13.3
Impact Factor
Theranostics 2018; 8(3):812-814. doi:10.7150/thno.24183 This issue Cite
Editorial
“Albumin Hitchhiking” with an Evans Blue Analog for Cancer Theranostics
1. Department of Medical Physics, University of Wisconsin - Madison, Madison, WI 53705
2. Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
3. Department of Radiology, University of Wisconsin - Madison, Madison, WI 53705
4. Carbone Cancer Center, University of Wisconsin - Madison, Madison, WI 53705
Received 2017-12-4; Accepted 2017-12-5; Published 2018-1-1
Abstract
Although 177Lu-DOTA-TATE was recently approved in Europe for the treatment of certain neuroendocrine tumors, continued development and optimization has been ongoing to further improve the therapeutic efficacy of somatostatin receptor 2 targeted peptide receptor radionuclide therapy, as well as reducing the renal toxicity. In this work, the use of an Evans blue analog for “albumin hitchhiking” resulted in significant improvement in both the imaging performance and therapeutic efficacy of radiolabeled octreotate, as well as reducing the toxicity since much less radioactivity was used for therapy. Upon clinical translation, such “albumin hitchhiking” could make significant impact in the near future for cancer patient management.
Keywords: somatostatin receptor 2 (SSTR2), peptide receptor radionuclide therapy (PRRT), octreotate (TATE), positron emission tomography (PET), theranostics, cancer, precision medicine
Precision medicine is the future for cancer patient management [1]. With the continued development of cancer diagnostic and therapeutic agents over the last several decades, many of these agents are already widely used in the clinic. One of the best examples is the targeting of somatostatin receptor 2 (SSTR2) with certain peptides for peptide receptor radionuclide therapy (PRRT) [2]. Two of the most commonly used peptides for PRRT are octreotide and octreotate (TATE), where TATE possesses higher binding affinity and selectivity towards SSTR2. In typical clinical practice, 68Ga-labeled octreotide or TATE is used first for positron emission tomography (PET) imaging to confirm that the neuroendocrine tumors in patients express high levels of SSTR2, before a large therapeutic dose (or multiple doses) of 177Lu-labeled TATE is administered for non-invasive and targeted cancer therapy.
Such SSTR2-targeted PRRT has been a shining example of cancer theranostics and precision medicine over the last decade. Currently, 177Lu-DOTA-TATE (Brand name: Lutathera) has already been approved in Europe for “the treatment of unresectable or metastatic, progressive, well differentiated (G1 and G2), somatostatin receptor positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in adults” [3]. In the Unites States, this agent is not yet approved by the FDA, although it may happen in the near future. Some of the well-recognized limitations for 177Lu-DOTA-TATE include the facts that: 1) the therapeutic efficacy is still limited, with modest complete response (CR) and partial response (PR) rate; 2) due to the fast renal clearance of the agent, there is significant renal toxicity associated with 177Lu-DOTA-TATE [4]. Therefore, continued development and optimization has been ongoing to further improve the therapeutic efficacy of SSTR2-targeted PRRT, as well as to reduce the undesirable toxicity.
In this issue of Theranostics, Chen and colleagues reported the use of an Evans blue analog (EB) for albumin hitchhiking to improve both the PET imaging performance and therapeutic efficacy of radiolabeled TATE in several animal tumor models, targeting SSTR2 [5]. Both the imaging and therapy results in these animal tumor models are very exciting, which could have significant clinical impact in the near future, once they are translated into the clinic.
Since human serum albumin is the most abundant protein in blood plasma (~ 50 mg/ml), it has been intensively investigated as a drug carrier for several decades. A large number of literature reports exist regarding the use of “albumin hitchhiking” to improve the pharmacokinetics of various imaging and/or therapeutic agents [6]. The agents that have been used for albumin binding include lipids, fatty acids, peptides, albumin binding motif, among many others, and many of these indeed led to significantly improved performance in vivo [6]. In this work [5], the researchers used an Evans blue analog (EB), which binds to albumin in a reversible manner, to improve the pharmacokinetics of TATE for neuroendocrine tumor imaging and therapy in several animal models. As for the radioisotope pair, the investigators used 86Y (a PET isotope with a decay half-life of 14.7 h) and 90Y (a beta-emitter with a decay half-life of 64.1 h and maximum energy of 2.28 MeV), which are from the same element. Hence, the imaging and therapy agents are the same chemical entity, truly embodying the notion of theranostics with exactly the same molecule. Such a perfectly matched theranostic pair will certainly facilitate clinical translation when compared to other theranostics agents that use radioisotopes from different elements (e.g. 68Ga/177Lu).
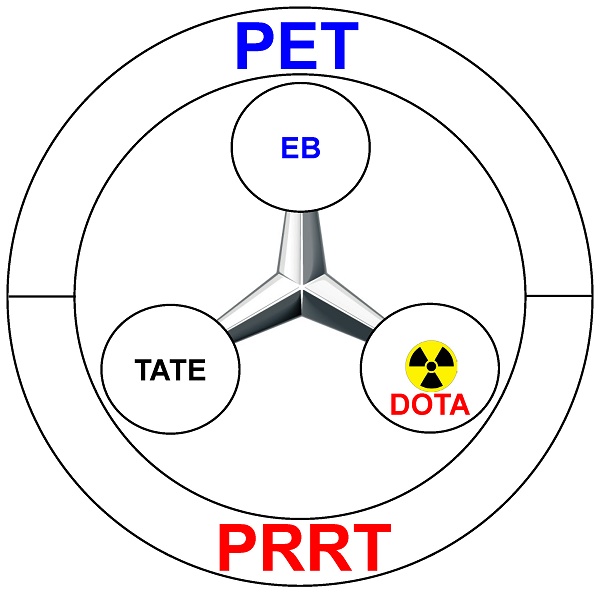
The chemical synthesis of the agent was quite straightforward, via a short linker that connects the three motifs: TATE (for SSTR2 binding), EB (for albumin hitchhiking), and DOTA (for 86Y/90Y labeling and PET/PRRT) (Figure 1). Various in vitro studies in multiple cell lines confirmed that such chemical conjugation did not significantly alter the SSTR2-binding affinity of TATE, or the albumin binding affinity of EB. Equally importantly, such modification did not significantly alter the internalization of TATE, which is critical for tumor uptake and PET imaging contrast, as well as the therapeutic efficacy of PRRT. A pleasant surprise in the experimental results is that although EB-TATE only exhibited about twice the circulation half-life when compared to that of TATE, the tumor uptake was much higher at about 4-fold. Even in a medium SSTR2-expressing model (i.e. HCT116/SSTR2), such a small molecule (i.e. 86Y-EB-TATE) was able to accumulate in the tumor at ~30 %ID/g within 24 h post-injection, outperforming many radiolabeled monoclonal antibodies in the literature [7-9]. In the model that expresses high level of SSTR2, the AR42J model, tumor uptake of 86Y-EB-TATE was about 60 %ID/g, which is an extremely impressive number!
Schematic representation of 86Y/90Y-EB-TATE for positron emission tomography (PET) and peptide receptor radionuclide therapy (PRRT).
Absolute tumor uptake of the radiopharmaceutical is only part of the story. According to the area under the curve (AUC) calculation, 86Y-EB-TATE had ~6-fold higher tumor exposure to the radioisotope than 86Y-TATE. Since it is almost impossible to measure the absolute biodistribution of 90Y-EB-TATE over time, hence it is very challenging to calculate the tumor radiation dose when 90Y is used, the difference in tumor radiation dose between the two agents (i.e. 90Y-EB-TATE vs. 90Y-TATE) could be much higher than the numbers calculated for 86Y, since 90Y has a much longer decay half-life. The decline in tumor radioactivity at late time points from mice injected with 86Y-EB-TATE was mostly due to radioactive decay, rather than biological clearance of the agent from the tumor.
With such high tumor uptake and persistent retention of 86Y/90Y-EB-TATE radioactivity in the tumor, it is of no surprise that the therapeutic effect was also very promising and long lasting. More importantly, the radioactivity dose administered was much lower than other studies reported in the literature. 0.2 mCi (i.e. 7.4 MBq) of 90Y-EB-TATE was able to achieve an almost 100% survival rate for at least 180 days in mice bearing either the HCT116/SSTR2 or AR42J tumors. Based on dosimetry estimations, the investigators concluded that treatment of a mouse with an average weight of 27 g with 7.4 MBq of 90Y-EB-TATE is equivalent to the treatment of a 60 kg patient with 1.3 GBq of radioactivity, which is more than 10 times lower than the cumulative ~30 GBq of radioactivity used in the clinic for 177Lu-DOTA-TATE [3]. Since a much lower amount of radioactivity is used for the investigated therapeutic applications, the potential (renal) toxicity of such treatment was also expected to be much lower. Indeed, histopathological staining of major organs and blood analysis showed no obvious difference between the treated groups and control mice.
Such exciting preclinical results clearly warrant clinical translation. According to personal communications with the investigators, clinical translation and investigation of this agent is currently ongoing in China. Furthermore, unpublished results by the investigators comparing 177Lu-EB-TATE and Lutathera were also quite encouraging, which further increased the potential clinical impact and future broad applications of this strategy of EB-based albumin hitchhiking. Based on clinicaltrials.gov, a Phase I trial of 177Lu-EB-TATE in patients with advanced metastatic neuroendocrine tumors is already ongoing in China (https://clinicaltrials.gov/show/NCT03308682). We look forward to the exciting clinical results in the near future.
Although this report is an excellent example of using EB to improve the pharmacokinetics of imaging/therapeutic agents, such albumin hitchhiking with EB is by no means limited to TATE. Over the last several years, the Chen group has published a large number of studies using EB to improve the performance of various agents [6, 10-15], which makes such EB-based albumin hitchhiking a generally applicable platform technology. Not only can this strategy be employed in oncologic applications, but it can also be utilized to improve the therapeutic efficacy of various agents in other diseases such as diabetes [12]. With continued development and application across many biomedical areas, as well as future clinical translation, this strategy holds tremendous promise as a platform technology for the new era of precision medicine and theranostics, not only in cancer patient management, but also in various other devastating diseases.
Acknowledgements
The authors are grateful for the financial support from the University of Wisconsin - Madison, the National Institutes of Health (NIBIB/NCI P30CA014520, T32GM008505, and T32CA009206), and the American Cancer Society (125246-RSG-13-099-01-CCE).
References
1. Ehlerding EB, Cai W. Harnessing the Power of Molecular Imaging for Precision Medicine. J Nucl Med. 2016;57:171-2
2. Smit Duijzentkunst DA, Kwekkeboom DJ, Bodei L. Somatostatin Receptor 2-Targeting Compounds. J Nucl Med. 2017;58:54s-60s
3. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-35
4. Fani M, Nicolas GP, Wild D. Somatostatin Receptor Antagonists for Imaging and Therapy. J Nucl Med. 2017;58:61s-6s
5. Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang Z, Zhu G. et al. Evans Blue Attachment Enhances Somatostatin Receptor Subtype-2 Imaging and Radiotherapy. Theranostics. 2018;8:735-45
6. Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432-56
7. England CG, Hernandez R, Eddine SB, Cai W. Molecular Imaging of Pancreatic Cancer with Antibodies. Mol Pharm. 2016;13:8-24
8. England CG, Rui L, Cai W. Lymphoma: current status of clinical and preclinical imaging with radiolabeled antibodies. Eur J Nucl Med Mol Imaging. 2017;44:517-32
9. Lamberts LE, Williams SP, Terwisscha van Scheltinga AG, Lub-de Hooge MN, Schroder CP, Gietema JA. et al. Antibody positron emission tomography imaging in anticancer drug development. J Clin Oncol. 2015;33:1491-504
10. Chen H, Jacobson O, Niu G, Weiss ID, Kiesewetter DO, Liu Y. et al. Novel "Add-On" Molecule Based on Evans Blue Confers Superior Pharmacokinetics and Transforms Drugs to Theranostic Agents. J Nucl Med. 2017;58:590-7
11. Chen H, Wang G, Lang L, Jacobson O, Kiesewetter DO, Liu Y. et al. Chemical Conjugation of Evans Blue Derivative: A Strategy to Develop Long-Acting Therapeutics through Albumin Binding. Theranostics. 2016;6:243-53
12. Liu Y, Wang G, Zhang H, Ma Y, Lang L, Jacobson O. et al. Stable Evans Blue Derived Exendin-4 Peptide for Type 2 Diabetes Treatment. Bioconjug Chem. 2016;27:54-8
13. Niu G, Lang L, Kiesewetter DO, Ma Y, Sun Z, Guo N. et al. In Vivo Labeling of Serum Albumin for PET. J Nucl Med. 2014;55:1150-6
14. Wang Y, Lang L, Huang P, Wang Z, Jacobson O, Kiesewetter DO. et al. In vivo albumin labeling and lymphatic imaging. Proc Natl Acad Sci U S A. 2015;112:208-13
15. Zhang J, Lang L, Zhu Z, Li F, Niu G, Chen X. Clinical Translation of an Albumin-Binding PET Radiotracer 68Ga-NEB. J Nucl Med. 2015;56:1609-14
Author contact
![]() Corresponding author: Xiaoli Lan: Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Email: LXL730724com. Weibo Cai: 1111 Highland Ave, Madison, WI 53705, USA; Phone: 608-262-1749; Fax: 608-265-0614. Email: wcaiorg
Corresponding author: Xiaoli Lan: Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Email: LXL730724com. Weibo Cai: 1111 Highland Ave, Madison, WI 53705, USA; Phone: 608-262-1749; Fax: 608-265-0614. Email: wcaiorg
 Global reach, higher impact
Global reach, higher impact