13.3
Impact Factor
Theranostics 2018; 8(1):237-255. doi:10.7150/thno.21945 This issue Cite
Review
Exosome Theranostics: Biology and Translational Medicine
1. Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, National Ministry of Education & Key Laboratory of Molecular Biology in Medical Sciences, Zhejiang Province), The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009 China;
2. Institute of Translational Medicine, Zhejiang University, Hangzhou 310029 China;
3. College of Basic Medical Sciences, School of Medicine, Zhejiang University, Hangzhou, 310058 China.
Received 2017-7-16; Accepted 2017-10-4; Published 2018-1-1
Abstract
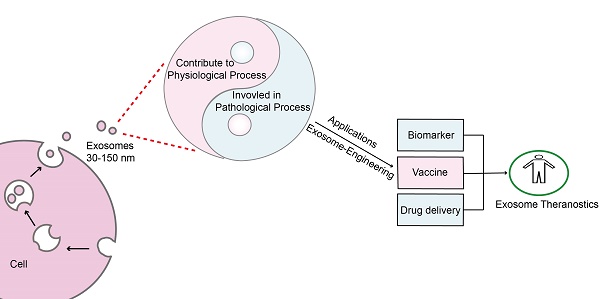
Exosomes are common membrane-bound nanovesicles that contain diverse biomolecules, such as lipids, proteins, and nucleic acids. Exosomes are derived from cells through exocytosis, are ingested by target cells, and can transfer biological signals between local or distant cells. Exosome secretion is a constitutive phenomenon that is involved in both physiological and pathological processes and determines both the exosomal surface molecules and the contents. Hence, we can exploit exosomes as biomarkers, vaccines and drug carriers and modify them rationally for therapeutic interventions. However, it is still a challenge to identify, isolate and quantify exosomes accurately, efficiently and selectively. Further studies on exosomes will explore their potential in translational medicine and provide new avenues for the creation of effective clinical diagnostics and therapeutic strategies; the use of exosomes in these applications can be called exosome theranostics. This review describes the fundamental processes of exosome formation and uptake. In addition, the physiological and pathological roles of exosomes in biology are also illustrated with a focus on how exosomes can be exploited or engineered as powerful tools in translational medicine.
Keywords: exosome, extracellular vesicle, translational medicine, biomarker, drug delivery.
Introduction
Exosomes are nanoscale extracellular lipid bilayer vesicles of endocytic origin, and they are secreted by nearly all cell types in physiological and pathological conditions. Initial studies regarded exosomes as a simple means for the disposal of unwanted cellular components [1]. They have now been shown to play a crucial role in intercellular communication through the intercellular transfer of nucleic acids and specific repertoires of proteins and lipids, which is important for protein and lipid homeostasis [2]. During these processes, exosomes can regulate the properties of target cells, which can be beneficial or detrimental [3]. Exosomes contribute to fundamental physiological processes, such as neuronal communication [4], antigen presentation [5], immune responses [6], organ development [7], and reproductive performances [8]. They also participate in some pathological disorders, including cancer progression [9], cardiovascular disease [10], and inflammation [11], and they even favor viral infection [12] and prion dissemination [13]. Given that exosomes can carry toxic damaged forms of aggregated proteins that are fated for destruction, they are also relevant to the progression of neurodegenerative diseases [14].
Exosome secretion occurs naturally, and cellular stress and activation signals can modulate the involved processes [15]. They can be found in multiple types of extracellular fluids, such as blood, urine, amniotic fluid, saliva, cerebrospinal fluid, and even breast milk [16-19]. The heterogeneity of exosome size and cargo reflect the state and types of the cells of origin. Thus, exosomes can be used as biomarkers for disease diagnostics and even fetal sex determination [20]. Since surface-bound proteins on exosomes stem from the plasma membranes of the cells from which they originated, exosomes released by antigen-presenting cells (APCs), dendritic cells (DCs) and tumor cells are promising for use in vaccines development. Moreover, exosomes can protect their cargoes from clearance or damage by the complement fixation or macrophages due to their double-layered membrane and nanoscale size, thus prolonging their circulation half-life and improving their biological activity. Hence, exosomes could potentially be used as drug delivery vesicles for treating disease. Furthermore, exosome engineering, i.e., the chemical or biological modification of these nanoscale extracellular lipid bilayer vesicles, may provide opportunities to enhance or broaden the innate therapeutic capability of exosomes.
Definition, biogenesis, uptake, and isolation of exosomes
Definition
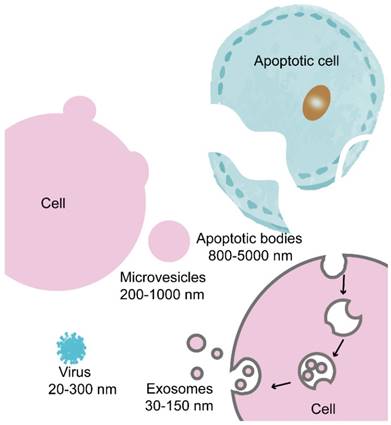
Cells are known to secrete various types of extracellular vesicles (EVs), which are differentiated based on their size, content, and formation mechanism [21, 22]. The primary EV class includes apoptotic bodies, microvesicles and exosomes (Figure 1). However, no precise methods are available to distinguish and isolate exosomes from other EVs. Many studies have convincingly shown that both exosomes and microvesicles contained specific sets of proteins and nucleic acids that act as agents of intercellular communication agents [1, 23]. Apoptotic bodies and microvesicles are generated from the plasma membrane by direct budding and exist in a broad range of dimensions (apoptotic bodies, 800-5000 nm; microvesicles, 200-1000 nm [24, 25]), whereas exosomes are vesicles of endocytic origin with a diameter of 30-150 nm [26-28]. Some vesicles that have a diameter of >150 nm can be generated by an endosomal pathway [29], and other vesicles of <150 nm in diameter can bleb directly from the plasma membrane [30, 31]. Therefore, the size-based differentiation of exosomes must be undertaken carefully.
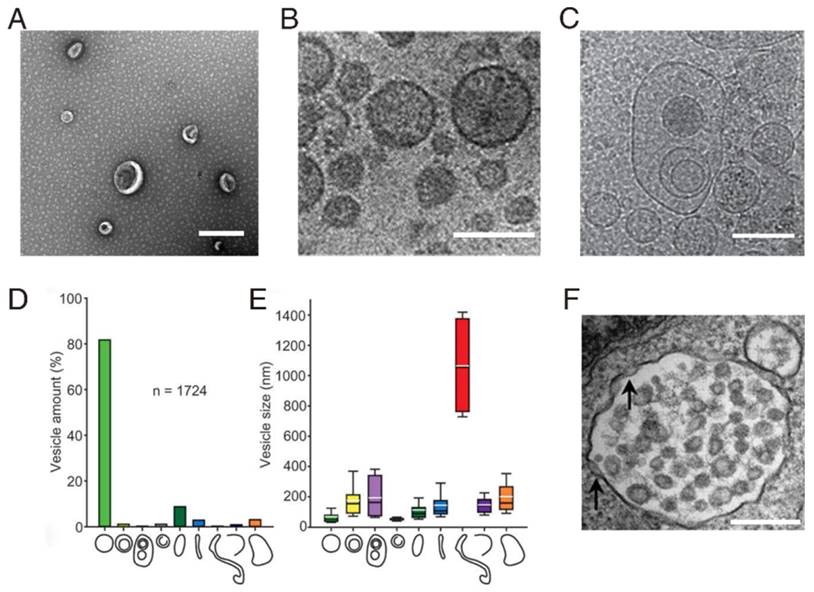
One of the standard methods for characterizing exosomes is negative staining and visualization by electron microscopy. Exosomes usually appear as cup-shaped entities by transmission electron microscopy (Figure 2A), but as round-shaped vesicles by cryoelectron microscopy (Figure 2B). A high level of morphological diversity among exosomes isolated from different body fluids has been described [33, 34], suggesting the existence of exosome subpopulations of exosomes with different functions and biochemical contents. Even when purified from a single cell type, exosome morphology remains diverse. Using multiple cryoelectron microscopy techniques, Zabeo et al. found nine different morphological categories morphology of exosomes (Figure 2C, D, and E) derived from the human mast cell line (HMC-1) [35].
Schematic representation of the biogenesis of apoptotic bodies, microvesicles and exosomes. Apoptotic bodies are vesicles that separate from post-apoptotic cells. Microvesicles are derived from the plasma membranes of most cell types. Exosomes are EVs of endocytic origin that are derived from most cell types. Viruses and exosomes are strikingly similar in structure and size.
Biogenesis
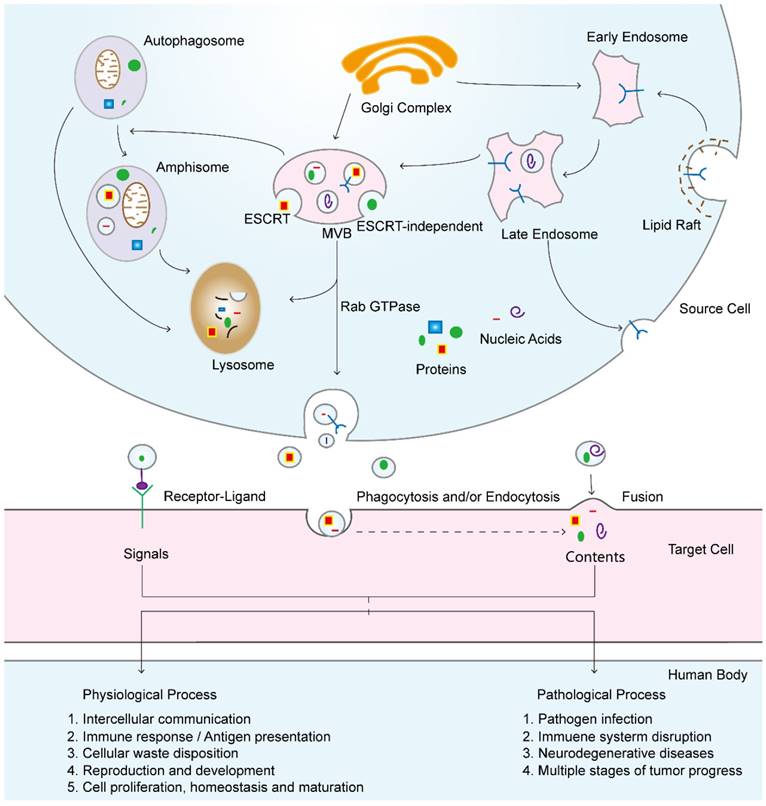
Some mechanisms have been recognized with respect to the progression of exosomes formation, but much remains to be understood. First, endocytic vesicles arise in lipid raft domains of the plasma membrane through endocytosis, leading to the intracellular formation of early endosomes. With the assistance of the Golgi complex, these early endosomes become late endosomes [6, 38], and intraluminal vesicles (ILVs) accumulated in their lumen during this process. The molecules that exist in early endosomes can be either recycled back to the plasma membrane or incorporated into ILVs [39]. Cargo sorting into the ILVs is mediated by endosomal sorting complexes required for transport (ESCRT)-dependent [40] and ESCRT-independent mechanisms [41, 42]. These vesicles accumulate in late endosomes by the inward budding of the early endosomal membrane and cytosol sequestration, thus transforming endosomes into multivesicular bodies (MVBs) (Figure 2F) [37]. Subsequently, these MVBs fuse with either lysosomes, in which the ILVs are degraded, or the plasma membrane, which results in the release of their internal vesicles (Figure 3), i.e., exosomes, into the extracellular space and the incorporation of the peripheral MVB membrane into the plasma membrane [23, 43]. Importantly, the mechanisms of MVB trafficking and fusion with the cell membrane are regulated by several Rab guanosine triphosphatase (GTPase) proteins and are coordinated with cytoskeletal and molecular motor activities [44, 45]. Although the mechanism that directs MVB traffic to the lysosomes instead of the plasma membrane for fusion remains elusive [46], some studies have indicated the possible simultaneous presence of different MVB subpopulations in cells, some of which are fated for degradation or exocytosis [47]. However, the mechanisms that are involved in the regulation of exosome secretion are poorly understood. A recent study showed that the actin cytoskeletal regulatory protein cortactin plays an important role in regulating exosome secretion. They found that cortactin, Rab27a, and coronin 1b coordinate to control the stability of cortical actin docking sites in multivesicular late endosomes, thus contributing to exosome secretion [48].
Uptake
Exosome selection and uptake by recipient cells is highly intriguing. According to the results of past studies, signals are transferred from exosomes to recipient cells by three methods: receptor-ligand interactions, direct membrane fusion, and endocytosis/ phagocytosis (Figure 3). Some studies have also described the pathways of transmembrane signal transduction between exosomes and recipient cells [49]. For example, a particular study showed that extracellular EVs, including exosomes, contributed to communication between neural stem/precursor cells and the microenvironment through receptor-ligand interactions [50]. First, free interferon (IFN)-γ binds to EV-associated interferon gamma receptor 1 (IFNGR-1) to form IFN-γ/IFNGR-1 complexes; then, the EV-associated complex activates signal transduction via the Stat1 pathway in target cells. In addition, some studies have suggested that exosomes transfer their contents into cells by direct fusion with the recipient cell membrane [51]. However, these observations do not clarify the molecular mechanism associated with the fusion of exosomes with target cells. An increasing number of studies suggest that internalization is the primary method for exosome uptake. A well-summarized review described several endocytic pathways, such as macropinocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, lipid raft-mediated endocytosis, and phagocytosis, used by cells for exosome uptake [52]. Remarkably, a different exosome internalization route was identified recently. Heusermann et al. showed that exosomes are recruited to the cell body by surfing on filopodia and are then endocytosed via a process that resembles virus entry [53], suggesting the existence of unanticipated routes for subcellular cargo delivery.
Under some conditions, such as low temperature, the proteolytic cleavage of exosomal surface proteins can inhibit exosome uptake [32]. Notably, these findings indicate that the internalization of exosomes depends on cell type and exosomal surface proteins [54]. Many proteins that facilitate exosome uptake have been found, such as the tetraspanin membrane proteins CD9 and CD81 and intercellular adhesion molecule (ICAM)-1 [55, 56]. In addition, some studies have shown that heparan sulfate proteoglycans (HSPGs) are used as internalizing receptors by cancer cell-derived exosomes [3]. The size distribution of exosomes can also profoundly affect their internalization; cells preferentially uptake smaller exosomes [57].
Exosome uptake by recipient cells is cell-specific. However, the mechanism of recipient cell selection is not yet clear. The interaction of surface molecules between specific cells and exosomes is critical for recipient cell targeting and adhesion. Proteins such as annexins and integrins are crucial for cell adhesion, similar to tetraspanins, which can directly target specific cells, e.g., endothelial cells, and subsequently promote angiogenesis and vasculogenesis [58]. Several studies have indicated that the tropism of exosomes might be determined by adhesion-associated molecules on the surface of exosomes, such as integrins, tetraspanins, and other glycoproteins [59]. For instance, some researchers have demonstrated that EVs containing the rabies viral glycoprotein show a tropism to the brain [60, 61]. Notably, one study showed that the distinct integrin expression patterns of tumor-derived exosomes were important contributors to organotropic metastasis. For example, exosomes that have α6β4 and α6β1 integrins can be taken up by lung-resident fibroblasts and epithelial cells to promote lung metastasis, while exosomes with the αvβ5 integrin are specifically taken up by Kupffer cells in the liver, thereby mediating liver tropism [62].
Characterization of exosome-like vesicles. (A) Transmission electron micrograph of exosomes isolated from urine; scale bar, 400 nm. (B) Cryoelectron microscopy image showing extracellular vesicles secreted by MLP-29 cells; scale bar, 100 nm. (Reproduced with permission from reference [36]. Copyright © 2008 American Chemical Society.) (C) Example of triple or higher-multiple vesicles; scale bar, 150 nm. (D) Percentage of each morphological category among the total number of vesicles. (E) Size distribution for each vesicle category. (C, D, E: reproduced with permission from reference [35]. Copyright © 2017 Taylor & Francis Group.) (F) Electron micrograph of double membrane-bound exosomes in multivesicular bodies (MVBs); inward invagination (arrows) in the MVB membrane indicates the beginning of exosome biogenesis, scale bar, 100 nm. (Reproduced from reference [37]. Copyright © 2011 American Heart Association, Inc.)
Isolation
Exosome purification is essentially based on immunoaffinity capture, size exclusion, polymeric precipitation, ultracentrifugation, and microfluidics techniques [63]. These techniques are not always mutually exclusive, and their combinations may be valuable [64]. In terms of these isolation methods, a standardization procedure proposed by the International Society for Extracellular Vesicles (ISEV) could provide detailed guidance [63]. To date, the most widely adopted and reliable method is ultracentrifugation, which involves a series of centrifugation steps that could be followed by flotation-sedimentation density gradient centrifugation [65]. However, this method is tedious, time consuming, and requires large biological sample volumes. Therefore, commercially available polymeric precipitation mixtures have been widely applied to isolate exosomes for various purposes [66, 67]. These methods use such reagents as ExoQuick and Total Exosome Isolation reagent are technically facile and less time consuming, but the purity of the product is inferior to that of ultracentrifugation. However, neither method allows the experimental separation or even the discrimination of different EVs similar in size, suggesting that these exosome preparations may also contain small extracellular vesicles or other components instead of only "pure" exosomes. Immunoaffinity isolation may have the potential to enrich a single type of exosome populations [68], but there are concerns about the yield of this method. Recently, Heller et al. demonstrated the rapid isolation and recovery of exosomes from human plasma with an alternating current electrokinetic microarray chip device [69]. This device requires only 30-50 μL of sample, and the entire isolation process can be completed within 15 minutes, which is suitable for point-of-care diagnostic applications. Furthermore, a microfluidic separator was used to purify and separate exosomes from larger EVs with a high efficiency via fractionation caused by the size-dependent elastic lifting forces in a viscoelastic medium [70]. Such microfluidics techniques are gradually advancing the exosome analysis for clinical applications.
Exosome composition
According to high-throughput exosome studies, exosomes contain numerous molecules, including proteins, lipids, metabolites, mRNA [71], mitochondrial DNA [72], microRNA (miRNA) [71] and many other non-coding RNAs [73]. In addition, there are three exosome databases that provide detailed information about the molecules inside exosomes: ExoCarta, EVpedia and Vesiclepedia. Exosomes are heterogeneous in their size and cargo, even when they are derived from the same cell; however, some partially common cargoes are found among exosomes of diverse origins [26].
Exosomal biogenesis and internalization mechanisms and their roles in physiological and pathological processes. Exosomes are formed by inward budding from the endosomal membrane, which leads to the formation of multivesicular bodies (MVBs). MVBs can be fated for lysosomal degradation or fusion with the plasma membrane, which is associated with the release of exosomes. In addition, MVBs also participate in autophagosome maturation as endocytic fusion partners that meet with autophagosomes. Target cells internalize exosomes by three methods, which can facilitate the signaling and content delivery from source to target cells, thus mediating the progression of many physiological and pathological processes.
Proteins
According to proteomic studies, exosomes have not only specific proteins that depend on the secreting cell type but also a specific subset of cellular proteins that are found in exosomes irrespective of the cell type. These proteins are involved in some basic cellular processes, such as cell adhesion, structural dynamics, membrane fusion, metabolism, and signal transduction [74]. Tetraspanin and integrin proteins (e.g., CD63, CD9, CD81, and CD82) are crucial for cell targeting and adhesion, while Rab GTPases, annexins, and flotillin are important for membrane fusion. Furthermore, heat shock proteins (HSP)70 and HSP90 are molecular chaperones, and tumor susceptibility gene 101 protein (TSG101) is involved in MVB biogenesis [23]. Exosomes also contain cytokines, transcription factor receptors, growth factor receptors, and other bioactive molecules [75].
The mechanism of sorting proteins into exosomes has not been extensively studied. There are two major pathways for this process: one is ESCRT dependent, and the other is ESCRT independent. ESCRT is composed of four multimeric complexes, including ESCRT-0 to ESCRT-III. Most transmembrane proteins in the ILVs of MVBs are complexes that are marked by a single ubiquitin or the subunits of two or three ubiquitins. The ESCRT machinery is important for recognizing the ubiquitination of transmembrane proteins and sorting them into the ILVs of MVBs [76, 77]. However, recent studies have indicated that proteins can also be packaged into the MVBs without the participation of ubiquitin or ESCRT [78, 79]. Lin et al. found that many ribosomal proteins were secreted by exosomes that were derived from embryonic fibroblasts in mammalian sirtuin 6 (SIRT6)-knockout mice, indicating that SIRT6 is involved in sorting proteins into exosomes [80].
Nucleic acids
In 2007, Valadi and colleagues first reported that exosomes derived from mast cells contained mRNA and miRNA [81]; after this report, many groups confirmed the presence of certain mRNAs and small non-coding RNAs, including microRNAs, in exosomes. In addition, exosomes contain many other types of RNA, a finding that was summarized in a previous review [32]. Exosomes contain specific subsets of cellular RNA. Some sets are distinct or tissue specific; in other cases, sets of RNA are present in exosomes regardless of cellular origin [82]. These findings indicate that specific RNAs are actively, not passively, sorted into exosomes. Interestingly, researchers have found that mRNA with a 3'-UTR could be packaged into exosomes via the specific RNA fragments [83]. Similarly, there is a tetranucleotide sequence that can induce the packaging of microRNA into exosomes and is recognized by heterogeneous nuclear ribonucleoproteins [84]. Another similar RNA-binding protein, synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP), which can control mRNA sorting into exosomes, has recently been demonstrated [85]. This protein binds specific miRNA repertoires through the recognition of their specific motif and loads them into exosomes, which may provide a potential way for the selective modulation of exosomal cargoes. Furthermore, these observations suggest that there may be different mechanisms related to the selection of RNA cargoes.
In the present context, microRNAs have received much attention as active EV cargoes, especially with respect to cancer. It is a widely-accepted hypothesis that microRNA can be directly delivered to target cells via exosomes, leading to the functional modulation of their mRNA targets. However, because of the lack of direct demonstrations of the functions of EV-mediated miRNA transfer, there are uncertainties that must be included in the hypothesis [86]. Central to this hypothesis is the processing of primary transcripts into active miRNAs that could directly function in a recipient cell, which occurs exclusively within mammalian cells. However, this dogma faces some challenges. Some studies have found that Dicer and Ago2, the key components of miRNA processing, are functionally present in exosomes [87]. Although it has yet to be confirmed, these findings suggest that exosomes are not only passive vehicles but also miRNA factories.
Physiological roles of exosomes
Exosomes are nearly ubiquitous and are secreted by almost all mammalian cells. They act as the medium for cell communication and the transmission of information to a multitude of cells and locations. The behavior of adjacent or distant cells can be modified by exosomes that are released from cells in a paracrine or an endocrine manner. In addition, donor cells can also re-uptake their own secreted exosomes. For example, in vitro experiments have shown that exosomes produced by pancreatic tumor cells exerted an autocrine effect on themselves [88]. Thus, exosomes play important roles in both physiological and pathological processes (Figure 3).
Immune response
Some studies have shown that exosomes can be involved in activating the immune system through different pathways. Such pathways include the induction of macrophages to release certain proinflammatory cytokines [89] and increase the release of tumor necrosis factors (TNFs) [90], the enhancement of natural killer (NK) cell activity [91], the promotion of dendritic cells (DC) maturation [92], the delivery of major histocompatibility complex (MHC) peptide molecules [93], and the presentation of antigens [94]. However, free EVs seem to be less efficient than the parent APCs in the stimulation of T cells [95].
Neural communication
Exosomes are also involved in the reciprocal signal transfer among sensory and motor neurons, interneurons, and glial cells, which means that exosomes play an important role in the communication of the nervous system [96]. In addition, the nervous system itself plays an important role in ageing, and the hypothalamus plays an especially critical role [97]; however, the relevant underlying cellular mechanisms are unclear. Recently, a remarkable study demonstrated that the endocrine functions of hypothalamic neural stem/progenitor cells are responsible for the progression of ageing. Moreover, miRNA delivery via exosomes derived from these cells partially participates in this process as a new type of endocrine function. Treatment with these exosomes was found to slow the progression of ageing, suggesting that exosomes play an important role in ageing [98].
Reproduction and development
Exosomes have also been shown to exert effects on reproduction and development. Exosomes are involved in multiple stages of reproduction, such as gamete maturation, fertilization and embryo implantation [8]. Even the processes of a successful pregnancy rely on the crucial function of exosomes in immunological communication between the mother and the fetus [99]. Exosomes have been shown to participate in fetal protection through regulating local and/or systemic immune responses [100]. In addition to communication between the mother and fetus, exosomes also mediate the crosstalk between the epithelium and mesenchyme, thus playing a crucial role in organ development. Epithelial and mesenchymal exosomes were preferentially adsorbed by reciprocal cell types in a developing tooth and reciprocally induced cell differentiation and matrix synthesis [101]. Moreover, a recent study has shown that keratinocyte-secreted exosomes are capable of enhancing melanin synthesis, revealing an unforeseen function of exosomes in human pigmentation [102].
Cell proliferation, homeostasis and maturation
The functions of exosomes relating to enhanced cell proliferation are widely known. According to previous studies, exosomes with these functions are mostly derived from stem cells [99]. As exosomes obtain their cargo of contents from their parental cells, it follows that exosomes could act as the alternative mediators of stem cells. In addition, hepatocytes-derived exosomes could transfer sphingosine kinase 2 to form sphingosine-1-phosphate in target hepatocytes, thus leading to cell proliferation and liver regeneration [103]. It is also worth mentioning that a type of CrkI-containing microvesicles (different from exosomes and apoptotic bodies) derived from apoptotic cells has been demonstrated to be capable of inducing proliferation in neighboring cells [104]. This compensatory proliferation is very important for restoring homeostasis.
Exosomes also participate in reticulocyte maturation. Originally, reticulocytes-derived exosomes were described as part of an excretory pathway for the disposal of unwanted cellular cargo [105]. When reticulocytes begin to mature into erythrocytes, transferrin receptors, membrane-associated enzymes and proteins are selectively removed through exosomes [106]. In addition, morphological evidence indicates that MVBs are major endocytic fusion partners that meet with autophagosomes, meaning that autophagosome interactions with endosomes are critical for its maturation [77].
Pathological roles of exosomes
Immune disturbance
Depending on the activation state of the original cells and the cellular cargo packaged into the vesicles, exosomes can be tolerogenic. Such immunosuppressive effects of exosomes can be diminished by reducing NK cell activity or DC differentiation, inducing Fas ligand (FasL)-mediated T cell apoptosis, inhibiting T cell proliferation, and inhibiting inflammation through the delivery of exosomal interleukin (IL)‑10 [107]. In addition, a recent study has indicated that tumor-derived exosomes expressing HSP72 at the surface can promote antigen-nonspecific immune suppression via myeloid-derived suppressor cells (MDSCs), a group of immature myeloid cells with the ability to suppress T cell activation [108]. Moreover, it has been shown that more exosomes are generated by breast cancer cells under hypoxic than normoxic conditions, and these exosomes act to suppress T cell proliferation via TGF-β pathways [109]. Many studies have shown that cancer exosomes can deliver multiple signals to immune cells that subsequently overcome immune system surveillance and induce the immune system to specifically ignore or tolerate cancer cells [39]. In addition, this tolerogenic function of cancer cell-derived exosomes contributes to immune evasion.
Tumor pathogenesis
Many studies have discussed the interplay of tumor cells and the microenvironment. Tumor cell-derived exosomes can be taken up by stromal cells, thus converting the microenvironment into one that is prone to tumors [110]. An interesting study used fluorescent protein imaging to provide direct evidence of exosomes derived from highly metastatic breast cancer cells being transferred to adjacent cancer cells and lung tissue cells both in vitro and in vivo. These findings confirmed the involvement of exosomes that are derived from metastatic cancer cells in the education of stromal cells [111]. The Warburg effect of cancer cells results in a high-energy demand and a low ATP-generating efficiency; therefore, the sufficient glucose uptake of cancer cells is indispensable. Interestingly, an amazing piece of work showed that cancer cell-derived exosomes containing miR-122 could suppress glucose uptake in non-tumor cells in the premetastatic niche, suggesting that the systemic energy metabolism can be reprogramed by exosomes and that disease progression can be facilitated accordingly [112].
Reciprocally, in addition to the fact that cancer-cell derived exosomes can modify premetastatic niches, it is worth emphasizing that stromal cells can also generate exosomes that are not only taken up by tumor cells but also facilitate tumor growth. Zhang et al. showed that astrocyte-derived exosomes could transfer miR-19a intracellularly to inhibit the expression of phosphatase and tensin homolog (PTEN) in metastatic tumor cells in breast cancer and melanoma brain metastasis models. Furthermore, this adaptive PTEN loss was found to increase secretion of the cytokine chemokine (C-C motif) ligand 2 (CCL2) and recruits Iba1-expressing myeloid cells, further reciprocally enhancing the proliferation and reducing the apoptosis of tumor cells, thereby triggering the outgrowth of brain metastasis [113]. This mechanism might also be involved in PTEN loss in other neurological diseases. However, whether other PTEN-targeting miRNAs that are derived from astrocytes share a similar function with miR-19a remains to be confirmed. Some researchers have recently shown that exosomes that were derived from cancer-associated fibroblasts could strikingly reprogram the metabolic machinery of cancer cells to inhibit mitochondrial oxidative phosphorylation and increase both glycolysis and glutamine-dependent reductive carboxylation. Exosomes also contribute to the survival of nutrient-deprived cancer cells by supplying them with amino acids via a mechanism similar to that of micropinocytosis [114].
In accordance with these observations, tumor-released exosomes play significant roles in multiple stages of tumor pathogenesis. In particular, exosomes derived from breast cancer cells contains Dicer, AGO2, and TRBP, whereby they are capable of processing precursor microRNAs into mature miRNAs for gene silencing in target cells and inducing non-tumorigenic epithelial cells to form tumors [115]. Moreover, exosomes are involved in the horizontal transfer of oncogenic activity via the delivery of oncogenic receptors, such as a truncated and oncogenic form of the epidermal growth factor receptor (EGFRvIII) [116]. Exosomes also play a role in the dissemination of cancer cells; they can contribute to extracellular matrix degradation, and stromal reprogramming, thus promoting cell motility [117]. Meanwhile, exosomes can promote adhesion assembly and control directional cell motility through the delivery of extracellular matrix components, such as fibronectin, via autocrine secretion [118]. Exosomes also facilitate immune surveillance escape. Exosomes derived from B-cell lymphoma cells carry some antigens in common with cancer cells, such as CD20, thus consuming anti-CD20 antibodies, and protecting cancer cells from antibody attack [119]. In addition, exosomes can mediate the transfer of miRNA or long non-coding RNA (lncRNA) to neighboring cells to alter biological phenotypes, such as drug resistance [120, 121].
Neurodegenerative pathogenesis
In addition to the transmission of pathogenic proteins, EV secretion is a primary method of internal communication between neurons in neurodegenerative diseases [122]. Recently, an interesting study recapitulated the pathology of Alzheimer's disease in a three-dimensional human neural cell culture model. This study suggested that the excessive accumulation of amyloid-β peptide can result in Alzheimer's disease [123]. Apparently, the cargo of EVs, including but not limited to toxic proteins, can be spread by exosomes and transferred to other neuronal cells. Accordingly, some investigations have shown that amyloid-β peptide can be delivered by exosomes, thereby exacerbating and extending neuronal injury [124, 125]. For instance, exosomes derived from primary phagocytes, namely, microglia, contribute to tau propagation, which is associated with Alzheimer's disease [126]. A similar finding suggests that the EVs are involved in the transfer of the α‑synuclein protein, which contributes to the spread of Parkinson's disease from enteric neurons to the brainstem and centers of higher cortical function [127].
Pathogenic infections
Similarly, beyond toxic proteins, exosomes have the potential to spread numerous pathogens. Many pathogenic factors, including viral proteins and fragments of viral genomes, can be incorporated into exosomes derived from virus-infected cells. The exosome-mediated delivery of these factors has been shown to affect the immune responses to infection and modulate recipient cells responses [128, 129]. For example, Epstein-Barr virus (EBV) represses the expression of EBV target genes in non-infected cells by the transfer of viral miRNAs to subcellular sites of gene repression in recipient cells via exosomes [130]; the vehicles are also involved in the N-terminal modification of prion proteins (PrP) and selectively deliver distinct PrP glycoforms into neuronal cells [13]. In addition, HIV‑1 achieves cell entry by exosome-mediated transfer of chemokine receptor 5 to recipient cells [131]. These findings suggest that viruses (retroviruses, in particular) and exosomes generated in virus-infected cells might be closely related. First, the size of a typical RNA virus is comparable to that of exosomes (Figure 1). Second, similar to exosomes, many viruses utilize the ESCRT machinery for their release [132]. Finally, exosomes can carry viral proteins and genetic materials, which can in turn modulate the responses of recipient cells. Despite these similarities, there is a fundamental difference: exosomes do not replicate. Therefore, exosomes could be considered defective retroviruses that have lost the ability to replicate [133]; as such, exosomes and viruses cannot be separated from each other based on size alone.
Certainly, several types of cells have developed sophisticated self-defense mechanisms [134]. The primary mechanism used by cells to sequester and clear microbial pathogens is engulfing the invaders in an autophagosome, which is then degraded by a lysosome. However, microbes have developed a counter-attack strategy by targeting autophagy proteins or regulators [135]. In such cases, exosomes become defenders. When bladder epithelial cells are infected by uropathogenic Escherichia coli, the host cells can sense lysosome malfunction through mucolipin TRP channel 3 and remove the invading bacteria via exosomes [136].
Exosomes in translational medicine
Many studies have demonstrated that exosomes can play significant roles in the treatment of many diseases, including cancer [137], cardiovascular diseases [138, 139], neurodegenerative diseases [61], tissue injury [140], and pathogenic infections [141]. Indeed, increasing evidence shows that the therapeutic activity of adult stem cells occurs partially through paracrine effects, which gives rise to the novel notion of “cell-free” stem cell therapy [142]. It is worth emphasizing that stem cells-derived exosomes are a good substitute for therapeutic stem cells as they are not associated with the clinical concern of tumorigenicity [143]. In this section, we will discuss exosomal versatility in translational medicine, such as applications of exosomes in the diagnosis, prevention, and treatment of disease (Figure 4).
Schematic representation of the primary strategies of exosome modification and therapeutic intervention in translational medicine. Exosomes have the potential to serve as biomarkers and vaccine and drug carriers engineered by different methods. Such methods include the transfer of target peptide-expressing plasmids into cells to generate exosomes with a target ligand, the direct loading of drugs into exosomes by electroporation, the extrusion of drug-loaded cells through a series of filters with diminishing pore sizes to generate exosome-mimetic vesicles, and the fusion of exosomes with modified liposomes via the freeze-thaw method to create hybrid exosomes.
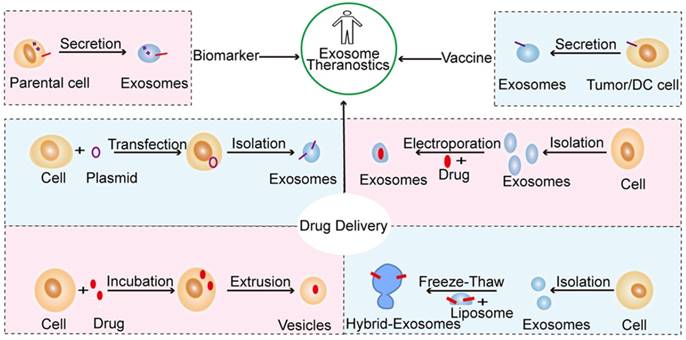
Biomarkers
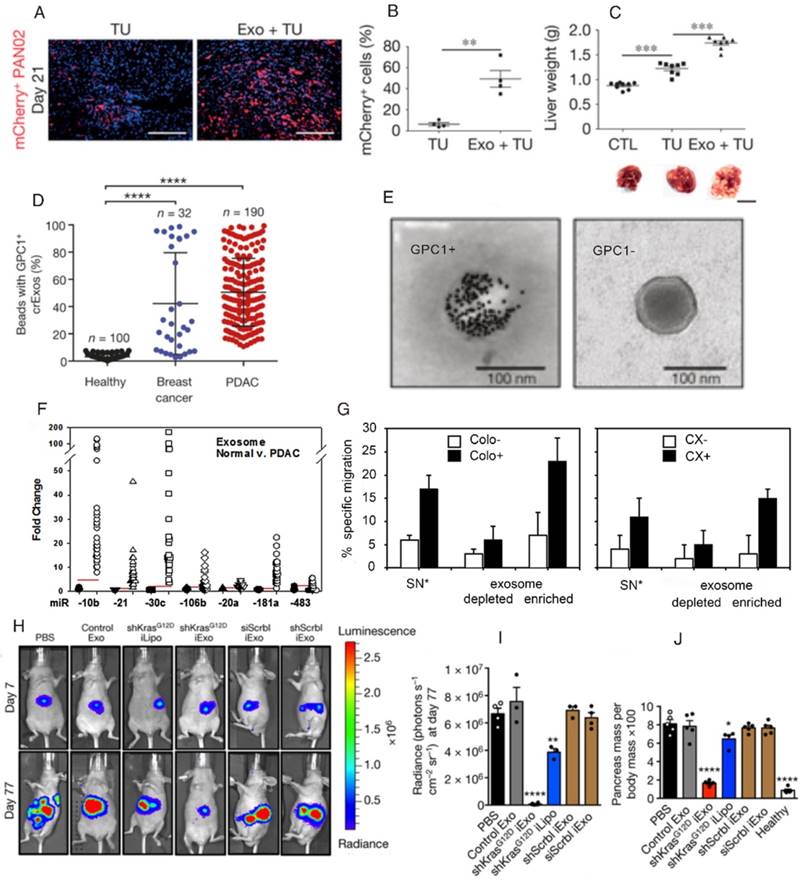
One potential clinical application of exosomes is their use as diagnostic and prognostic biomarkers. As mentioned above, exosomes exist in various body fluids and likely reflect the status of their parental cells. Therefore, exosomes are ideal non-invasive biomarkers for disease diagnosis. For example, exosomes that express CD63 and caveolin-1 in plasma can be considered non-invasive markers of melanoma and a novel tool for the clinical management of cancer patients [144]. Additionally, mRNA that encodes the zinc finger protein ZFY and is contained in exosomes of amniotic fluid can be used to determine the sex of the fetus [20]. Although no specific markers for differentiating tumor exosomes from normal exosomes are known, one important study showed that exosomes derived from pancreatic cancer cells were enriched with a proteoglycan, glypican 1 (GPC1), from the surface of the parental cells and that GPC1 could be used as a highly specific biomarker for pancreatic cancer. GPC1 is an overexpressed, membrane-anchored protein in both breast and pancreatic cancer.
Exosomes derived from pancreatic cancer cells are also involved in the progression of metastasis to the liver (Figure 5A, B, and C) [110]. The levels of GPC1+ circulating exosomes in the serum of patients correlate with pancreatic cancer with absolute specificity and sensitivity (Figure 5D and E) [145]. Moreover, the levels of circulating exosomes correlate with tumor burden and the survival of pre- and post-surgical patients. Therefore, these levels can be utilized to determine the stage of pancreatic cancer. Although GPC1 needs to be validated as a biomarker of pancreatic cancer in larger patient cohorts, this finding exemplifies the power of utilizing exosomes for cancer diagnosis. Notably, a recent study found that in contrast with individuals without pancreatic disease and chronic pancreatitis (CP) patients, exosomal GPC1 levels from pancreatic ductal adenocarcinoma (PDAC) patients tended to be slightly high, but not significantly so. Moreover, they found that a miRNA signature in circulating exosomes is superior to exosomal GPC1 or plasma CA 19-9 levels in diagnosing pancreatic cancer and differentiating between PDAC and CP (Figure 5F) [146]. However, the true sensitivity and specificity of this signature requires further assessment using a larger number of PDAC and control samples. Interestingly, a new finding indicates that exosomal miRNAs present in saliva can be used as ageing biomarkers. The fold-change values were validated by comparing individual total RNA contents of 15 young and 13 old individuals, and the results suggested that exosomal miRNAs in saliva could be a novel and potential biomarkers for ageing [147]. Furthermore, the concentration of exosomal miR-92a represents a potential serum biomarker for brown fat activity in mice and humans [148]. The use of exosomes as biomarkers has broad implications for personalized disease diagnosis and monitoring. Notably, the first blood-based cancer diagnostic method based on free-floating exosomes has recently become commercially available [149]. Significantly, by integrating microfluidic technology, exosomes can be detected rapidly and specifically for advanced interventions, accordingly based on precise clinical diagnoses [150].
Exosomes can also contain lncRNAs, serving as natural non-coding RNAs carriers. These functional RNAs are more than 200 nucleotides long and do not have the potential to code proteins. In addition, they are currently partially characterized as potential diagnostic and prognostic biomarkers. In particular, exosomal lncRNA and miR-217 are differentially expressed in the serum of colorectal carcinoma patients and are correlated with tumor classification (T3/T4), clinical stage (III/IV), and lymph node or distant metastasis [151]. Similarly, exosomal lncRNAs are differentially expressed in the cervicovaginal lavage samples of cervical cancer patients and cancer-free volunteers, indicating the potential of exosomal lncRNAs to serve as biomarkers for the early diagnosis of cervical cancer [152]. The combined detection of exosomal miR-21 and lncRNA Hox transcript antisense intergenic RNA (HOTAIR) as diagnostic biomarkers for laryngeal squamous cell carcinoma achieved a sensitivity and specificity of 94.2% and 73.5%, respectively [153]. Recently, exosomes were found to be enriched with another high-profile class of functional non-coding RNAs, circular RNAs (circRNAs), much more than they were with linear RNA compared with the parental cells [154]. circRNAs have a covalent loop structure that confers resistance to RNA exoribonuclease [155]. Moreover, circRNAs can serve as sponges for microRNAs and proteins with stable, conservative, and tissue/developmental-stage-specific expression patterns [156]. Hence, they have potential to regulate gene expression and are related to tumorigenesis. One study demonstrated that the serum exosomal circRNAs profiles of cancer patients were significantly different from those of individuals without cancer, suggesting that exosomal circRNAs are potential diagnostic biomarkers for cancer [154].
The concentration of exosomes is another important parameter that can indicate pathological circumstances. Recently, a study showed that the concentration and origin of circulating vesicles depended on cancer evolution, meaning that the concentration of exosomes can be used as a complex biomarker to reflect the evolution of diseases [157]. Inspiringly, the accuracy of discriminating malignant from nonmalignant common bile ducts based on the threshold of the median concentration of EVs in bile reaches 100%, and when based on the serum concentration of EVs, the accuracy reaches 63.3% [158]. In addition, the threshold values of bile and serum EV concentrations are 9.46×1014 and 6.39×1013 nanoparticles per liter, respectively. Apart from pathological conditions, physiological conditions also impact the concentration of exosomes. When undergoing exercise stress testing, the concentrations of serum and plasma EVs increase, and these EVs were identified to provide protective effects against acute ischemia/reperfusion injury in mice [159]. These features suggest that EV concentration is an extremely promising parameter to be evaluated for clinical applications and that EV-measuring technologies need to be standardized.
Vaccines
Owing to the presence of peptide-MHC complexes and their ability to activate responses from T cells and NK cells in experimental animals and in cancer patients [160], DC-derived exosomes can induce anti-tumor immunity and have been used in clinical trials [161, 162]. Furthermore, the anti-tumor immunity can be improved by maturing these DCs with Toll-like receptor agonists [163]. The potency of DC-derived exosomes is also promising regarding malignancies that respond poorly to immunotherapy. For instance, in three hepatocellular carcinoma mouse models with antigenic and pathological heterogeneity, exosomes that were derived from antigen-expressing DCs triggered strong antigen-specific immune responses, revealing a significant anti-tumor function and leading to prolonged survival rates [164]. DCs are also involved in the immune response against transplanted allografts by migrating to recipient lymphoid tissues and directly activating alloreactive T cells to produce donor MHC molecules. Astoundingly, with the contribution of donor DC-derived exosomes, limited populations of donor DCs caused acute rejection in a murine heart transplant model [12].
In general, tumor cells can release exosomes that interfere with the immune system, indicating that tumor-derived exosomes could also serve as tumor-tailored vaccines for cancer immunotherapy. In the immune disturbance section, tumor-derived exosomes were described as immunosuppressive and capable of promoting tumor growth in various ways. In contrast, exosomes derived from cancer cells can be engineered to exert immunostimulatory effects because they share tumor rejection antigens with their parental cells. These exosomes not only induce a potent anti-tumor effect mediated by CD8+ T cells but also suppress other associated tumors that are equipped with the same tumor rejection antigens [94]. Tumor-derived exosomes also stimulate NK cell activity; exosomes derived from pancreatic carcinoma cell sublines expressing HSP70 stimulate migration and lytic activity in NK cells against HSP70 surface-positive tumors (Figure 5G) [91]. However, these immune responses are relatively weak and prone to inducing tolerance. Therefore, researchers have worked toward artificially modifying exosomes, which could improve their immunogenicity. Common strategies include genetic modification, the external stimulation of original cells, and the direct fusion of exosomes with antigens [150].
Drug delivery
Liposomes or lipid-based nanoparticles have been effectively used as conventional drug delivery platforms to encapsulate various macromolecular drugs. However, the widespread use of artificial drug carriers has been prevented by their potential toxicity, immunogenicity and inability to penetrate and target specific organs. These disadvantages may be largely absent when these drug carriers are properly functionalized with biological entities. Thus, cell-membrane-camouflaged nanoparticles might offer a promising delivery strategy for clinical use. Proteins derived from the plasma membrane of leukocytes were successfully incorporated into lipid nanoparticles by Tasciotti et al. to formulate biomimetic vesicles to target inflamed vasculature [165]. Hu et al. developed a top-down approach to disguise poly(lactic-co-glycolic acid) (PLGA) nanoparticles with natural erythrocyte membranes [166]. These membrane-camouflaged nanoparticles exhibited an excellent ability to reside in blood; modification with membrane lipids and associated membrane proteins prevented particle clearance by macrophages. Furthermore, these researchers cloaked PLGA nanoparticles with the plasma membranes of human platelets, thereby endowing these nanoparticles with the immunomodulatory and adhesive antigens that are associated with platelets. These nanoparticles were used to repair damaged blood vessels and to treat systemic infections caused by opportunistic pathogens in vivo [167].
Exosome theranostics in pancreatic cancer. (A, B, C) Exosomes derived from the serum of pancreatic cancer (PC) patients initiate premetastatic niche formation in the liver, as evaluated by liver weight and the percentage of mCherry+ PAN02 cells in mice. (Reproduced with permission from reference [110]. Copyright © 2015 Nature Publishing Group.) (D, E) Exosomes with surface-anchored glypican 1 (GPC1) proteins from the serum of PC patients are used as biomarkers to detect early PC. (Reproduced with permission from reference [145]. Copyright © 2015 Nature Publishing Group.) (F) Change in the exosomal miRNAs level of the normal controls and PC patients. (Reproduced with permission from reference [146]. Copyright © 2017 Elsevier) (G) HSP70/Bag-4+ exosomes that specifically induced the migration of natural killer cells. (Reproduced from reference [91]. Copyright © 2005 American Association for Cancer Research.) (H, I, J) Exosomes that are loaded with short interfering RNA restrain PANC-1 tumor growth. (Reproduced with permission from reference [178]. Copyright © 2017 Nature Publishing Group.)
The possibility of using exosomes as nucleic acid or drug delivery carriers has attracted wide attention due to their excellent biodistribution and biocompatibility. They are membrane-permeable and can also cross the blood-brain barrier [168]. Most importantly, normal cell-derived exosomes have low immunogenicity and are well tolerated [114]. Therefore, exosomes are favorable candidate carriers of treatment for cancer and other diseases. Exosome can carry proteins, miRNAs, small interfering RNAs (siRNAs) and other therapeutic compounds because these cargoes are more stable inside than outside exosomes [169]. In addition, sonic hedgehog (Shh), a factor with angiogenic properties, has been shown to be enriched in exosomes generated by modified CD34+ cells; exosomes that transported Shh to other cells reversed ventricular dilation and cardiac dysfunction post-myocardial infarction in a mouse model. Interestingly, no protective effect is induced by the intramyocardial injection of recombinant Shh protein. The results show that in this context, hitchhiking in exosomes, as a delivery system is key for conferring therapeutic efficacy [170]. TNF-related, apoptosis-inducing, ligand-armed exosomes can also be exploited to transfer pro-apoptotic signals to different tumor cell types with the aim of inducing cancer cell apoptosis and thus suppressing tumor progression in vivo [171]. In addition, other therapeutic compounds, such as paclitaxel [172] and doxorubicin [173], can be packaged into exosomes for crossing the blood-brain barrier and achieving targeted delivery. However, drug resistance restricts on the therapeutic applications of exosomes. To overcome imatinib resistance in chronic myeloid leukemia (CML) patients, Bellavia D. et al. utilized modified exosomes to deliver functional siRNA for BCR-ABL to imatinib-resistant CML cells. These modified exosomes were harvested from engineered HEK293T cells expressing human Lamp2b and were then fused to a fragment of IL-3. As CML cells overexpress the IL-3 receptor, they could effectively be targeted by the exosomes modified with IL-3 [174]. Additionally, Ma et al. developed a novel approach for eliminating drug resistance characteristics in tumor-repopulating cells (TRCs) using tumor cell-derived vesicles loaded with anti-tumor drugs. To promote nuclear entry of the drugs, chemotherapeutic drugs were packaged into tumor cell-derived vesicles. These vesicles were preferentially internalized by TRCs and subsequently released the anti-tumor drugs, thereby contributing to the reversal of drug resistance in TRCs in vitro [175].
RNA delivery via exosomes could be developed further in different ways. For example, monocytes/macrophages were transfected by lipofection with the desired RNA, miR-143BP [176]. Then, miR-143BP was detected in EVs that were derived from the monocytes/macrophages. Lamp2b is a protein that is enriched in exosomal membranes and can fuse with neuron-specific rabies viral glycoprotein (RVG) peptide, which can specifically bind to the acetylcholine receptor. Some researchers have used electroporation to load exogenous siRNA into exosomes derived from DCs engineered to overexpress Lamp2b [61]. siRNAs can be specifically delivered via RVG-targeting exosomes by intravenous injection, resulting in gene knockdown in the brain and especially in neurons, microglia, and oligodendrocytes. The efficacy of RNA delivery also depends on the origin of the exosomes. Exosomes derived from normal fibroblast-like mesenchymal cells are positive for CD47, a widely expressed transmembrane protein that is well known to protect cells from phagocytosis [177], and have excellent retention in the circulation; in contrast, liposomes are rapidly eliminated from the circulation. Such properties of exosomes with an enhanced potency for delivering miRNA to pancreatic tumors, along with the use of these types of exosomes as RNA interference (RNAi) mediators specifically targeting oncogenic KRAS in PDAC, have significantly improved overall survival in many mouse models (Figure 5H, I, and J) [178].
Electroporation and chemical treatment are the primary methods used to load exosomes with cargo, although the corresponding loading efficiencies are not desirable [179] (Figure 4). It is worth emphasizing that transfecting exosome-producing cells rather than therapeutic vesicles might offer increased efficiency [32]. However, clinical applications of exosomes remain challenging due to inadequate targeting. Rational elevation of target ligand expression on the surfaces of exosomes can further improve their cell- or tissue-targeting specificity. Cells with special receptor expression can be targeted precisely by plasma membrane-extracted vesicles from engineered donor cells that express transmembrane-targeting ligands [180]. Several studies have successfully modified exosomes by anchoring targeting ligands on their membrane and then introduced the exosomes to diseased cells [181, 182]. For instance, exosomes were engineered to express targeting ligands, such as iRGD-Lamp2b, to enhance tumor-specific uptake; the intravenous injection of these exosomes obviously suppressed tumor growth [183]. Moreover, Yim et al. developed a strategy to intracellularly deliver target proteins to newly generated exosomes by integrating a reversible protein-protein interaction module during the endogenous process of exosome biogenesis [184]. In addition, a recent study has reported a strategy for producing exosomes with robust targeting ability for circulation in the blood by anchoring multiple superparamagnetic nanoparticles onto them such that they form a cluster [185].
Rational design of biomimetic exosomes
Recently, a group of researchers developed a method to produce exosome-mimetic vesicles, which can overcome exosomal limitations, such as low loading efficiency and low exosome production yields. These chemotherapeutic-loaded nanovesicles, which are 100-200 nm in diameter, were generated by a breaking down cells via serial extrusion through filters with diminishing pore sizes (Figure 4) [186]. It was further suggested that these nanoscale vesicles with exosome-mimetic characteristics can be used as a platform for RNAi transportation to the cell cytoplasm [187]. However, the high level of cholesterol, ganglioside, and sphingomyelin in exosomal membranes leads to a more rigid bilayer structure than that of their parent cells [188], which suggests that their fusion with lipid-based particles requires harsher conditions [189]. For example, a novel study that developed hybrid exosomes by fusing exosomes with liposomes required aggressive freeze-thaw processes (Figure 4) [190]. To avoid such conditions, Yang et al. designed a virus-mimetic fusogenic exosome platform to deliver membrane proteins to target cell membranes. They integrated vascular stomatitis virus G protein, a viral fusogen, into an exosomal membrane to enhance its fusion efficiency [191]. Inspiringly, these methods allow easy exosome modification by fusing exosomes derived from modified cells with liposomes embedded with peptides, antibodies or poly(ethylene glycol) (PEG).
Exosome elimination and settlement in new therapeutics
Given the growing evidence regarding the contribution of exosomes to pathological processes, three promising strategies for treating disease can be investigated thoroughly: inhibiting exosome assembly, release, and uptake; altering harmful compositions; and blockading exosome dissemination and elimination of exosomes from the circulatory system.
The first strategy has been summarized well in a previous review [107]. Here, we explore the latter two strategies. Regarding alterations in the harmful compositions of exosomes, promising methods include the viral manipulation of a host's exosomal content that occurs by either directly altering the host's exosomal composition or incorporating viral proteins or RNAs into secreted exosomes [28]. In addition, owing to the impairment of the ubiquitin-proteasome system, which could alter the process of sorting cargo into ILVs, the immunosuppressive activities of exosomes against NK cell tumors in breast cancer can be reduced by the dietary polyphenol curcumin [192].
Recently, an interesting study showed that subcapsular sinus (SCS) CD169+ macrophages can bind tumor-derived EVs, including exosomes, thus restricting their interactions with B cells. Because B cells may contribute to tumor progression, SCS macrophages can suppress cancer progression under specific circumstances by blocking exosomal dissemination [193], indicating that blockading the dissemination and elimination of exosomes is beneficial in some cases. Another possible option that can be used to eliminate exosomes from the circulatory system via extracorporeal purification is the utilization of microfluidic techniques. For example, a recent study discussed a new method based on microscale acoustic standing wave technology for the rapid noncontact capture of microvesicles from samples despite their limited flux needs to be resolved in the future [194]. Another study has suggested that the clearance of exosomes in vivo is likely controlled by the innate immune system and assisted by the opsonization effects of complement proteins [195].
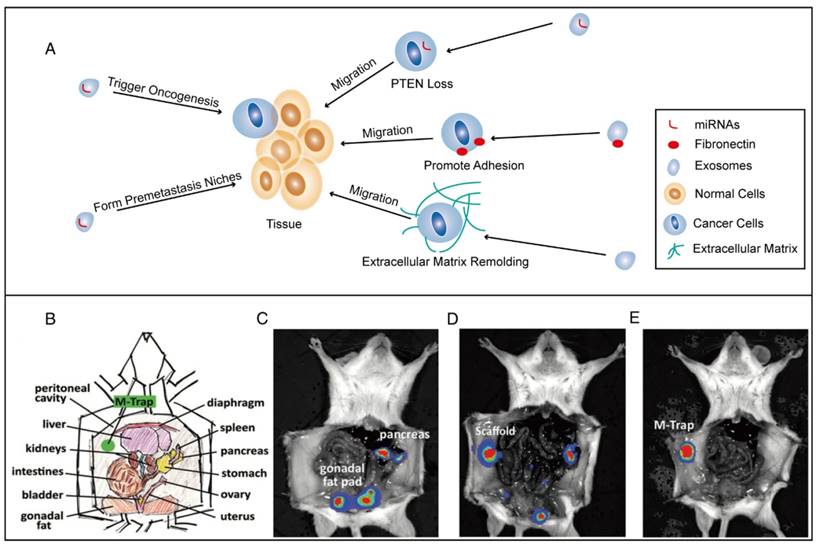
In some ways, the introduction of exosomes favors disease treatment. As we have mentioned above, exosomes play a crucial role in premetastatic niche formation of tumors (Figure 6A). Fuente et al. studied the effect of exosomes on redirecting tumor cell dissemination and recruiting tumor cells to non-deleterious locations, which now represents a strategy for suppressing tumor metastasis (Figure 6B, C, D, and E). First, the tumor EVs were embedded in a scaffold to form a premetastatic niche. Then, this niche was implanted in the mouse peritoneum to trap metastatic cells that were disseminating in the peritoneal cavity, thus preventing them from metastasizing to the organ they normally target and leading to a statistically significant improvement in survival [196].
Conclusions and perspectives
Exosomes are widely disseminated, heterogeneous entities. However, the complexity of exosomes is not thoroughly understood, especially the mechanisms responsible for sorting cargo into exosomes and releasing cargo into cells after exosome internalization. Various recent studies have focused on protein sorting; nevertheless, the predominant functions of exosomes might be associated with RNA delivery. Therefore, determining the mechanism that underlies RNA sorting holds great potential for the development of various applications. Exosomes are emerging as important mediators of intercellular communication and playing significant roles in both physiological and pathological processes. However, the current understanding of exosomes is incomplete.
Because of the costs, technical challenges, and lack of suitable biomarkers for particular exosomes, it is difficult to isolate large quantities of pure and specific exosomes from mixtures of different vesicle types in a large volume of solution. Thus, numerous studies have provided insights into the functions of EVs but without much information on exosomes alone. If only a small subtype of exosomes has a desirable efficiency, but this subtype is obscured by an abundance of non-functional EVs, how can the specific or prominent functions of a specific category of exosomes be determined? Toward this end, the next important step is to comprehensively define the many subtypes of EVs.
Although there are currently many challenges in the treatment of cancer and other refractory diseases, exosomes are considered valid diagnostic biomarkers and potential therapeutic tools. Moreover, along with chemical, cellular and genetic engineering techniques, many existing exosomal modification strategies are promising for the development of improved clinical therapies.
Tumor metastases formation mediated by exosomes and metastatic tumor cell capturing in situ by a scaffold embedded with EVs (M-Trap) in an ovarian cancer dissemination model. (A) Schematic representation of tumor metastases. (B) Schematic of the peritoneal anatomy of mice, indicating M-Trap implantation. (C) Representative bioluminescent distribution of metastatic cells in the natural pattern of dissemination (D), in the presence of an empty scaffold (E) and in the presence of M-Trap. (B, C, D, E were reproduced from reference [196]. Copyright © 2015 Oxford University Press.)
Abbreviations
APCs: antigen-presenting cells; DCs: dendritic cells; EVs: extracellular vesicles; HMC-1: human mast cell line; MVBs: multivesicular bodies; ILVs: intraluminal vesicles; ESCRT: endosomal sorting complexes required for transport; GTPase: guanosine triphosphatase; IFN: interferon; IFNGR1: interferon gamma receptor 1; ICAM: intercellular adhesion molecule; HSPGs: heparan sulfate proteoglycans; ISEV: International Society for Extracellular Vesicles; HSP: heat shock proteins; TSG101: tumor susceptibility gene 101 protein; SIRT6: sirtuin 6; SYNCRIP: synaptotagmin-binding cytoplasmic RNA-interacting protein; TNFs: tumor necrosis factors; NK cell: natural killer cell; MHC: major histocompatibility complex; FasL: Fas ligand; IL: interleukin; MDSCs: myeloid-derived suppressor cells; PTEN: phosphatase and tensin homolog; CCL2: cytokine chemokine (C-C motif) ligand 2; EGFRvIII: truncated and oncogenic form of the epidermal growth factor receptor; EBV: Epstein-Barr virus; PrP: prion proteins; GPC1: glypican 1; CP: chronic pancreatitis; PDAC: pancreatic ductal adenocarcinoma; lncRNAs: long noncoding RNAs; HOTAIR: Hox transcript antisense intergenic RNA; circRNAs: circular RNAs; PLGA: poly(lactic-co-glycolic acid); siRNAs: small interfering RNAs; Shh: sonic hedgehog; CML: chronic myeloid leukemia; TRCs: tumor-repopulating cells; RVG: rabies viral glycoprotein; RNAi: RNA interference; PEG: poly(ethylene glycol); SCS: subcapsular sinus.
Acknowledgements
This work was supported by the National Science Foundation of China [81401541, 81570168], the National Key R&D Program [2016YFC1100800], the Distinguished Young Scientist Award of the Zhejiang Provincial Natural Science Foundation [LR16H180001], and the Major Project of the Science and Technology Department of Zhejiang Province [2014C03048-2].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ludwig A-K, Giebel B. Exosomes: Small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11-5
2. Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406-15
3. Christianson HC, Svensson KJ, van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci. 2013;110:17380-5
4. Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS. et al. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte-Neuron Communication. PLoS Biol. 2013;11:e1001604
5. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ. et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-72
6. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208
7. Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N. et al. Trans-Synaptic Transmission of Vesicular Wnt Signals through Evi/Wntless. Cell. 2009;139:393-404
8. Machtinger R, Laurent LC, Baccarelli AA. Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum Reprod Update. 2016;22:182-93
9. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT. et al. Glioblastoma microvesicles transport RNA and protein that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-6
10. Ailawadi S, Wang X, Gu H, Fan G-C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta - Mol Basis Dis. 2015;1852:1-11
11. Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131:1194-203 1203.e1-14
12. Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and Biologic Characterization of Exosomes and Microvesicles as Facilitators of HIV-1 Infection in Macrophages. J Immunol. 2012;189:744-54
13. Vella L, Sharples R, Lawson V, Masters C, Cappai R, Hill A. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582-90
14. György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667-88
15. Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83
16. Lässer C, Seyed Alikhani V, Ekström K, Eldh M, Torregrosa Paredes P, Bossios A. et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9
17. Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS. et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5
18. Jia S, Zocco D, Samuels ML, Chou MF, Chammas R, Skog J. et al. Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Rev Mol Diagn. 2014;14:307-21
19. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta - Gen Subjects. 2012;1820:940-8
20. Keller S, Ridinger J, Rupp A-K, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86
21. Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83
22. Al-Nedawi K, Meehan B, Rak J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014-8
23. Simons M, Raposo G. Exosomes - vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575-81
24. Livshts MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE. et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319
25. Camussi G, Deregibus M-C, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98-110
26. Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907-20
27. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581-93
28. Sampey GC, Meyering SS, Asad Zadeh M, Saifuddin M, Hakami RM, Kashanchi F. Exosomes and their role in CNS viral infections. J Neurovirol. 2014;20:199-208
29. Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta (BBA) - Reviews on Biomembranes. 1985;822:203-18
30. Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci. 2012;109:4146-51
31. Booth AM, Fang Y, Fallon JK, Yang J-M, Hildreth JEK, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923-35
32. Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977-9
33. Hoog JL, Lotvall J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J Extracell Vesicles. 2015;4:28680
34. Yuana Y, Koning RI, Kuil ME, Rensen PC, Koster AJ, Bertina RM. et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles. 2013;2:21494
35. Zabeo D, Cvjetkovic A, Lasser C, Schorb M, Lotvall J, Hoog JL. Exosomes purified from a single cell type have diverse morphology. J Extracell Vesicles. 2017;6:1329476
36. Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M. et al. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J Proteome Res. 2008;7:5157-66
37. Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M. et al. Exosomes From Human CD34+ Stem Cells Mediate Their Proangiogenic Paracrine Activity. Circul Res. 2011;109:724-8
38. Nilsson P, Sekiguchi M, Akagi T, Izumi S, Komori T, Hui K. et al. Autophagy-Related Protein 7 Deficiency in Amyloid β (Aβ) Precursor Protein Transgenic Mice Decreases Aβ in the Multivesicular Bodies and Induces Aβ Accumulation in the Golgi. Am J Pathol. 2015;185:305-13
39. Marleau AM, Chen C-S, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134
40. de Gassart A, Géminard C, Hoekstra D, Vidal M. Exosome Secretion: The Art of Reutilizing Nonrecycled Proteins? Traffic. 2004;5:896-903
41. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F. et al. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science. 2008;319:1244-7
42. Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic. 2009;10:925-37
43. Advani RJ, Yang B, Prekeris R, Lee KC, Klumperman J, Scheller RH. Vamp-7 Mediates Vesicular Transport from Endosomes to Lysosomes. J Cell Biol. 1999;146:765-76
44. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883-91
45. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30
46. Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015;2015:1-10
47. Colombo M, Raposo G, Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89
48. Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M. et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197-213
49. Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y. et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158-68
50. Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D. et al. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol Cell. 2014;56:193-204
51. Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W. et al. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation. 2015;131:2120-30
52. Ann Mulcahy L, Charles Pink R, Raul Francisco Carter D. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641
53. Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A. et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213:173-84
54. Lässer C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Expert Opin Biol Ther. 2015;15:103-17
55. Segura E. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216-23
56. Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y. et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349-60
57. Caponnetto F, Manini I, Skrap M, Palmai-Pallag T, Di Loreto C, Beltrami AP. et al. Size-dependent cellular uptake of exosomes. Nanomedicine. 2017;13:1011-20
58. Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559-62
59. Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108
60. El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C. et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7:2112-26
61. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5
62. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-35
63. Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013:2
64. Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C. et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211-26
65. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3.22 3.22.1-3.22.29
66. Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang XY. et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361-7
67. da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86:71
68. Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM. et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293-304
69. Ibsen SD, Wright J, Lewis JM, Kim S, Ko SY, Ong J. et al. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS nano. 2017;11:6641-51
70. Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y. et al. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS nano. 2017;11:6968-76
71. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9
72. Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna). 2010;117:1-4
73. Ferguson SW, Nguyen J. Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179-90
74. Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267-83
75. Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352:33-47
76. Baixauli F, López-Otín C, Mittelbrunn M. Exosomes and Autophagy: Coordinated Mechanisms for the Maintenance of Cellular Fitness. Front Immunol. 2014;5:403
77. Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70-8
78. Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF. et al. A Lumenal Domain-Dependent Pathway for Sorting to Intralumenal Vesicles of Multivesicular Endosomes Involved in Organelle Morphogenesis. Dev Cell. 2006;10:343-54
79. Reggiori F, Pelham HRB. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 2001;20:5176-86
80. Zhang X, Khan S, Jiang H, Antonyak MA, Chen X, Spiegelman NA. et al. Identifying the functional contribution of the defatty-acylase activity of SIRT6. Nat Chem Biol. 2016;12:614-20
81. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9
82. Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143-9
83. Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3'-untranslated regions. Biol Direct. 2013;8:12
84. Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980
85. Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R. et al. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell reports. 2016;17:799-808
86. Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-32
87. Tran N. Cancer Exosomes as miRNA Factories. Trends in Cancer. 2016;2:329-31
88. Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016-26
89. Bhatnagar S, Schorey JS. Exosomes Released from Infected Macrophages Contain Mycobacterium avium Glycopeptidolipids and Are Proinflammatory. J Biol Chem. 2007;282:25779-89
90. Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C. et al. Hsp70 Translocates into the Plasma Membrane after Stress and Is Released into the Extracellular Environment in a Membrane-Associated Form that Activates Macrophages. J Immunol. 2008;180:4299-307
91. Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA. Heat Shock Protein 70 Surface-Positive Tumor Exosomes Stimulate Migratory and Cytolytic Activity of Natural Killer Cells. Cancer Res. 2005;65:5238-47
92. Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G. et al. Mast Cell-Derived Exosomes Induce Phenotypic and Functional Maturation of Dendritic Cells and Elicit Specific Immune Responses In Vivo. JImmunol. 2003;170:3037-45
93. Segura E, Amigorena S, Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89-93
94. Wolfers J, Lozier A, Raposo Ga, Regnault A, Th?ry C, Masurier C. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297-303
95. Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713-22
96. Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160-72
97. Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y. et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211-6
98. Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C. et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52-7
99. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066
100. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. 2013;19:548-56
101. Jiang N, Xiang L, He L, Yang G, Zheng J, Wang C. et al. Exosomes Mediate Epithelium-Mesenchyme Crosstalk in Organ Development. ACS nano. 2017;11:7736-46
102. Cicero AL, Delevoye C, Gilles-Marsens F, Loew D, Dingli F, Guéré C. et al. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun. 2015;6:7506
103. Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ. et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60-8
104. Gupta KH, Goldufsky JW, Wood SJ, Tardi NJ, Moorthy GS, Gilbert DZ. et al. Apoptosis and Compensatory Proliferation Signaling Are Coupled by CrkI-Containing Microvesicles. Dev Cell. 2017;41:674-84
105. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-78
106. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-20
107. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-57
108. Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin J-P. et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457
109. Rong L, Li R, Li S, Luo R. Immunosuppression of breast cancer cells mediated by transforming growth factor-β in exosomes from cancer cells. Oncol Lett. 2015;11:500-4
110. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-26
111. Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E. et al. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell. 2012;151:1542-56
112. Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183-94
113. Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100-4
114. Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T. et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 2016;5:e10250
115. Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A. et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell. 2014;26:707-21
116. Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619-24
117. Yue S, Mu W, Erb U, Zoller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 2015;6:2366-84
118. Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164
119. Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M. et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci. 2011;108:15336-41
120. Qu L, Ding J, Chen C, Wu Z-J, Liu B, Gao Y. et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653-68
121. Au Yeung CL, Co N-N, Tsuruga T, Yeung T-L, Kwan S-Y, Leung CS. et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150
122. Guo JL, Lee VMY. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20:130-8
123. Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C. et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274-8
124. Bellingham SA, Guo BB, Coleman BM, Hill AF. Exosomes: Vehicles for the Transfer of Toxic Proteins Associated with Neurodegenerative Diseases? Front Physiol. 2012;3:124
125. Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. The Exosome Secretory Pathway Transports Amyloid Precursor Protein Carboxyl-terminal Fragments from the Cell into the Brain Extracellular Space. J Biol Chem. 2012;287:43108-15
126. Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584-93
127. Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH. et al. Cell-Produced -Synuclein Is Secreted in a Calcium-Dependent Manner by Exosomes and Impacts Neuronal Survival. J Neurosci. 2010;30:6838-51
128. Meckes DG, Shair KHY, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370-5
129. Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C. et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558-70
130. Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL. et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328-33
131. Mack M, Kleinschmidt A, Brühl H, Klier C, Nelson PJ, Cihak J. et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769-75
132. Mori Y, Koike M, Moriishi E, Kawabata A, Tang H, Oyaizu H. et al. Human herpesvirus-6 induces MVB formation, and virus egress occurs by an exosomal release pathway. Traffic. 2008;9:1728-42
133. Nolte-'t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci U S A. 2016;113:9155-61
134. Randow F, MacMicking JD, James LC. Cellular Self-Defense: How Cell-Autonomous Immunity Protects Against Pathogens. Science. 2013;340:701-6
135. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323-35
136. Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell. 2015;161:1306-19
137. Munson P, Shukla A. Exosomes: Potential in Cancer Diagnosis and Therapy. Medicines. 2015;2:310-27
138. Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P. et al. Embryonic Stem Cell-Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circul Res. 2015;117:52-64
139. Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W. et al. Heat Shock Improves Sca-1 + Stem Cell Survival and Directs Ischemic Cardiomyocytes Toward a Prosurvival Phenotype Via Exosomal Transfer: A Critical Role for HSF1/miR-34a/HSP70 Pathway. Stem Cells. 2014;32:462-72
140. Tan C, Lai R, Wong W, Dan Y, Lim S-K, Ho H. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76
141. Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H. et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. 2013;14:793-803
142. Maguire G. Stem cell therapy without the cells. Commun Integr Biol. 2013;6:e26631
143. Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS. et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-22
144. Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M. et al. High Levels of Exosomes Expressing CD63 and Caveolin-1 in Plasma of Melanoma Patients. PLoS One. 2009;4:e5219
145. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
146. Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86-93
147. Machida T, Tomofuji T, Ekuni D, Maruyama T, Yoneda T, Kawabata Y. et al. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int J Mol Sci. 2015;16:21294-309
148. Chen Y, Buyel JJ, Hanssen MJW, Siegel F, Pan R, Naumann J. et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun. 2016;7:11420
149. Sheridan C. Exosome cancer diagnostic reaches market. Nat Biotechnol. 2016;34:359-60
150. Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. OncoImmunology. 2015;4:e1027472
151. Yu B, Du Q, Li H, Liu HY, Ye X, Zhu B. et al. Diagnostic potential of serum exosomal colorectal neoplasia differentially expressed long non-coding RNA (CRNDE-p) and microRNA-217 expression in colorectal carcinoma. Oncotarget. 2017 [Epub ahead of print]
152. Zhang J, Liu SC, Luo XH, Tao GX, Guan M, Yuan H. et al. Exosomal Long Noncoding RNAs are Differentially Expressed in the Cervicovaginal Lavage Samples of Cervical Cancer Patients. J Clin Lab Anal. 2016;30:1116-21
153. Wang JT, Zhou YD, Lu JG, Sun YN, Xiao H, Liu M. et al. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31:148
154. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J. et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981-4
155. Suzuki H, Tsukahara T. A view of pre-mRNA splicing from RNase R resistant RNAs. Int J Mol Sci. 2014;15:9331-42
156. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A. et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333-8
157. Mege D, Panicot-Dubois L, Ouaissi M, Robert S, Sielezneff I, Sastre B. et al. The origin and concentration of circulating microparticles differ according to cancer type and evolution: A prospective single-center study. Int J Cancer. 2016;138:939-48
158. Severino V, Dumonceau JM, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X. et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology. 2017;153:495-504
159. Bei Y, Xu T, Lv D, Yu P, Xu J, Che L. et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol. 2017;112:38
160. Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O. et al. Dendritic Cell-Derived Exosomes for Cancer Immunotherapy: What's Next? Cancer Res. 2010;70:1281-5
161. Escudier B, Dorval T, Chaput N, Andr? F, Caby M-P, Novault S. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10
162. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4:594-600
163. Damo M, Wilson DS, Simeoni E, Hubbell JA. TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep. 2015;5:17622
164. Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z. et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739-48
165. Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S. et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15:1037-46
166. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980-5
167. Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118-21
168. Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC. et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol Ther. 2011;19:1769-79
169. Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Del Rev. 2012;64:206-12
170. Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A. et al. Sonic Hedgehog-Modified Human CD34+ Cells Preserve Cardiac Function After Acute Myocardial Infarction. Circul Res. 2012;111:312-21
171. Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B. et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL)-Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin Cancer Res. 2016;22:3499-512
172. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Controlled Release. 2014;192:262-70
173. Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R. et al. Exosome Delivered Anticancer Drugs Across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm Res. 2015;32:2003-14
174. Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A. et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7:1333-45
175. Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y. et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713-27
176. Akao Y, Iio A, Itoh T, Noguchi S, Itoh Y, Ohtsuki Y. et al. Microvesicle-mediated RNA Molecule Delivery System Using Monocytes/Macrophages. Mol Ther. 2011;19:395-9
177. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271-85
178. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
179. Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Controlled Release. 2013;172:229-38
180. Zhao C, Busch DJ, Vershel CP, Stachowiak JC. Multifunctional Transmembrane Protein Ligands for Cell-Specific Targeting of Plasma Membrane-Derived Vesicles. Small. 2016;12:3837-48
181. Kooijmans SAA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:1-11
182. Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N. et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol Ther. 2013;21:185-91
183. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-90
184. Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H. et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277
185. Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X. et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS nano. 2016;10:3323-33
186. Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J. et al. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS nano. 2013;7:7698-710
187. Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lässer C. et al. RNAi delivery by exosome-mimetic nanovesicles - Implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231-8
188. Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211-22
189. Armstrong JP, Holme MN, Stevens MM. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS nano. 2017;11:69-83
190. Sato YT, Umezaki K, Sawada S, Mukai S-a, Sasaki Y, Harada N. et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933
191. Yang Y, Hong Y, Nam GH, Chung JH, Koh E, Kim IS. Virus-Mimetic Fusogenic Exosomes for Direct Delivery of Integral Membrane Proteins to Target Cell Membranes. Adv Mater. 2017;29:1605604
192. Zhang H-G, Kim H, Liu C, Yu S, Wang J, Grizzle WE. et al. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116-23
193. Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C. et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352:242-6
194. Evander M, Gidlöf O, Olde B, Erlinge D, Laurell T. Non-contact acoustic capture of microparticles from small plasma volumes. Lab Chip. 2015;15:2588-96
195. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Controlled Release. 2015;199:145-55
196. de la Fuente A, Alonso-Alconada L, Costa C, Cueva J, Garcia-Caballero T, Lopez-Lopez R. et al. M-Trap: Exosome-Based Capture of Tumor Cells as a New Technology in Peritoneal Metastasis. J Natl Cancer Inst. 2015;107:djv184
Author contact
![]() Corresponding authors: Tel & Fax: +86-571-86971683, E-mail: bwangedu.cn (B.W.), luoyan2011edu.cn (Y.L.), zhengshuedu.cn (S.Z.)
Corresponding authors: Tel & Fax: +86-571-86971683, E-mail: bwangedu.cn (B.W.), luoyan2011edu.cn (Y.L.), zhengshuedu.cn (S.Z.)
 Global reach, higher impact
Global reach, higher impact