13.3
Impact Factor
Theranostics 2018; 8(4):1131-1145. doi:10.7150/thno.22078 This issue Cite
Research Paper
Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery
1. Center for Biomimetic Medicine, Houston Methodist Research Institute, 6670 Bertner Ave. Houston, TX 77030 USA.
2. Department of Cardiovascular Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA
3. Escuela de Ingeniería y Ciencias, Tecnológico de Monterrey, Av. Eugenio Garza Sada 2501 Sur Col. Tecnológico, Monterrey, Nuevo León, México
4. Department of Cardiovascular Sciences, Houston Methodist Research Institute, Houston, TX 77030 USA
5. Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX 77030 USA
6. Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX 75390 USA
7. Department of Surgery, University of Alabama at Birmingham, Birmingham, AL 35294 USA
8. Houston Methodist Orthopedic and Sports Medicine, Houston Methodist Hospital, 6565 Fannin Street Houston, TX 77030 USA.
*Shared authorship
Abstract
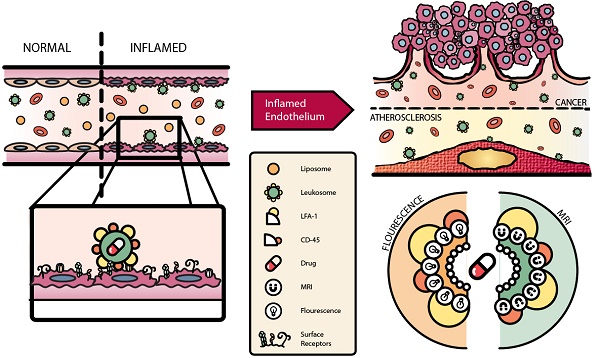
Activation of the vascular endothelium is characterized by increased expression of vascular adhesion molecules and chemokines. This activation occurs early in the progression of several diseases and triggers the recruitment of leukocytes. Inspired by the tropism of leukocytes, we investigated leukocyte-based biomimetic nanoparticles (i.e., leukosomes) as a novel theranostic platform for inflammatory diseases.
Methods: Leukosomes were assembled by combining phospholipids and membrane proteins from leukocytes. For imaging applications, phospholipids modified with rhodamine and gadolinium were used. Leukosomes incubated with antibodies blocking lymphocyte function-associated antigen 1 (LFA-1) and CD45 were administered to explore their roles in targeting inflammation. In addition, relaxometric assessment of NPs was evaluated.
Results: Liposomes and leukosomes were both spherical in shape with sizes ranging from 140-170 nm. Both NPs successfully integrated 8 and 13 µg of rhodamine and gadolinium, respectively, and demonstrated less than 4% variation in physicochemical features. Leukosomes demonstrated a 16-fold increase in breast tumor accumulation relative to liposomes. Furthermore, quantification of leukosomes in tumor vessels demonstrated a 4.5-fold increase in vessel lumens and a 14-fold increase in vessel walls. Investigating the targeting mechanism of action revealed that blockage of LFA-1 on leukosomes resulted in a 95% decrease in tumor accumulation. Whereas blockage of CD45 yielded a 60% decrease in targeting and significant increases in liver and spleen accumulation. In addition, when administered in mice with atherosclerotic plaques, leukosomes exhibited a 4-fold increase in the targeting of inflammatory vascular lesions. Lastly, relaxometric assessment of NPs demonstrated that the incorporation of membrane proteins into leukosomes did not impact the r1 and r2 relaxivities of the NPs, demonstrating 6 and 30 mM-1s-1, respectively.
Conclusion: Our study demonstrates the ability of leukosomes to target activated vasculature and exhibit superior accumulation in tumors and vascular lesions. The versatility of the phospholipid backbone within leukosomes permits the incorporation of various contrast agents. Furthermore, leukosomes can potentially be loaded with therapeutics possessing diverse physical properties and thus warrant further investigation toward the development of powerful theranostic agents.
Keywords: Biomimetic, Leukocyte, Endothelium, Nanoparticles, Magnetic Resonance Imaging, Inflammation
 Global reach, higher impact
Global reach, higher impact