδTCR*FITC (clone 11F2, BD), Vδ2*PE and *FITC (clone B6, BD), Vδ1*FITC (fluorescein isothiocyanate, clone R9.12, Beckman Coulter), αβTCR*PE-Cy5 (IP26A, Beckman Coulter), CD3*eFluor450 (clone OKT3, eBioscience), CD3*pacific blue (clone SP34-2, BD), CD4*PE-Cy7 (clone RPA-T4, BD), CD8β*PE (clone 2ST8.5H7, BD), All samples were processed with FACSCanto-II or LSR-II flow cytometers (BD) and analyzed using the FACS Diva™ software (BD) as described earlier[30].
Results
Clinical observations
The ten patients included in this clinical phase I study (EudraCT no.: 2010-018884-40) were 34 to 69 years old (median: 62 years) at the time of first vaccination, comprising nine male patients and one female. Table 1 depicts the underlying hematological malignancies of the patients along with the CMV-serostatus of recipient (R) and donor (D), respectively. All patients but one received peripheral blood stem cells, patient #003 received a bone marrow graft. Grafts were donated from HLA-matched sibling or unrelated donors as indicated in Table 2, with the exception of patient #005 and #010 who received grafts from DQB1 or DRB1 mismatched unrelated donors. Patient #001 obtained the classical myeloablative conditioning regimen with 12 Gy total body irradiation (TBI) and cyclophosphamide (120mg/kg BW). All other patients were conditioned with fludarabine-based reduced intensity conditioning (RIC) regimens as indicated in Table 2.
CMV Clearance and Immunological Responses of the Patients
| Pat # | Gender/Age at Vaccination (years) | Underlying disease | Time from transplant to first vaccine (days) | CMV status | Enduring clearance of CMV | CMV- specificCD8+ T cells % | IFNγ release (ELISPOT) | Neutralizing antibodies(normalized) | Vd2-γδT cells % | Immune response (p*) |
|---|---|---|---|---|---|---|---|---|---|---|
| 001 | f/51 | ALL | 273 | R+/D- | Yes | 0.08->0.11 | 0->0 | 1->1 | 1.7->4.4 | 1/4 - yes |
| 002 | m/34 | AML | 125 | R+/D+ | Yes | 0.53->1.32 | 23->19 | 1->1 | 1.7->1.0 | 1/4 - yes |
| 003 | m/68 | FL | 503 | R+/D- | Yes | 0.20->0.20 | 4->13 | 1->10 | 8.5->11.0 | 3/4 - yes |
| 004§ | m/67 | TPLL | 190 | R+/D- | No | 0.15->0.05 | 4->0 | 1->1 | 1.7->1.0 | 0/4 - no |
| 005 | m/69 | CLL | 183 | R+/D+ | Yes | 0.23->0.07 | 15->2 | 1->4 | 3.0->1.9 | 1/4 - yes |
| 006 | m/62 | CLL | 119 | R+/D- | Yes | 2.00->1.40 | 161->186 | 1->2 | 2.2->13.7 | 3/4 - yes |
| 007 | m/58 | CMML | 76 | R+/D- | Yes | 0.03->0.73 | 0->0 | 1->1 | 2.6->10.4 | 2/4 - yes |
| 008† | m/64 | AML | 61 | R+/D- | NA† | 0.00->0.00 | 7->0 | 1->2 | 0.5->0.9 | 2/4 - yes |
| 009 | m/56 | PMF | 77 | R+/D- | Yes | 0.33->1.05 | 3->76 | 1->1 | 1.0->0.8 | 2/4 - yes |
| 010§ | m/64 | MDS | 62 | R+/D- | No | 0.36->0.19 | 2->0 | 1->1 | 0.4->0.2 | 0/4 - no |
ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; CLL: Chronic lymphocytic leukemia; CMML: Chronic myelomonocytic leukemia; D: Donor; f: female; FL: Follicular lymphoma; m: male; MDS: Myelodysplastic syndrome; NA: Not applicable; PAT: Patient; PMF: Primary myelofibrosis; R: Recipient; TPLL: T-cell-prolymphocytic leukemia; §All response parameters negative, clinical non-responder; †not applicable, since prophylactic vaccination; all other vaccinations were preemptive; p=0.01 (*; yes compared to no)
Clinical Characteristics of the Patients
| Pat # | Gender/ Age (years) | Underlying disease | Clearance of CMV | Skinreaction | Stem cell Source | Conditioning regimen | Stem cell donor | cGVHD before /after vaccination (time of onset after 1st vaccine) | Disease status before / after Tx (Donor Chimerism) |
|---|---|---|---|---|---|---|---|---|---|
| 001 | f/51 | ALL | Yes | No | PBSC | TBI 12 Gy/Cy | MRD (brother) | LD skin, resolved/none | CR/CR (100%) |
| 002 | m/34 | AML | Yes | Yes | PBSC | TBI 12 Gy/Thio/ Flu | MRD (brother) | None/LD skin, CSA, Steroids, ECP, MMF, sirolimus (30d) | CRi/CR (100%) |
| 003 | m/68 | FL | Yes | No | BM | Treo/Flu/ATG | MUD | LD skin resolved/none | PR/CR (100%) |
| 004 | m/67 | TPLL | No | No | PBSC | Flu/Bu/Cy | MUD | none/LD skin, resolved (86d) | CR/CR (100%) dead from MOF |
| 005 | m/69 | CLL | Yes | Yes | PBSC | Treo/Flu/ATG | MMUD (DQB1) | none/LD skin, resolved (75d) | CRu/CR (100%) |
| 006 | m/62 | CLL | Yes | Yes | PBSC | Treo/Flu/ATG | MUD | none/none | CR/CR (100%) |
| 007 | m/58 | CMML | Yes | Yes | PBSC | TBI 8 Gy/Flu/ATG | MUD | none/none | PR/CR (100%) |
| 008 | m/64 | AML | NA† | Yes | PBSC | TBI 2 Gy/Flu | MUD | none/LD skin, resolved (99d) | CR/CR (100%) |
| 009 | m/56 | PMF | Yes | No | PBSC | Treo/Flu/ATG | MUD | none/LD mucosa, resolved (124d) | PD/CR (100%) |
| 010 | m/64 | MDS | No | Yes | PBSC | Bu/Flu/ATG | MMUD (DRB1) | none/none | PR/CR (100%) |
ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; ATG: Anti-thymocyte globulin; BM: Bone marrow; Bu: Busulfan; (c)GvHD: (Chronic) Graft-versus-host disease; CLL: Chronic lymphocytic leukemia; CMML: Chronic myelomonocytic leukemia; CR: Complete remission; Cri: Complete remission with incomplete blood count recovery; Cru: Complete remission unconfirmed; CSA: Cyclosporine A; Cy: Cyclophosphamide; ECP: Extracorporeal photopheresis; f: female; FL: Follicular lymphoma; Flu: Fludarabine; Gy: Gray; LD: limited disease; m: male; MDS: Myelodysplastic syndrome; MMUD: Miss-matched unrelated donor; MOF: Multiple organ failure (sepsis, non-transplant related mortality); MRD: Matched related donor; MUD: Matched unrelated donor; NA: Not applicable; PAT: Patient; PBSC: Peripheral blood stem cells; PMF: Primary myelofibrosis; PR: Partial remission; TBI: Total body irradiation; Thio: Thiotepa; TPLL: T-cell-prolymphocytic leukemia; Treo: Treosulfan; †not applicable, since prophylactic vaccination; all other vaccinations were preemptive
Chronic graft-versus-host disease (cGvHD) was observed in seven out of ten patients. In two out of these seven cases cGvHD had developed prior to vaccination with no aggravation by vaccination. In five cases cGvHD-developed post vaccination. In all seven patients cGvHD was graded limited disease (LD) and was responsive to steroids or calcineurin inhibitors. Only patient #002 required additional immunosuppressive therapy with mycophenolate mofetil (MMF), sirolimus and extracorporeal photophoresis (ECP). Three patients did not show any signs of GvHD, before or after vaccination.
Six patients were transplanted in CR, patients #003, #007 and #010 in PR and patient #009 with myelofibrosis was refractory to prior treatment. After transplantation all patients reached full donor chimerism and remained in CR with the exception of patient #004. This patient suffered from a severe immunodeficiency resulting in recurrent infectious complications, i.e. several episodes of CMV reactivation and severe norovirus infection with grade IV diarrhea. Cumulative nephrotoxicity from both calcineurin inhibitors and antiviral therapy resulted in renal insufficiency which required hemodialysis. The patient eventually died from multi-organ failure. This was not considered to be related to vaccination.
All ten patients received all four vaccinations. The time point of the first vaccination was dependent on the inclusion criteria CD4+ cells > 50/µl, absence of florid GvHD and prednisolone < 30 mg/d. The time from allogeneic stem cell transplantation to first vaccination ranged from 61 to 503 days (mean: 168d and median: 125d). As characteristic for Montanide™-based vaccines [31, 32] no side effects were observed with the exception of CTC (common toxicity criteria) grade I rash and induration of the skin at the site of injection. These side effects resolved. No other toxicities were observed.
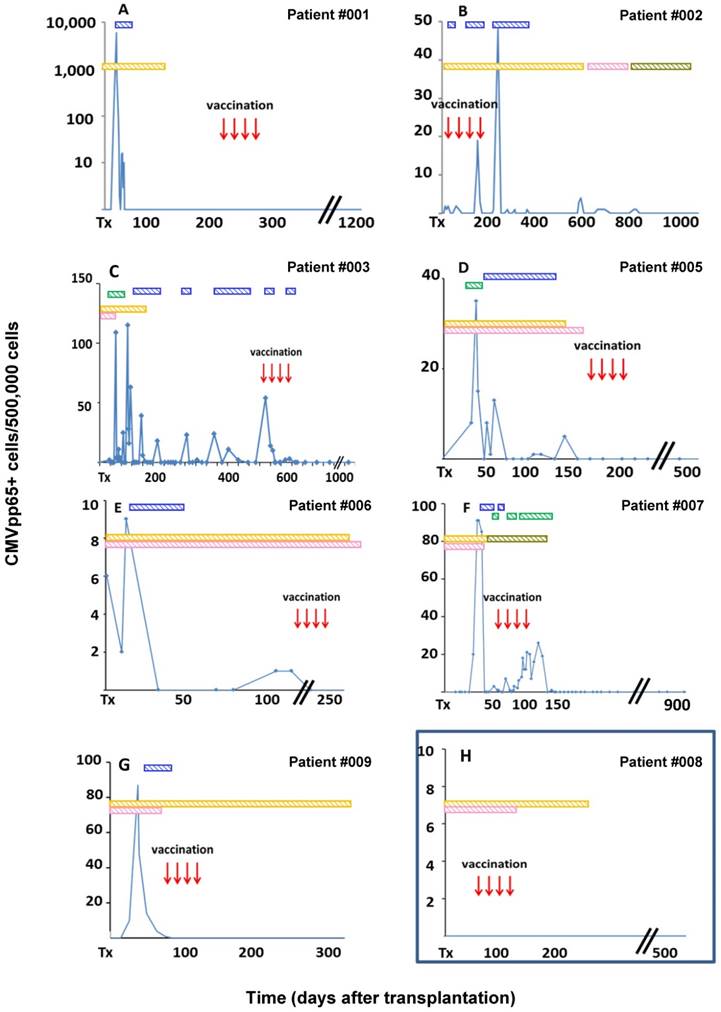
Seven of nine patients with CMVpp65 antigenemia cleared the virus after four vaccinations and have been free from antigenemia (maximum 2.5 years) despite cessation of antiviral prophylaxis (Fig. 1, A to G).The patient receiving prophylactic vaccination never developed antigenemia (Fig. 1 H). Two patients were not able to clear CMV antigenemia (Fig. 2).
The time point of inclusion showed a broad range because the CD4+ cell count needed to be >50/µl. Besides that, because of all the inclusion criteria heterogeneity was inevitable. Moreover the patients had dismal prognosis owing to diverse mutations and a high percentage of the patients had GvHD in their course, associated with a high risk of CMV reactivation.
Immunological Responses
Immunological responses to CMV infection or reactivation are complex. T cells recognizing CMVpp65 derived epitopes are considered to play a key role. Neutralizing antibodies are also important for the clearance of the viral load.
Clinical responders to preemptive vaccination and prophylactic vaccination in patients with a high risk for reactivation of CMV. (A)-(G) Seven of nine patients with CMVpp65 antigenemia assessed by immune fluorescence tests (IFT) responded to a sequence of four repetitive preemptive CMVpp65-peptide vaccinations by permanently clearing CMV despite cessation of antiviral therapy. (H) One out of ten patients received a sequence of four repetitive prophylactic CMVpp65 peptide vaccinations due to a high-risk constellation for CMV reactivation soon after transplantation. The patient did not develop CMV viremia. Antiviral drugs were administered over the time periods indicated by horizontal bars in the upper part of the respective panel: blue bars - valganciclovir and green bars - foscavir. Immunosuppressive drugs were given over the time periods indicated by yellow bars - cyclosporine A, pink bars - mycophenolate mofetil and olive green bars - tacrolimus.
Clinical non-responders to preemptive vaccination. (A, B) Two out of nine patients with CMVpp65 antigenemia assessed by immune fluorescence tests did not respond to a sequence of four repetitive preemptive CMVpp65-peptide vaccinations. CMVpp65 antigenemia could not be cleared by the patients' immune system. Antiviral drugs were administrated over the time periods indicated by horizontal bars in the upper part of the respective panel. Blue bars - valganciclovir, red bars - ganciclovir, green bars - foscavir. Immunosuppressive drugs were given over the time periods indicated by orange bars - cyclosporine A, pink bars - mycophenolate mofetil and olive green bars - tacrolimus.
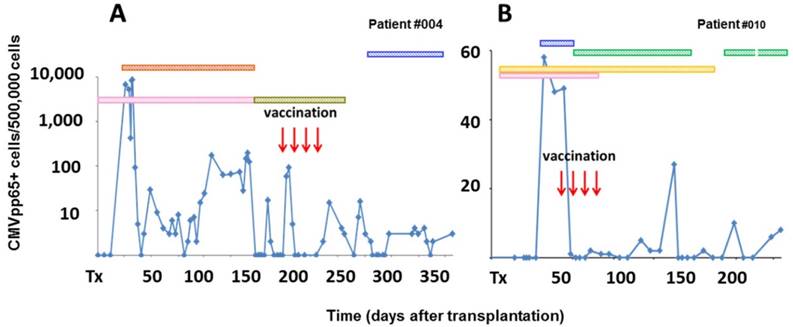
Of ten vaccinated patients, eight patients responded clinically: seven cleared the viral load and one patient never developed a virus reactivation after prophylactic vaccination (Fig. 1). In all of the eight clinical responders immune responses were observed (marked in red in Table 1; Fig. 3 to 5). In contrast, cellular or humoral immune responses could not be detected in the two non-responder patients #004 and #010 (Table 1; Fig. 2, 4 and 5). These two patients had multiple episodes of CMV reactivation, even after four vaccinations.
Classical CMV-specific T cells and CMV-specific neutralizing antibodies
Classical CMV-specific αβ CD8+ T cells (Fig. 3 and 4; Fig. S1), γδ T cells (Fig. 4 and Fig. S2) and CMV-specific neutralizing antibodies (Fig. 4 and Fig. S3) were assessed.
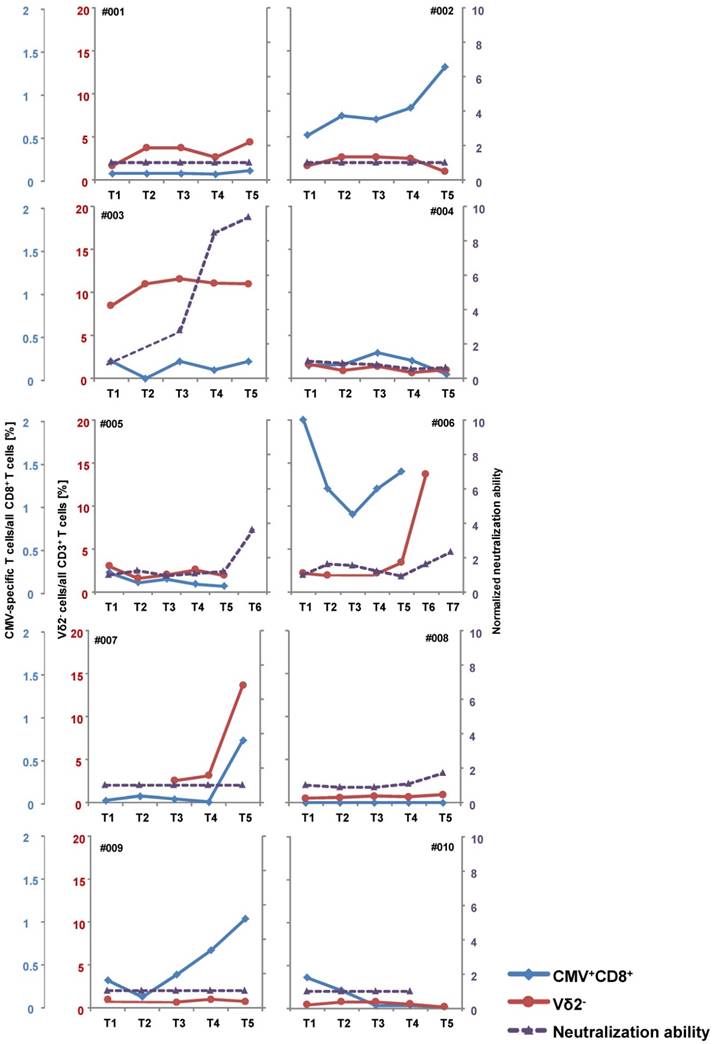
Patient #008 (receiving a prophylactic vaccination) never developed a population of CMV-specific T cells. The other patients already had a measurable frequency of CMV-specific T cells before vaccination. The frequency in patients #003, #004 and #005 ranged below 0.3% with no definite increase during vaccination. In patients #002, #007 and #009 the frequency of CMV-specific T cells clearly increased after vaccination. This increase was observed after at least two vaccinations. In patient #010 - a clinical non-responder, i.e. a patient with recurrent antigenemia - the initial frequency of 0.3% CMV-specific T cells decreased to zero. Patient #006 showed a fluctuation of the CMV-specific T cell subpopulation as displayed in Fig. 4 and Fig. S1.
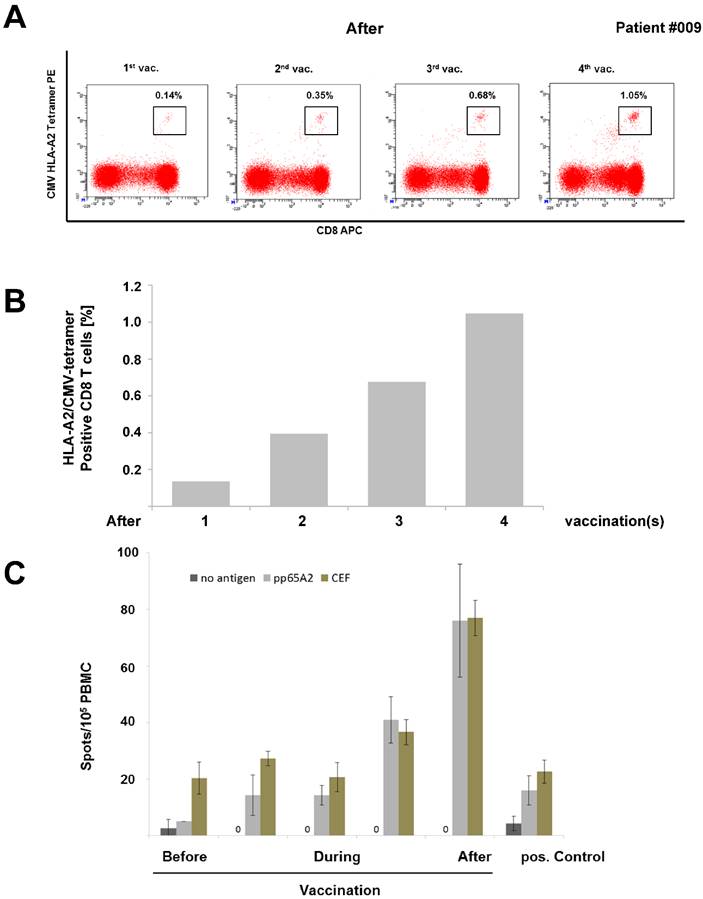
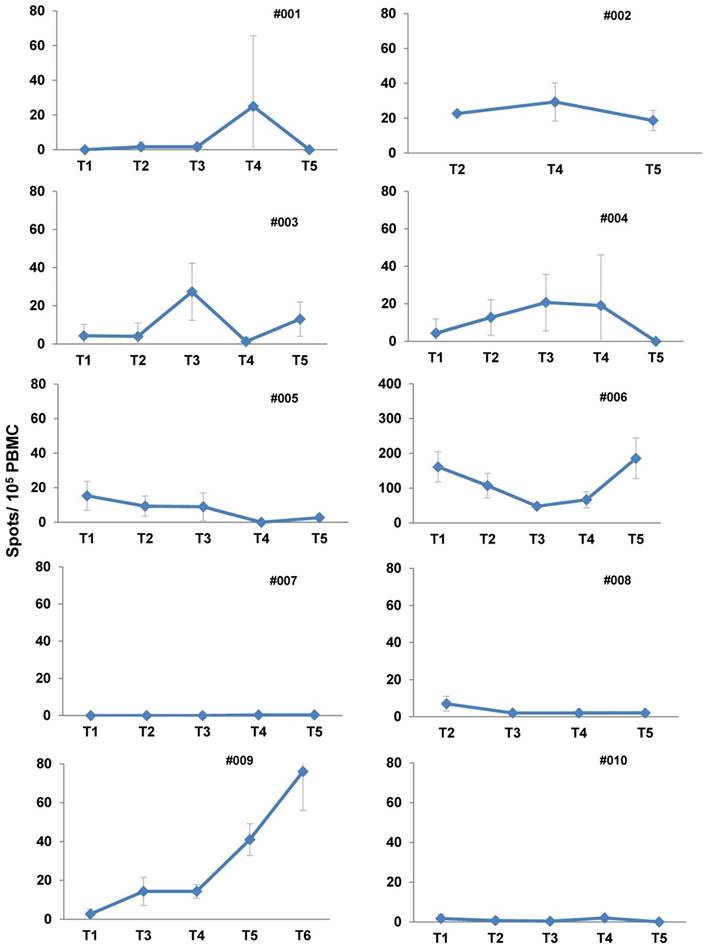
In two patients the increase in frequency of CMV-specific T cells assessed by tetramer-based flow cytometry was paralleled by an increase of IFNγ release as demonstrated in ELISPOT assay (Fig. 4 and 5), thus indicating that these T cells were activated. Fig. 3 (Patient #009) shows a representative increase in frequency of IFNγ secreting CMV-specific T cells over the vaccination period.
γδ CD3+ T cells were assessed in general as well as divided into Vδ2+ and Vδ2- subpopulations. An increase in total number of γδ T cells was observed in patients #001, #006 and #007, while the frequency of this population was fluctuating in patients #003, #004 and #010, and decreasing in patients #002, #005, #008 and #009 (blue lines Fig. S2). The subpopulation of Vδ2-/Vδ1+ cells (red lines in Fig. 4, Table 1) is of particular importance for the clearance of the CMV load [33]. These cells increased in patients #001, #003, #006 and #007, decreased in patients #002, #004 and #005, and did not show distinct dynamic changes in all other patients.
As for humoral immune responses, all nine patients enrolled in the preemptive arm of this study tested CMV-seropositive in standard commercial assays. These titers did not show any kinetics. To further assess humoral immune responses against CMV, an additional cell-based assay for neutralizing antibodies was established as described in the Methods section. Patient #003 showed robust neutralizing responses, nevertheless also in patients #005 and #008 an increase was seen. In patient #006 the titer of neutralizing antibodies fluctuated. Patients #003, #005 and #006 with increasing titers cleared the virus while patient #008 received prophylactic vaccinations and never developed a viremia. The most pronounced increase was observed in patients #003 and #005 who interestingly did not show either αβ or γδ T cells effective against CMV. In line with this, Meyers et al. demonstrated that there is not necessarily an antibody response involved in the protective mechanisms in allo-HSCT recipients [12, 34].
Increase of CMV-specific CD8+ T cells during vaccination. (A: dot plots, B: histogram) Multi-parametric flow cytometry revealed a several-fold increase of CD8+ / HLA-A2 CMVpp65-specific tetramer-positive T lymphocytes over the course of repetitive preemptive CMVpp65 vaccinations in patient #009 corresponding to a clearance of CMV. (C) ELISPOT assays for the release of IFNγ were performed after stimulation with the HLA-A2 restricted CMVpp65 peptide. Stimulation with CEF (CMV/EBV/FLU) control peptide pools served as a positive control, no peptide stimulation as a negative control. Peptide-specific T cell activity was measured by IFNγ and showed accordingly an increase over the course of vaccinations in patient #009. Wells were performed in triplicate for each group in the ELISpot assay.
Immunomonitoring. The frequency of CMV-specific CD8+ T cells (blue curves), Vδ2 negative CD3+ γδ T cells (red curves) and CMV neutralizing antibody titers (purple curves) were assessed over the course of CMV peptide vaccination as described in the Materials & Methods section. Time points of assessment: T1 - before 1st vaccination; T2/T3/T4/T5 - after 1st/2nd/3rd/4th vaccination; T6/T7 - approximately one or three months after 4th vaccination. CMV-specific CD8+ T cells increased in patients #002, #006, #007 and #009 which was paralleled by an increase of γδ CD3+ T cells in patients #006 and #007. Also in patients #001, #003 and #008 the γδ T cell frequency increased. Increasing titers of neutralizing antibodies were observed in patient #003, #005, #006 and #008.
Enzyme linked immunospot (ELISPOT) assays. The number of IFNγ secreting CD8+ T cells was assessed by ELISPOT assays over the course of CMV peptide vaccination as described in the Methods section. Time points of assessment: T1 - before 1st vaccination; T2/T3/T4/T5 - after 1st/2nd/3rd/4th vaccination; T6/T7 - approximately one or three months after 4th vaccination. Wells were performed in triplicate for each group in the ELISpot assay.
When taking these three parameters αβ and γδ T cells synoptically together with neutralizing antibodies (Table 1) we could differentiate three categories of immune responses: 1) a clear increase of CMV-specific αβ CD8+ T cells in three patients (#002, #007 and #009), 2) a clear increase in Vδ2- γδ T cells in four patients (#001, #003, #006 and #007) and 3) increasing titers of neutralizing antibodies in four patients (#003, #005, #006 and #008). Both categories of immune responses 1) plus 2) overlapped in patient #007, as well as types 2) plus 3) in patient #006. Early responses after one to two vaccinations were observed in patients #001, #002 and #003. In all other patients clinical and immunological responses were observed at later time points. Patients #004 and #010 were clinical and immunological non-responders without cellular or humoral responses. All patients showing at least a response in one of these three categories of immune responses also had a clinical benefit.
Discussion
Our group designed and manufactured a novel CMV peptide vaccine. The major finding of the phase I clinical trial employing this vaccine was that 80% of the vaccinated patients cleared the virus and showed positive results in immunomonitoring for γδ T cells, CMV-specific αβ T cells and neutralizing antibodies. Antiviral drugs could be discontinued and the control of CMV was enduring (Fig. 1, Table 1). In contrast the two non-responders did not clear the virus and did not show any immune response (Fig. 2, Table 1).
The example of two non-responders (Fig. 2) is quite paradigmatic for patients at highest risk with the CMV sero-constellation R(ecipient)+/D(onor)-: these patients are prone to multiple reactivations; cessation of immunosuppressive drugs might not necessarily improve this situation. Multiple reactivations cause repetitive or continuous use of antiviral drugs resulting in myelosuppression opening the door for further infectious complications. In this situation, antiviral drug therapy does not result in enduring clearance of the virus, but may select for resistant viruses.
Humoral and cellular immune responses play a role in the clearance of the CMV load [2, 14, 35].
The induction of such multifactorial responses is highly desirable. Despite the fact that we used solely nonamer peptide derived from CMVpp65 we were able to induce both cellular and humoral immune responses. It is of particular note that eight of ten stem cell donors were CMV seronegative and therefore at particularly high risk to CMV reactivation. This R+/D- combination could not be sufficiently treated by any adoptive transfer of CMV recognizing T cells so far. Third party donor lymphocytes reactive to viruses were often rejected by the recipient.
Hitherto, no commercial vaccine against CMV is available. Several products are under current investigation in phase I to phase III clinical trials including attenuated viruses, truncated proteins as well as DNA vaccines [23, 36-38]. Despite the fact that we used a mere peptide vaccine, we observed good clinical effects in 80% of our patients after vaccination. Compared with 25 to 50% immune responses to other vaccines comprising truncated pp65, IE1 and gB proteins or DNA encoding these viral proteins under current development [23, 36-38], our results are very promising.
Our study is an early phase I trial including only ten patients. However, this small cohort groups patients at highest risk for CMV reactivation after allogeneic HSCT. The risk constellation of the serostatus is R+/D- in eight of ten patients. These patients normally develop a deep and long lasting lymphopenia, with an absence of CMV-specific T cells and even B cells, particularly after administration of antibodies against CD20 and CD52 as in patients #005 and #006. Administration of antiviral drugs is temporarily effective, but CMV antigenemia returns immediately after cessation of the antiviral drug therapy. Moreover, these drugs are myelo- and nephrotoxic. The fatal outcome of patient #004 illustrates this dilemma of anti-CMV drug therapy.
Interestingly standard care with antiviral and immunosuppressive drugs did not hamper the development of a CMV-specific immune response. This is in line with recent in vitro observations [39]. Moreover cessation or reduction of immunosuppressive drugs in patients #003 and #007 did not automatically result in a cessation of CMV antigenemia. Under continuous immunosuppression in patients #002, #006 and #009 CMV antigenemia was eventually cleared after four vaccinations. These findings clearly demonstrate the impact of peptide vaccination on the clearance of the virus.
We assumed that a vaccination only with the nonamer epitope peptide derived from CMVpp65 would not be sufficient to elicit specific T cell immune responses to our vaccine. However, emulsification with Montanide® and adding GM-CSF as a second adjuvant might even elicit or augment cellular or humoral immune responses against CMV in the whole context/immunological environment.
In our study very limited side effects occurred as one would expect in the context of Montanide®-based peptide vaccines [40]. This is in keeping with the lack of adverse events (AE) we observed when vaccinating patients with RHAMM-R3 [32, 41, 42]. Our CMV vaccine was used rather as a therapeutic vaccine (at least in 9 of 10 patients). Only one patient received the vaccine prophylactically. The problem after allo-stem cell transplantation is that viral drugs of CMV reactivated patients have strong toxic effects (myelosuppression, nephrotoxicity and mortality) and CMV vaccination avoids these, therefore there is a high medical need for such therapeutic approaches as these spare the patients many strong and adverse side effects.
Immunological responses corresponded with clinical responses. As shown in Table 1 the development of CMVpp65-specific T cell responses was preceded or coincided by γδ T cell responses. CMV-specific CD8+ T cells were functional as demonstrated in ELISPOT assays.
At the time of the first vaccination, patients #003, #004, #005, #006 and #010 had already experienced multiple episodes of CMV reactivation despite the presence of CMV-specific CD8+ T cells at least at low frequency. This observation underlines that CD8+ T cell responses might not be sufficient in all cases and other mechanisms might help to clear the virus from the bloodstream.
Next to the adaptive immune system the innate immune system in form of γδ T cells can also contribute to clearance of CMV load [30]. The traditional view is that a peptide vaccine if successful would elicit α/β T cells. Exact mechanisms for stimulating γ/δ T cells and neutralizing antibodies still need to be elucidated. There is an increasing body of evidence that γ/δ T cells do play a role in this setting [43].
Montanide and GM-CSF generally activate class II long peptide epitope recognition leading to CD4+ helper T cell and subsequent B cell activation resulting in class switch from IgM to IgG antibodies eventually leading to neutralizing antibodies. Only patients with CD4+ T cell recovery > 50/µl were eligible to participate, it is possible that this is an important factor to take in account to explain the results. γ/δ T cells are also activated through the adjuvants Montanide and GM-CSF.
In four of ten patients we also observed humoral responses augmented under vaccination. This is in line with reports from Spanish colleagues [35] who described a synergy of humoral and cellular immune responses against the virus.
There is an ongoing debate on the issue that in cancer patients both immune responses against CMV and tumor/leukemia were observed [44]. This might suggest a cross-reactivity of T cells. As recently overlapping epitopes of CMVpp65 and tumor/leukemia antigens could be detected, the basis of this twofold immune response might rather be the T cell stimulatory milieu created by T cell reactivity against the virus and thus also stimulate anti-leukemia T cell clones. Of note all patients in our clinical trial remained in CR.
In responsive patients four vaccinations were needed to elicit cellular or humoral responses. For non-responders a higher number of vaccinations should be considered for further trials in line with observations made in the field of therapeutic cancer vaccines [38].
Modulations of the immune system like CMV vaccination employing general activation of the immune system like GM-CSF or Montanide® might elicit GvHD. Nemunaitis demonstrated that even doses of up to 500µg/d GM-CSF over three to four weeks after allo-HSCT did not result in a higher frequency or intensity of GvHD in the patients [45].
In our cohort of ten patients we saw seven cases of GvHD, five of them occurring after transplantation (Table 2). All GvHD cases had limited disease (LD) and were responsive to steroids alone or in combination with calcineurin inhibitors. Only patient #002 required more and complex GvHD treatment. Because of the onset 30 to 124 days after the first vaccination an effect of the vaccine on GvHD cannot be completely ruled out. But GvHD might also be caused in these cases by CMV reactivation per se or by the reduced intensity conditioning (RIC) regimen where we see GvHD in up to 70% of the cases. Incidence of this frequency and intensity of GvHD should not hamper further testing of our vaccine in larger cohorts.
Taken together, administration of CMVpp65 peptide in Montanide™ plus GM-CSF as vaccination was safe and well tolerated in all patients with allogeneic stem cell transplantation. The clinical effects were very encouraging. We were able to detect an expansion of activated CMVpp65-specific CD8+ T cells as well as γδ CD3+ T cells, as well as an increase in titers of neutralizing antibodies. This indicates that our approach of peptide in water-in-oil emulsion can induce multiple immune responses even in immunocompromised CMV-seropositive patients transplanted from seronegative stem cell donors. Therefore, our (peptide) vaccine constitutes a promising option for patients at risk for CMV reactivation warranting further studies, even in the field of solid organ transplantation. We have therefore already initiated a phase I trial in patients before kidney Tx. Currently we are planning a phase II trial in which we will incorporate a placebo group.
Supplementary Material
Supplementary figures.
Acknowledgements
The authors thank the NIH tetramer facility for providing the CMVpp65495-503 monomer, Mandy Hinkelbein for taking care of the graphical work, Dr. Amy Publicover for proofreading the manuscript as native speaker, and Ulrike Gern, Cornelia Herbst, Stefanie Mechler, Petra Richter and Vanessa Schneider for technical assistance.
This work was supported by generous grants from the Bundesministerium für Bildung und Forschung (BMBF), i.e. the German Federal Ministry for Education and Research. Additional support was granted from the German Cancer Consortium/Deutsches Konsortium für Translationale Krebsforschung (DKTK). (BMBF 01KI0772, DKTK-GMP).
This study was designed by MS and JG. MS, MW, BM, MG, AS, AH, PS were critically involved in obtaining manufacturing licenses and manufacturing the vaccines. MS, AS, PW, JG, SH were study physicians. ZW and TM developed an assay to measure the CMV-neutralizing antibodies. JK assessed the γδ T cell frequencies. Further lab research was performed by MG, SH and LW. The manuscript was written by MS, AS and JG. PD and DB included patients and helped writing the manuscript. ADH, HD and HS discussed the manuscript. VE was critically involved in the set-up of flow cytometry assays.
Competing Interests
J.K. is inventor of multiple patents dealing with γδ TCRs and co-founder as well as chief scientific officer (CSO) of Gadeta (http://www.gadeta.nl/). The other authors have declared that no competing interest exists.
References
1. Boeckh M, Murphy WJ, Peggs KS. Reprint of: Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant. 2015;21:S19-24
2. Ljungman P, Brand R, Hoek J, de la Camara R, Cordonnier C, Einsele H. et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis. 2014;59:473-81
3. Einsele H, Mielke S, Grigoleit GU. Diagnosis and treatment of cytomegalovirus 2013. Curr Opin Hematol. 2014;21:470-5
4. Bacigalupo A, Bregante S, Tedone E, Isaza A, Van Lint MT, Trespi G. et al. Combined foscarnet-ganciclovir treatment for cytomegalovirus infections after allogeneic hemopoietic stem cell transplantation. Transplantation. 1996;62:376-80
5. Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L. et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11:284-92
6. Chemaly RF, Ullmann AJ, Stoelben S, Richard MP, Bornhauser M, Groth C. et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370:1781-9
7. Boeckh M, Murphy WJ, Peggs KS. Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant. 2015;21:24-9
8. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G. et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64:87-91
9. Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D. et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407-14
10. Quinnan GV Jr, Kirmani N, Rook AH, Manischewitz JF, Jackson L, Moreschi G. et al. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307:7-13
11. Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373-80
12. Meyers JD, Flournoy N, Thomas ED. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986;153:478-88
13. Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J Immunol. 2002;168:5455-64
14. Hanley PJ, Bollard CM. Controlling cytomegalovirus: helping the immune system take the lead. Viruses. 2014;6:2242-58
15. Riddell SR, Rabin M, Geballe AP, Britt WJ, Greenberg PD. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795-804
16. Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102-8
17. Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238-41
18. Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C. et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6:242ra83
19. Odendahl M, Grigoleit GU, Bonig H, Neuenhahn M, Albrecht J, Anderl F. et al. Clinical-scale isolation of 'minimally manipulated' cytomegalovirus-specific donor lymphocytes for the treatment of refractory cytomegalovirus disease. Cytotherapy. 2014;16:1245-56
20. Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M. et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591-9
21. Grigoleit GU, Kapp M, Hebart H, Fick K, Beck R, Jahn G. et al. Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J Infect Dis. 2007;196:699-704
22. Salguero G, Daenthanasanmak A, Munz C, Raykova A, Guzman CA, Riese P. et al. Dendritic cell-mediated immune humanization of mice: implications for allogeneic and xenogeneic stem cell transplantation. J Immunol. 2014;192:4636-47
23. Nakamura R, Rosa CL, Longmate J, Drake J, Slape C, Zhou Q. et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol. 2016;3:e87-98
24. Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK. et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:290-9
25. Cardenoso L, Pinsky BA, Lautenschlager I, Aslam S, Cobb B, Vilchez RA. et al. CMV antigenemia and quantitative viral load assessments in hematopoietic stem cell transplant recipients. J Clin Virol. 2013;56:108-12
26. Wu Z, Sinzger C, Reichel JJ, Just M, Mertens T. Natural killer cells can inhibit the transmission of human cytomegalovirus in cell culture by using mechanisms from innate and adaptive immune responses. J Virol. 2015;89:2906-17
27. Schmitt A, Bechter C, Yao J, Goetz M, Maccari B, Schauwecker P. et al. Cytomegalovirus vaccination of leukemia and lymphoma patients after allogeneic stem cell transplantation-validation of a peptide vaccine. J Immunol Methods. 2009;343:140-7
28. Greiner J, Li L, Ringhoffer M, Barth TF, Giannopoulos K, Guillaume P. et al. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood. 2005;106:938-45
29. Schmitt M, Schmitt A, Reinhardt P, Thess B, Manfras B, Lindhofer H. et al. Opsonization with a trifunctional bispecific (alphaCD3 x alphaEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM positive tumor cells by cytotoxic T lymphocytes. Int J Oncol. 2004;25:841-8
30. Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S. et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27:1328-38
31. Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111-8
32. Schmitt M, Schmitt A, Rojewski MT, Chen J, Giannopoulos K, Fei F. et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood. 2008;111:1357-65
33. Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S. et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116:2164-72
34. Meyers JD, Bowden RA, Counts GW. Infectious complications of marrow transplant: risk factors for infection. Prog Clin Biol Res. 1989;309:357-66
35. Gimenez E, Blanco-Lobo P, Munoz-Cobo B, Solano C, Amat P, Perez-Romero P. et al. Role of cytomegalovirus (CMV)-specific polyfunctional CD8+ T-cells and antibodies neutralizing virus epithelial infection in the control of CMV infection in an allogeneic stem-cell transplantation setting. J Gen Virol. 2015;96:2822-31
36. Bernstein DI, Munoz FM, Callahan ST, Rupp R, Wootton SH, Edwards KM. et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine. 2016;34:313-9
37. Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML. et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191-9
38. Wloch MK, Smith LR, Boutsaboualoy S, Reyes L, Han C, Kehler J. et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197:1634-42
39. Stachel D, Stevens-Ayers T, Boeckh M. In vitro studies of the impact of maribavir on CMV-specific cellular immune responses. J Clin Virol. 2016;75:53-9
40. Parmiani G, Pilla L, Castelli C, Rivoltini L. Vaccination of patients with solid tumours. Ann Oncol. 2003;14:817-24
41. Giannopoulos K, Dmoszynska A, Kowal M, Rolinski J, Gostick E, Price DA. et al. Peptide vaccination elicits leukemia-associated antigen-specific cytotoxic CD8+ T-cell responses in patients with chronic lymphocytic leukemia. Leukemia. 2010;24:798-805
42. Greiner J, Schmitt A, Giannopoulos K, Rojewski MT, Gotz M, Funk I. et al. High-dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Haematologica. 2010;95:1191-7
43. Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L. et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol. 2017
44. Schuessler A, Walker DG, Khanna R. Cytomegalovirus as a novel target for immunotherapy of glioblastoma multiforme. Front Oncol. 2014;4:275
45. Nemunaitis J, Anasetti C, Storb R, Bianco JA, Buckner CD, Onetto N. et al. Phase II trial of recombinant human granulocyte-macrophage colony-stimulating factor in patients undergoing allogeneic bone marrow transplantation from unrelated donors. Blood. 1992;79:2572-7
Author contact
![]() Corresponding author: Univ.-Prof. Dr. med. Michael Schmitt, MHBA Cellular Immunotherapy, GMP Core Facility, Department of Internal Medicine V University Hospital of Heidelberg Im Neuenheimer Feld 410 69120 Heidelberg, Germany Tel. +49-(0)6221-56-6614 FAX +49-(0)6221-56-5740 michael.schmittuni-heidelberg.de
Corresponding author: Univ.-Prof. Dr. med. Michael Schmitt, MHBA Cellular Immunotherapy, GMP Core Facility, Department of Internal Medicine V University Hospital of Heidelberg Im Neuenheimer Feld 410 69120 Heidelberg, Germany Tel. +49-(0)6221-56-6614 FAX +49-(0)6221-56-5740 michael.schmittuni-heidelberg.de
 Global reach, higher impact
Global reach, higher impact