13.3
Impact Factor
Theranostics 2017; 7(17):4289-4300. doi:10.7150/thno.21092 This issue Cite
Research Paper
Label-Free Biochips for Accurate Detection of Prostate Cancer in the Clinic: Dual Biomarkers and Circulating Tumor Cells
1. Institute of Medical Science and Technology, National Sun Yat-sen University, Kaohsiung 80424, Taiwan
2. Division of Urology, Department of Surgery, Linkou Chang Gung Memorial Hospital, Taoyuan 33305, Taiwan.
School of Medicine, Chang Gung University, Taoyuan 33302, Taiwan.
* These authors contributed equally.
Abstract
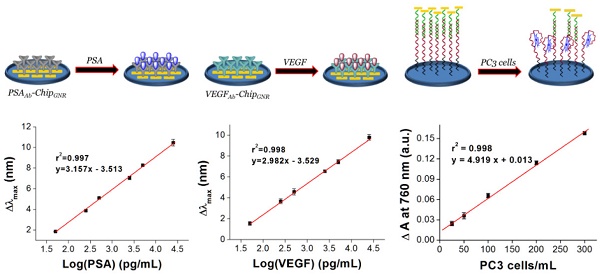
Purpose: Early diagnosis of prostate cancer (PCa) is essential for the prevention of metastasis and for early treatment; therefore, we aimed to develop a simple, accurate, and multi-analyte assay system for early PCa diagnosis in this study. Experimental design: We fabricated three kinds of biochips then integrated into microfluidic device for simultaneous detection of vascularendothelial growth factor (VEGF), prostate-specific antigen (PSA), and PCa circulating tumor cells (CTC) in human serum for accurate diagnosis of PCa. Then the integrated device can be put in the ELISA reader for signal analysis after sample incubation, no necessary of further fluorescence staining or microscopy counting. Result: The integrated device has wide liner detection ranges (0.05-25 ng/mL for both PSA and VEGF, and 5-300 cells/mL for PCa CTC), as well as high levels of sensitivity and selectivity, and demonstrated a high correlation with an enzyme-linked immunosorbent assay for sample detection in patients. Also, the presented biochips could maintain their stability when stored at 37°C for 49 days without significant differences in the red-shift (<5%). Conclusions: We have successfully developed a multi-analyte sensing system for rapid and easy detection of PSA, VEGF, and PC3 cells in PCa samples using label-free glass-based chips. This method presents the advantages of a broad working range, high specificity, label-free, high-speed, stability, and low cost detection method for point-of-care testing of PCa.
Keywords: label-free biochip, prostate cancer diagnosis, PSA, VEGF, circulating tumor cells.
 Global reach, higher impact
Global reach, higher impact